Introduction
Breast cancer is the most commonly diagnosed cancer among women and accounts for approximately 25% of all new cancer cases and 13% of all cancer deaths in Canadian women.Reference Brenner, Weir and Demers1,2 In 2020, it is estimated that about 27,400 Canadian women will be diagnosed with breast cancer (an average of 75 new cases per day) and an estimated 5,100 women will die from the disease (an average of 14 deaths per day).Reference Brenner, Weir and Demers1,2 The most commonly used treatment modalities to effectively treat and control the disease include surgery, systemic therapy and radiation therapy or any combination depending on the stage of the tumour.Reference Kagkiouzis, Platoni and Kantzou3 Surgery is often the first line of treatment for low-risk early stage patients, when the cancer is localised within the breast, followed by adjuvant radiation therapy to reduce the risk of local recurrence and prevent metastasis after lumpectomy or mastectomy.2,Reference Kagkiouzis, Platoni and Kantzou3 This usually implies treatment of the surgical bed or the treatment of the intact breast or chest-wall and a local radiation boost to the surgical bed. When treating the intact breast or chest-wall for low-risk early stage patients, a pair of opposed tangential fields are usually employed.Reference Haciislamoglu, Colak and Canyilmaz4–Reference Osei, Darko and Fleck18 However for high-risk patients with node positive disease or are at greater risk of nodal metastasis, radiation therapy will involve treatment of the intact breast or chest-wall as well as the regional lymph nodes. This is achieved using two tangential fields to treat the intact breast or chest-wall and an anterior and sometimes opposed posterior fields to treat the regional lymph nodes in the upper axillary and supraclavicular regions.Reference Kagkiouzis, Platoni and Kantzou3,Reference Poortmans, Collette and Kirkove19–Reference Zheng, Lai and Zhou27
There are several techniques used for breast radiation therapy including the use of wedges, field-in-field (FIF), hybrid technique (involving open and optimised fields), intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT).Reference Kagkiouzis, Platoni and Kantzou3–Reference Zheng, Lai and Zhou27 Several studies have reported that using IMRT, VMAT, FIF or the hybrid technique to treat the breast and regional lymph nodes have greater target coverage, reduced hotspots and reduced doses to the organs at risk (OAR) compared to conventional treatment with wedges.Reference Haciislamoglu, Colak and Canyilmaz4,Reference Jin, Chen and Deng6,Reference Fogliata, Seppälä and Reggiori7,Reference Zhang, Hu and Xie10,Reference Smith, Estoesta and Kader11,Reference Ma, Zhang and Lu14,Reference Osei, Darko and Fleck18,Reference Deseyne, Speleers and De Neve23,Reference Zheng, Lai and Zhou27–Reference Bergom, Currey and Desai30 Some studiesReference Jin, Chen and Deng6,Reference Nokhasteh, Nazemi and Hejazi9,Reference Xie, Ouyang and Wang12–Reference Ma, Zhang and Lu14,Reference Tanaka, Hayashi and Kajiura16,Reference Ghadimi, Jabbari and Karimkhani17,Reference Csenki, Újhidy and Cserháti21,Reference Rafic, Peace and Babu26,Reference D’Avenia, Nigro and Camarda31 that have evaluated and compared the dosimetric parameters of three-dimensional conformal radiation therapy using FIF technique have reported significant maximum dose and hotspots reduction, improved dose coverage and a more homogenous distribution within the target. Other studiesReference Jin, Chen and Deng6,Reference Bahrainy, Kretschmer and Jöst8,Reference Smith, Estoesta and Kader11,Reference Xie, Ouyang and Wang12,Reference Yim, Suttie and Bromley15,Reference Osei, Darko and Fleck18,Reference Rafic, Peace and Babu26,Reference Zheng, Lai and Zhou27 have also used the hybrid technique for breast radiotherapy and observed similar improved dose coverage, reduced hotspots in the target and less dose to the OARs. The impact of respiratory motion during breast radiation therapy has also been investigated comparing free-breathing (FB) and deep inspiration breath hold (DIBH) techniques.Reference Mansouri, Naim and Glaria5,Reference Fogliata, Seppälä and Reggiori7,Reference Smith, Estoesta and Kader11,Reference Xie, Ouyang and Wang12,Reference Osei, Darko and Fleck18,Reference Hjelstuen, Mjaaland and Vikström20,Reference Surmann, van der Leer and Branje25,Reference Bergom, Currey and Desai30 Mansouri et al.,Reference Mansouri, Naim and Glaria5 Osei et al.Reference Osei, Darko and Fleck18 and Hjelstuen et al.Reference Hjelstuen, Mjaaland and Vikström20 reported low dose to the heart with the DIBH technique as the heart is pushed out of the high dose region (increased distance from the field edge to the heart) due to increased lung volume during inspiration. Other studiesReference Csenki, Újhidy and Cserháti21,Reference Deseyne, Speleers and De Neve23 have also investigated and evaluated radiation doses to the heart for patients treated in either the supine or prone positions during treatment. Csenki et al.Reference Csenki, Újhidy and Cserháti21 used the 4-field technique to treat patients in either the supine or prone positions and observed that the prone position approach resulted in significantly less dose to the heart and lung compared to the FB supine position although the target dose coverage was similar.
One of the challenges in radiation treatment planning is the lack of consistency among different institutions and individuals with regard to what is considered an acceptable treatment plan in terms of target coverage and doses to the OAR. Clinical trialsReference Chen, White and Vicini32,Reference Mamounas, White and Bandos33 usually resolve this issue by providing well-defined criteria for treatment plans acceptability within the trial whereby any plan fulfilling the criteria is considered acceptable, whereas any plan not fulfilling all the criteria may be considered unacceptable. The availability of these criteria lessens the stress on dosimetrists, as they can present treatment plans to radiation oncologists, which are less likely to be rejected and therefore could potentially improve confidence in dosimetrists, reduce variation in treatment plans and improve workflow and patient care.Reference Osei, Darko and Fleck18 Despite these benefits, several institutions are yet to develop local institutional criteria for accepting treatment plans. Therefore, there is a growing need for the development of local site-specific treatment plan acceptability criteria in order to minimise significant variations in patient treatment plans.
Aim of study
In a previous study,Reference Osei, Darko and Fleck18 we described the establishment of well-defined criteria for accepting treatment plans of intact breast and chest-wall for low-risk early stage breast cancer patients’ treatment plans. In order to develop similar institutional criteria for accepting volume-based breast radiation therapy treatment plans for high-risk patients with nodal involvement based on our current experiences and resources, we conducted this comprehensive retrospective dosimetric analysis of 3 and/or 4 field radiation therapy plans for high-risk breast cancer patients. This study therefore reports on the dosimetric evaluation of the 3 and/or 4 field technique for high-risk breast cancer treatment at our centre over a 4-year period and suggests criteria for treatment plans acceptability. The implementation of such criteria would establish an evaluation process to define a consistent and transparent treatment path for all patients that reduces significant variations in the acceptability of treatment plans.
Materials and Methods
We retrospectively evaluated the treatment plans of 354 high-risk breast cancer patients with nodal involvement who were treated at our cancer centre over a 4-year period. All patients were treated with a prescription dose of 50 Gy in 25 fractions to the intact breast or chest-wall and 50 Gy in 25 fractions to the supraclavicular region and, based on patient suitability and tolerance, were treated either using the DIBH technique or the FB technique.
Patient positioning for computer tomography scan
The standard protocol for patient positioning during computer tomography (CT) scan for breast cancer patients with nodal involvement at our institution is usually the supine position with both arms raised above the patient’s head. The arms are supported in position with a custom made Vac-Lok cushion, and a Kneefix cushion is usually placed under the knees. If breast tissue falls above the second intercostal space as a result of patient positioning, then a breast board is used at an appropriate inclined angle. The use of a head rest is optional but may be used depending on patient comfort and folds in the skin tissue. Usually, the patient’s head will be slightly turned to the contralateral side. If the patient cannot be scanned through the CT simulator bore diameter due to the arms positioning, the positioning may be modified such that the ipsilateral arm is raised above the head and the contralateral arm is placed by the patient’s side. In some cases, a breast sling may be used to support the breast when a pendulous breast falls too far laterally that result in a large lung volume being included in the treatment field or if the breast falls inferiorly and creates a fold.
Patient CT simulation
The detailed CT scan protocol used for low-risk breast cancer patients at our institution has been described by Osei et al.Reference Osei, Darko and Fleck18 In addition, for high-risk patients, radio-opaque markers are placed on the scar and the surrounding breast tissue for visualisation on the radiograph. A marker is also placed at the level of the second intercostal space to guide the placement of the isocentric tattoo. All patients are scanned using the institutional ‘breast’ CT scan protocol with a slice thickness of 3 mm. The scan extends superiorly from the mandible to inferiorly to include the breast and lung tissue. A reproducible 3-point setup is marked on the patient for tattoo localisation. Tattoos are placed at the anterior, right lateral and left lateral setup points. Additional tattoos are placed at the isocentre and inferiorly for leveling if the patient’s anatomy requires a more stable setup point. The CT scan dataset and all points were then exported into the Eclipse TPS (Version 13·6; Varian Medical Systems, Palo Alto, CA, USA).
Field arrangements and borders for treatment planning
Supraclavicular field(s)
The isocentre is placed at the base of the clavicle. The supraclavicular area is usually treated with a single anterior field (for a 3 field arrangement) which is usually angled slightly away from the vertebral bodies. The superior border of this field is placed at the inferior level of the cricoid cartilage and the inferior border at the isocentre at the base of the clavicle. The lateral borders are placed at the neck of the humeral head and the medial borders at 1 cm from the spinal cord. Multileaf collimators are used to shield the humeral head and larynx (Figure 1). Currently, the lymph nodes in the supraclavicular region are not contoured; therefore, the radiation oncologist will usually examine the isodose lines coverage to ensure adequate coverage of the lymph nodes. Depending on the acceptability of the dose coverage in the axilla, a posterior field (opposed anterior field) may be added to the supraclavicular area creating a 4 field monoisocentric field arrangement.
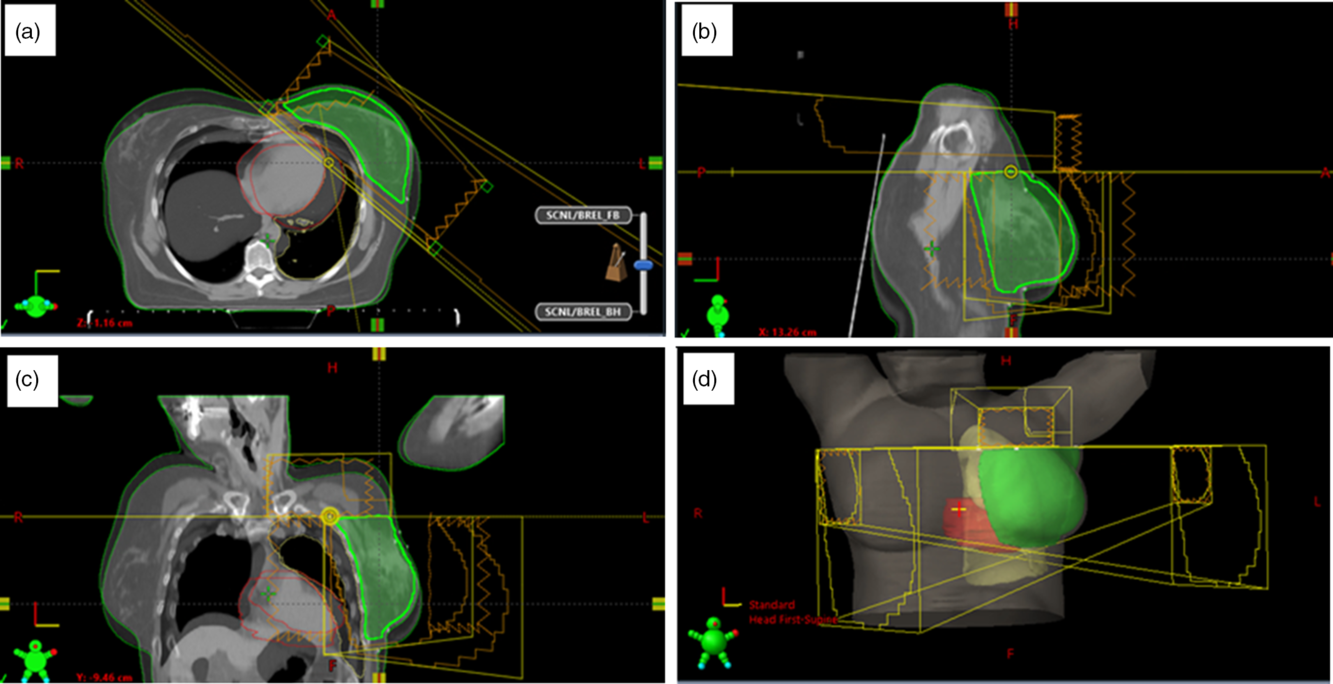
Figure 1. Beams arrangement for a 3 field radiation therapy of the breast and the supraclavicular lymph nodes: two tangential fields to treat the intact breast or chest-wall and a third field to treat the supraclavicular lymph nodes. The figure shows the blended images of the CT datasets for both free breathing and deep inspiration breathe hold for the same patient in the transverse (a), sagittal (b), coronal (c) views and the 3-D visualisation of the breast, lung, heart and the field placements (d).
Tangential fields
The intact breast or chest-wall is treated with an opposing pair of tangential fields, and the detailed description of the field borders for these fields has been described by Osei et al.Reference Osei, Darko and Fleck18 In summary, the superior field border is set at the level of the second intercostal space but may be adjusted to clinically cover the entire breast tissue. The inferior border is set at 1·5 cm inferior to the inframammary fold. The medial tangent field border is set medially at midline and the lateral border is set at the mid-axillary line or 1·0 cm posterior to palpable breast tissue (Figure 1).
Target volumes and OAR
The detailed description of the contouring of the targets and OAR volumes has been described by Osei et al.Reference Osei, Darko and Fleck18 The following structures are usually generated for treatment plan evaluation; the body, the ipsilateral lungs, a treated volume (i.e., 50% isodose line converted to a structure to help create the breast volume), PTV_eval [breast volume for evaluation, which is a contraction of 5 mm (in all directions) of the breast contour] and the heart for left sided breast patients. The ipsilateral lung is usually contoured with auto-segmentation with manual verification. Currently, the lymph nodes in the supraclavicular region are not contoured and are therefore not evaluated in this study.
Radiation treatment planning
A 3-field or 4-field monoisocentric plan is generated by the treatment planner using either 6, 10 and 15 MV beam energies or a combination of energies depending on patient breast size or separation to ensure acceptable dose coverage; however, low-energy beams are usually used for the optimised field. All patients were treated with a prescription dose of 50 Gy in 25 fractions to the intact breast or chest-wall using two tangential fields and 50 Gy in 25 fractions to the supraclavicular region with a 3rd and/or 4th fields. Usually, a 0·5 cm or 1 cm thickness of bolus is placed on the chest-wall to ensure adequate skin dose coverage. A hybrid IMRT technique using both ‘open’ and optimised tangential fields and the dose distribution from the supraclavicular field(s) used as the based dose plan are used for generating the 3-field or 4-field monoisocentric treatment plans. The details of the hybrid IMRT technique have been described elsewhereReference Osei, Darko and Fleck18 and the technique has the potential to produce a homogeneous dose distribution within the breast or chest-wall PTV_eval.
Daily treatment
Patients are set up as per the CT simulation using the tattoos on the patient which were marked during the CT and the in-room lasers as per institutional protocol. Patients are ensured to be straight using a single isocentre and inferior straightening tattoos and the target-to-skin distances and clearance of breast tissue are also checked. Images are taken on Day 1 and weekly thereafter for verification of field portals for all patients: An anterior kV image is taken for supraclavicular field verification and it is matched to bony anatomy and a medial MV image is taken to verify and match lung volume. Although there are standard images taken for all patients, other images may be repeated more often at the discretion of the treating radiation therapists to ensure the correct target is being treated.
Indices for PTV_eval
The plan quality in this study was quantitatively evaluated by calculating the homogeneity index (HI), uniformity index (UI) and the conformity index (CI) for the PTV_eval. The HI, CI and UI evaluate the dose homogeneity, conformity and uniformity, respectively, within the PTV_eval and are calculated as
 $$HI = {{{D_2} - {D_{98}}} \over {DPD}}$$
$$HI = {{{D_2} - {D_{98}}} \over {DPD}}$$
 $$UI = {{{D_5}} \over {{D_{95}}}}$$
$$UI = {{{D_5}} \over {{D_{95}}}}$$
 $$CI = {{{V_{RI}}} \over {TV}}$$
$$CI = {{{V_{RI}}} \over {TV}}$$
where D2, D5, D95 and D98 are the doses received by 2, 5, 95 and 98% of the PTV_eval, respectively. DPD is the prescribed dose, VRI is the volume of PTV_eval covered by the reference isodose line (in this case the 95% isodose line) and TV is the target volume (in this case the PTV_eval). The values of CI and UI close to unity indicate greater conformity and uniformity, and values of HI close to zero indicate greater homogeneity.
Treatment plan data analysis
We retrospectively evaluated the treatment plans of 354 high-risk breast cancer patients with nodal involvement or at risk of nodal involvement who were treated at our cancer centre over a 4-year period. For each patient, we determined the mean, median, maximum, minimum and standard deviation of the breast separation, the HI, UI and the CI for the PTV_eval and volumes of the PTV_eval, ipsilateral lung and heart (for patients whose left-sided breast was treated and for whom the heart is usually contoured). We also analysed the dose-volume histograms (DVHs) for the PTV_eval and the OARs. We stratified patients into those who received treatment using the DIBH and those who used the FB technique for their treatment. The PTV_eval coverage is evaluated to ensure that the plan meets the following criteria for the normalised PTV_eval receiving 92 (V92%), 95 (V95%) and 105 (V105%) of the prescription dose such that V92% ≥ 99, V95% ≥ 95 and V105% < 5% and adequate dose coverage in the axilla region as determined by the radiation oncologist based on the isodose lines coverage. For the OARs, plan acceptability is determined based on the following organs volume dose constraints: The volume dose constraints used for the ipsilateral lung are V5Gy ≤ 65, V10Gy ≤ 45 and V20Gy ≤ 25% (ideal) or V20Gy ≤ 35%, (acceptable) and the heart volume dose constraints are V25Gy ≤ 10% and the mean heart dose ≤ 3 Gy (ideal) or V30Gy ≤ 10% and the mean heart dose ≤ 5 Gy (acceptable).Reference Parulekar, Berrang, Kong and Rakovitch34
Results and Discussions
The treatment plans of high-risk breast cancer patients treated with the 3 and/or 4 field technique at our cancer centre over a 4-year period using either the FB or the DIBH techniques were evaluated. Figure 1 shows typical beams arrangement for a 3 field radiation therapy of the breast or chest-wall and supraclavicular lymph nodes: two tangential fields to treat the intact breast or chest-wall and a third field to treat the supraclavicular lymph nodes. Based on patients suitability and tolerance, 130 (36·7%) patients were treated using the DIBH technique and 224 (63·3%) were treated with the FB technique. There were 169 (47·7%) patients treated with intact breast, whereas 185 (52·3%) were treated for post-mastectomy chest-wall.
Analysis of PTV_eval Dose
Figure 2 shows the DVH plots of the intact breast and chest-wall PTV_eval for patients treated using either the DIBH or FB techniques. A summary of the measures of central tendency and dispersion of patients breast separation, PTV_eval volume and normalised PTV_eval volume receiving 90, 92, 95, 100, 101, 102, 103, 104, 105 and 106% of the prescription dose for all patients and stratified by breast separation into small (< 20 cm), medium (20 ≤ x ≥ 25) and large (> 25 cm) groups are shown in Table 1. A similar summary of the measures of central tendency and dispersion is shown in Tables 2 and 3 when the patients were stratified into those treated for the intact breast and those treated for the chest-wall post-mastectomy, respectively. The mean patient breast separation and PTV_eval are 21·1 ± 3·1 cm and 868·9 ± 506·0 cc, respectively (Table 1). When the patients were stratified into those who received treatment to the intact breast (Tables 2) and those who received treatment to the chest-wall post-mastectomy (Table 3), the mean breast separations were 21·5 ± 3·3 cm and 20·8 ± 3·0 cm and the PTV_eval volumes were 1079·1 ± 557·2 cc and 676·9 ± 360·7 cc for intact breast and chest-wall patients, respectively. A two tail student t-test of the mean breast separations (p-value = 0·04) and mean PTV_eval volumes (p-value < 0·001) indicates that there is significant difference in the patients breast separations and the PTV_eval volumes when patients were stratified into intact breast and post-mastectomy chest-wall.
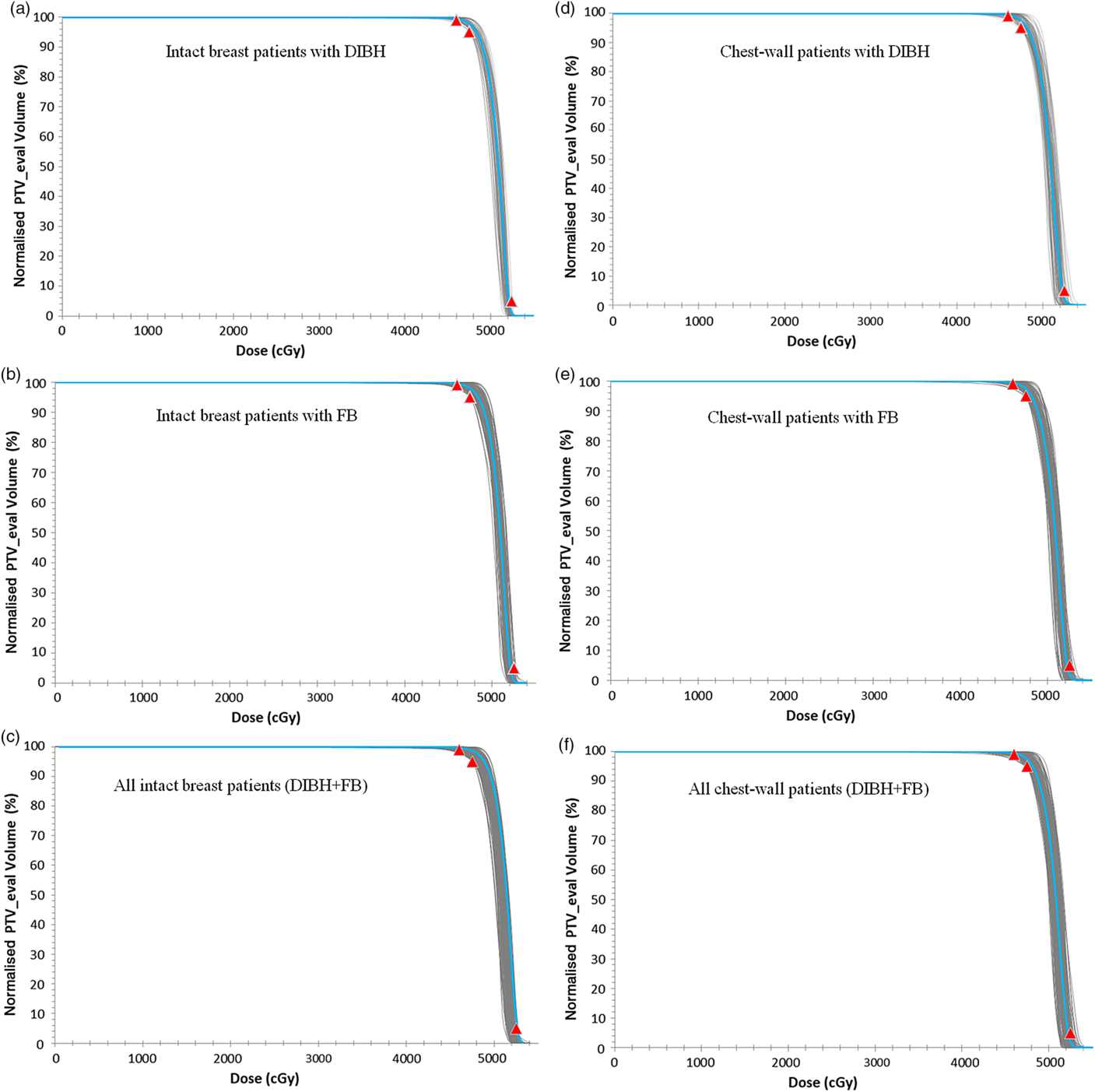
Figure 2. A plot of the dose-volume histogram (DVH) of the intact breast (a, b and c) and chest-wall (d, e and f) PTV_eval for patients treated using either the DIBH technique (a and d) or FB technique (b and e). (c) All treated intact breast patients and (f) all treated chest-wall patients. The grey lines represent individual patients DVH and the blue lines in each plot are the mean DVHs. The red data points are the planning dose objectives of V92% ≥ 99, V95% ≥ 95 and V105 < 5%.
Abbreviations: DIBH, deep inspiration breathe hold; FB, free breathing.
Table 1. A summary of the measures of central tendency and dispersion of patient breast separation, PTV_eval volume and normalised PTV_eval volume receiving 90, 92, 95, 100, 101, 102, 103, 104, 105 and 106% of the prescription dose for all patients and stratified by breast separation into small (< 20 cm), medium (20 ≤ x ≥ 25) and large (> 25 cm) main groups
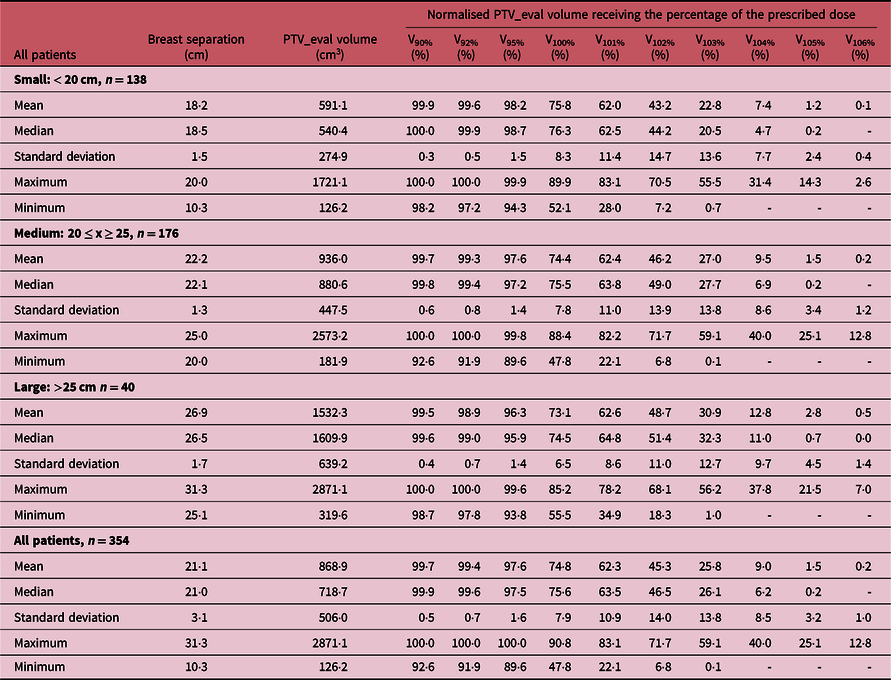
‘-’ Indicates the value is less than 0·1%.
Table 2. A summary of the measures of central tendency and dispersion of patient breast separation, PTV_eval volume and normalised PTV_eval volume receiving 90, 92, 95, 100, 101, 102, 103, 104, 105 and 106% of the prescription dose for patients treated with intact breast and stratified by breast separation into small (< 20 cm), medium (20 ≤ x ≥ 25) and large (> 25 cm) main groups
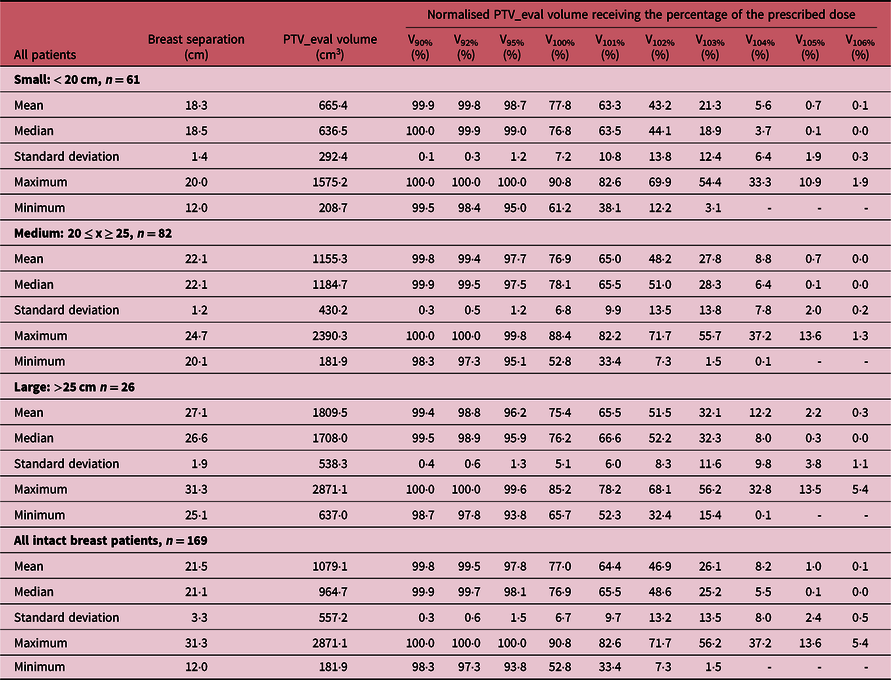
‘-’ Indicates the value is less than 0·1%.
Table 3. A summary of the measures of central tendency and dispersion of patient breast separation, PTV_eval volume and normalised PTV_eval volume receiving 90, 92, 95, 100, 101, 102, 103, 104, 105 and 106% of the prescription dose for post-mastectomy chest-wall patients and stratified by breast separation into small (< 20 cm), medium (20 ≤ x ≥ 25) and large (> 25 cm) main groups

‘-’ Indicates the value is less than 0.1%.
The mean V92%, V95%, V100%, V101%, V102%, V103%, V104% and V105%, for all patients are 99·4 ± 0·7, 97·6 ± 1·6, 74·8 ± 7·9, 62·3 ± 10·9, 45·3 ± 14·0, 25·8 ± 13·8, 9·0 ± 8·5 and 1·5 ± 3·2, respectively. When patients were stratified into the three cohorts based on the breast separation of small (PTV_eval < 20 cm), medium (20 cm ≤ PTV_eval ≤ 25 cm) and large (PTV_eval > 25 cm) groups, the mean V92% (which is our current minimum dose coverage to the PTV_eval of V92% ≥ 99%) were 99·6 ± 0·5, 99·3 ± 0·8 and 98·9 ± 0·7% for the small, medium and large breast (Table 1). Furthermore, when patients were stratified based on whether they were treated for intact breast (Table 2) or chest-wall (Table 3), the target coverages were found to meet our current minimum target coverage and the hotspots (V106) were also very similar. Adequate target coverage is associated with tumour control which leads to improve biochemical relapse-free survival, cancer progression-free survival and cancer-specific survival. Several studiesReference Bahrainy, Kretschmer and Jöst8–Reference Tanaka, Hayashi and Kajiura16,Reference Hjelstuen, Mjaaland and Vikström20,Reference Banaei, Hashemi and Bakhshandeh22,Reference Deseyne, Speleers and De Neve23,Reference Rafic, Peace and Babu26,Reference Zheng, Lai and Zhou27,Reference Bergom, Currey and Desai30 have reported similar PTV_eval coverage for breast cancer patients. Tanaka et al.Reference Tanaka, Hayashi and Kajiura16 investigated the FIF technique with lung blocks for breast radiotherapy and reported V95% and V100% of 93·2 and 62·7% for patients treated with the FB technique. Zheng et al.Reference Zheng, Lai and Zhou27 investigated the dosimetry of seven radiation therapy planning techniques for breast cancer after mastectomy and reported V95%, V100%, V105% of 95 ± 0·0, 32·0 ± 14·9 and 0·16 ± 0·16% for the hybrid technique and 95 ± 0·0, 72·9 ± 8·2 and 15·7 ± 12·5% for FIF-IMRT. Ma et al.Reference Ma, Zhang and Lu14 compared radical mastectomy radiotherapy treatment plans for left-sided breast cancer patients using three-dimensional conformal radiotherapy with FIF technique and reported a V95% of 78·2 ± 4·3%. Hjelstuen et al.Reference Hjelstuen, Mjaaland and Vikström20 also evaluated the reduction in cardiopulmonary radiation doses using the DIBH technique and reported V95% of 98·9 ± 0·5 and 98·8 ± 0·5% for treatment plans based on patient FB and DIBH techniques, respectively.
Analysis of lung dose
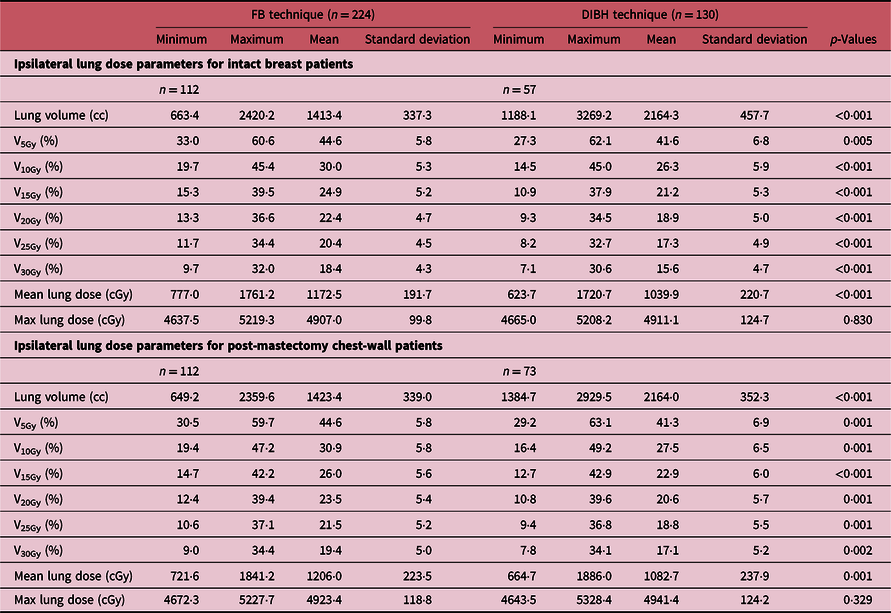
The DVH plots of the ipsilateral lung for patients treated using either the DIBH or the FB techniques are shown in Figure 3, and Figure 4 shows a 3-D visualisation of the ipsilateral lung volume in the tangential and supraclavicular treatment fields during FB and DIBH for the same patient. Table 4 shows a summary of the measures of central tendency and dispersion of the ipsilateral lung volumetric doses for all patients stratified into patients who were treated with the FB and those treated with the DIBH techniques. The mean ipsilateral lung volumes for patients treated with intact breast and post-mastectomy chest-wall are 1413·4 ± 337·3 cc and 1423·4 ± 339·0 cc, respectively, using FB technique, and the corresponding values for patients treated with DIBH are 2164·3 ± 457·7 cc and 2164·0 ± 352·3 cc, respectively. A two tail student t-test of the means of the ipsilateral lung volumes for intact breast and chest-wall patients with FB (p-value = 0·83) and with DIBH (p-value = 0·99) techniques shows no significant difference in the mean lung volumes when comparing intact breast and chest-wall patients irrespective of the technique (Table 4). However, there was a significant difference (about 53% increased) in patients mean lung volumes between FB and DIBH techniques for both intact breast and chest-wall patients (Table 4, Figure 3). A two tail student t-test of the means of the lung volumes with FB and DIBH techniques for intact breast patients (p-value < 0·001) and for chest-wall patients (p-value < 0·001) indicates a significant difference in mean lung volumes between FB and DIBH techniques irrespective of the target volume (i.e., intact breast or chest-wall). The dose to the ipsilateral lung was indexed by V5Gy, V10Gy, V15Gy, V20Gy, V25Gy and V30Gy (i.e., the percentage volume of the ipsilateral lung receiving at least the indicated dose). The mean ipsilateral lung V5Gy, V10Gy, V15Gy, V20Gy, V25Gy and V30Gy for patients treated with intact breast and using the FB technique are 44·6 ± 5·8, 30·0 ± 5·3, 24·9 ± 5·2, 22·4 ± 4·7, 20·4 ± 4·5, 18·4 ± 4·3 and 44·6 ± 5·8%, 30·9 ± 5·8, 26·0 ± 5·6, 23·5 ± 5·4, 21·5 ± 5·2 and 19·4 ± 5·0% for post-mastectomy chest-wall patients, respectively. Similarly, the V5Gy, V10Gy, V15Gy, V20Gy, V25Gy and V30Gy for patients treated using the DIBH technique are 41·6 ± 6·8, 26·3 ± 5·9, 21·2 ± 5·3, 18·9 ± 5·0, 17·3 ± 4·9 and 15·6 ± 4·7% for patients treated with intact breast and 41·3 ± 6·9, 27·5 ± 6·5, 22·9 ± 6·0, 20·6 ± 5·7, 18·8 ± 5·5 and 17·1 ± 5·2% for post-mastectomy chest-wall patients, respectively. The p-values using a two tail student t-test analyses of the means for the V5Gy, V10Gy, V15Gy, V20Gy, V25Gy and V30Gy for the ipsilateral lung comparing the DIBH and FB techniques are well below 0·05 (i.e., p < 0·05) indicating that there is significant differences in the lung volumetric doses when comparing the two treatment techniques. The reason for the increased dose with DIBH being that during inspiration the lung takes in more air and hence increases in volume, thereby extending into both the tangential and supraclavicular treatment fields (Figure 4). Banaei et al.Reference Banaei, Hashemi and Bakhshandeh22 compared monoisocentric and dual isocentric techniques for mastectomy patients undergoing chest-wall radiotherapy using the 3 field technique and reported ipsilateral lung V10Gy = 26·6, V15Gy = 22·9% and mean lung dose of 13·0 Gy. Takano et al.Reference Takano, Omura and Suzuki35 compared the dosimetry of TomoDirect, TomoHelical and 3D-CRT plans in left breast cancer patients who received postoperative radiation therapy to the chest-wall and supraclavicular lymph node areas and reported mean ipsilateral lung V5Gy, V10Gy, V15Gy, V20Gy and mean lung dose of 55·37 ± 5·37, 46·56 ± 5·98, 42·62 ± 6·25, 39·95 ± 6·34 and 19·64 ± 3·04% for 3D-CRT plans. Ma et al.Reference Ma, Zhang and Lu14 also reported V5Gy, V10Gy and V20Gy of 49·6 ± 7·8, 37·5 ± 7·1 and 31·4 ± 6·0%, whereas Hjelstuen et al.Reference Hjelstuen, Mjaaland and Vikström20 reported V20Gy of 44·5 ± 5·0 and 32·7 ± 4·8% and mean doses of 21·7 ± 2·5 Gy and 16·4 ± 2·3 Gy for patients treated using the FB and DIBH techniques, respectively. According to Emami’sReference Emami36 the tolerance of normal tissue to therapeutic radiation revealed that symptomatic radiation pneumonitis is one of the most common toxicities in radiotherapy of patients with breast cancer. Therefore, it is very important to minimise the dose to the lung during radiation therapy of the breast.
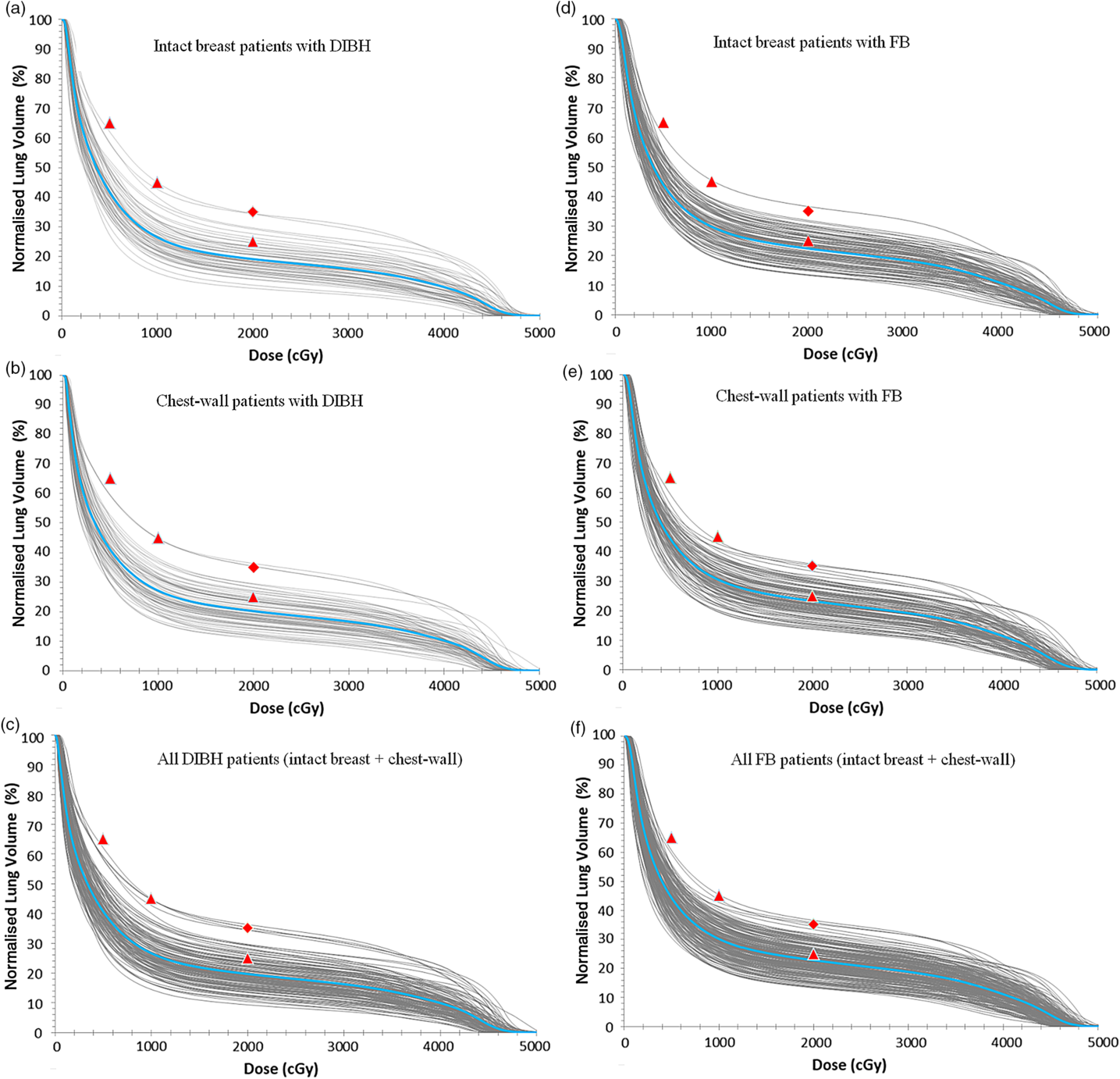
Figure 3. A plot of the dose-volume histogram (DVH) of the ipsilateral lung for patients treated using either the DIBH technique (a, b and c) or the FB technique (d, e and f). (c) All patients treated with the DIBH and (f) all patients treated with the FB techniques. The grey lines represent individual patients DVH and the blue lines in each plot are the mean DVHs. The red data points are the planning dose objectives of V5Gy ≤ 65, V10Gy ≤ 45 and V20Gy ≤ 25% (ideal: triangles) or V20Gy ≤ 35% (acceptable: diamonds).
Abbreviations: DIBH, deep inspiration breathe hold; FB, free breathing.
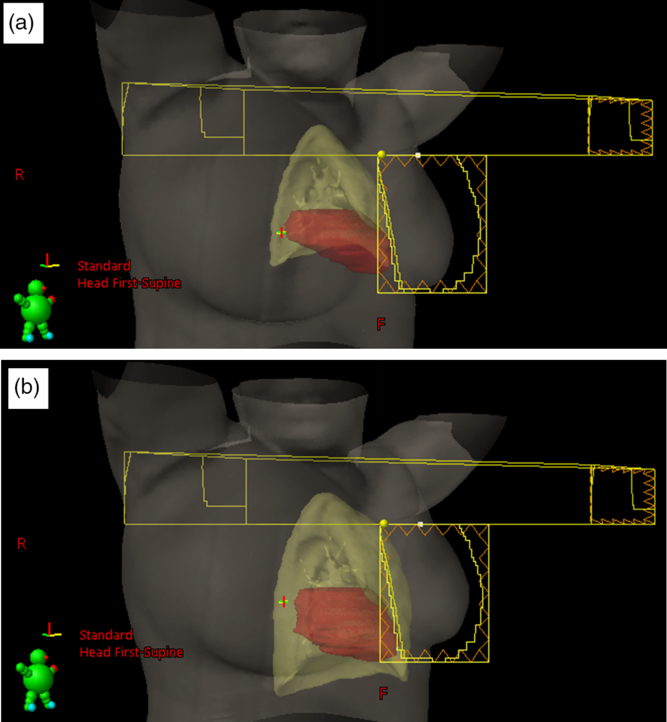
Figure 4. 3-D visualisation of the lung and heart positions and size in the tangential and supraclavicular treatment fields during free breathing (a) and deep inspiration breathe hold (b) for the same patient.
Table 4. A summary of the measures of central tendency and dispersion of the ipsilateral lung volume dose parameters for both intact breast and chest-wall patients stratified into patients who were treated with the free-breathing (FB) technique and those treated with the deep inspiration breath hold (DIBH) technique. The p value analysis of the tabulated results for the various doses when comparing the DIBH and the FB techniques is also shown in the table
Analysis of heart dose
The DVH plots of the heart for patients treated using either the DIBH or the FB techniques are shown in Figure 5. A summary of the measures of central tendency and dispersion of the heart volumetric doses for all patients stratified into patients who were treated with the FB technique and those treated with the DIBH technique is shown Table 5. The mean heart volumes for patients treated with intact breast and post-mastectomy chest-wall are 653·1 ± 139·9 cc and 625·4 ± 134·9 cc, respectively, using FB ,and the corresponding values for patients treated with DIBH are 570·5 ± 94·5 cc and 558·5 ± 90·3 cc, respectively. A two tail student t-test of the means of the heart volume for intact breast and chest-wall patients with FB (p-value = 0·45) and with DIBH (p-value = 0·43) technique shows no significant difference in the mean heart volumes when comparing intact breast and chest-wall patients (Table 5). However, there was a significant difference in patients mean heart volumes between FB and DIBH techniques for both intact breast and chest-wall patients (Table 5). A two tail student t-test of the means of the heart volumes with FB and DIBH techniques for intact breast patients (p-value = 0·005) and for chest-wall patients (p-value = 0·0025) shows significant difference in mean heart volumes irrespective of the target volume (i.e., intact breast or chest-wall). The dose to the heart was evaluated by estimating the V5Gy, V10Gy, V15Gy, V20Gy, V25Gy and V30Gy values. The mean heart V5Gy, V10Gy, V15Gy, V20Gy, V25Gy and V30Gy for patients using the FB technique are 5·5 ± 3·5, 1·8 ± 1·7, 1·2 ± 1·3, 0·9 ± 1·0, 0·7 ± 0·8 and 0·5 ± 0·7% for patients treated with intact breast and 7·5 ± 3·7, 3·1 ± 2·2, 2·2 ± 1·8, 1·7 ± 1·6, 1·3 ± 1·4 and 1·0 ± 1·2% for post-mastectomy chest-wall patients, respectively. Similarly, the V5Gy, V10Gy, V15Gy, V20Gy, V25Gy and V30Gy for patients using the DIBH technique are 3·5 ± 2·0, 0·5 ± 0·7, 0·2 ± 0·5, 0·1 ± 0·4, 0·1 ± 0·3 and 0·1 ± 0·2% for patients treated with intact breast and 4·8 ± 3·2, 1·1 ± 1·4, 0·6 ± 0·9, 0·4 ± 0·7, 0·2 ± 0·6 and 0·2 ± 0·4% for post-mastectomy chest-wall patients, respectively. The p-values obtained using a two tail student t-test analyses of the means for the V5Gy, V10Gy, V15Gy, V20Gy, V25Gy and V30Gy for the heart comparing the DIBH and FB techniques are well less than 0·05 (i.e., p < 0·05) indicating that there is significant difference in heart volumetric doses comparing the two treatment techniques. The reason being that during DIBH the lung takes in more air and hence increases in volume, thereby pushing the heart away from the treatment beams with the corresponding reduction in heart dose (Figure 4, Table 5). Banaei et al.Reference Banaei, Hashemi and Bakhshandeh22 reported heart V5Gy, V20Gy and mean heart dose of 11·5%, 5·1% and 3·5 Gy, respectively, for mastectomy patients who received chest-wall and lymph nodes radiotherapy with a 3 field technique. Ma et al.Reference Kim, Park and Kim24 on the other hand reported V5Gy, V10Gy, V20Gy and V30Gy of 22·1 ± 8·6, 15·0 ± 6·7, 12·5 ± 6·4 and 10·7 ± 5·9%, respectively. Hjelstuen et al.Reference Hjelstuen, Mjaaland and Vikström20 also reported V25Gy of 6·7 ± 6·8 and 1·2 ± 2·8%, mean doses of 6·2 ± 3·6 Gy and 3·1 ± 1·9 Gy, maximum doses of 47·4 ± 4·1 Gy and 33·5 ± 13·9 Gy for treatment plans using the FB approach and the DIBH technique, respectively. Takano et al.Reference Takano, Omura and Suzuki35 also evaluated dosimetry of 3D-CRT plans in left breast cancer patients who received postoperative radiation therapy to the chest-wall and supraclavicular lymph node areas and reported mean heart V15Gy, V25Gy, V30Gy, V35Gy and mean heart dose of 21·32 ± 7·6%, 18·68 ± 7·13%, 17·47 ± 6·86% and 16·19 ± 6·59 Gy. Deseyne et al.Reference Deseyne, Speleers and De Neve23 also investigated breast treatment plans for prone and supine positions for whole breast plus lymph node irradiation and reported mean heart V5Gy, V10Gy, V20Gy, V30Gy and mean heart dose of 7·10 ± 1·93%, 3·47 ± 0·92%, 1·04 ± 0·39%, 0·10 ± 0·18% and 2·38 ± 0·28 Gy for prone treatment and 6·83 ± 3·56%, 3·51 ± 2·35%, 1·23 ± 1·20%, 0·06 ± 0·05% and 2·52 ± 0·59 Gy for supine treatment. According to Emami’s,Reference Emami36 radiotherapy of the breast could result in cardiac symptoms such as clinical pericarditis and death from a myocard infarctus due to previous radiotherapy. Therefore, it is of crucial importance to ensure there is very minimal radiation dose to the heart when treating patients with breast cancer.
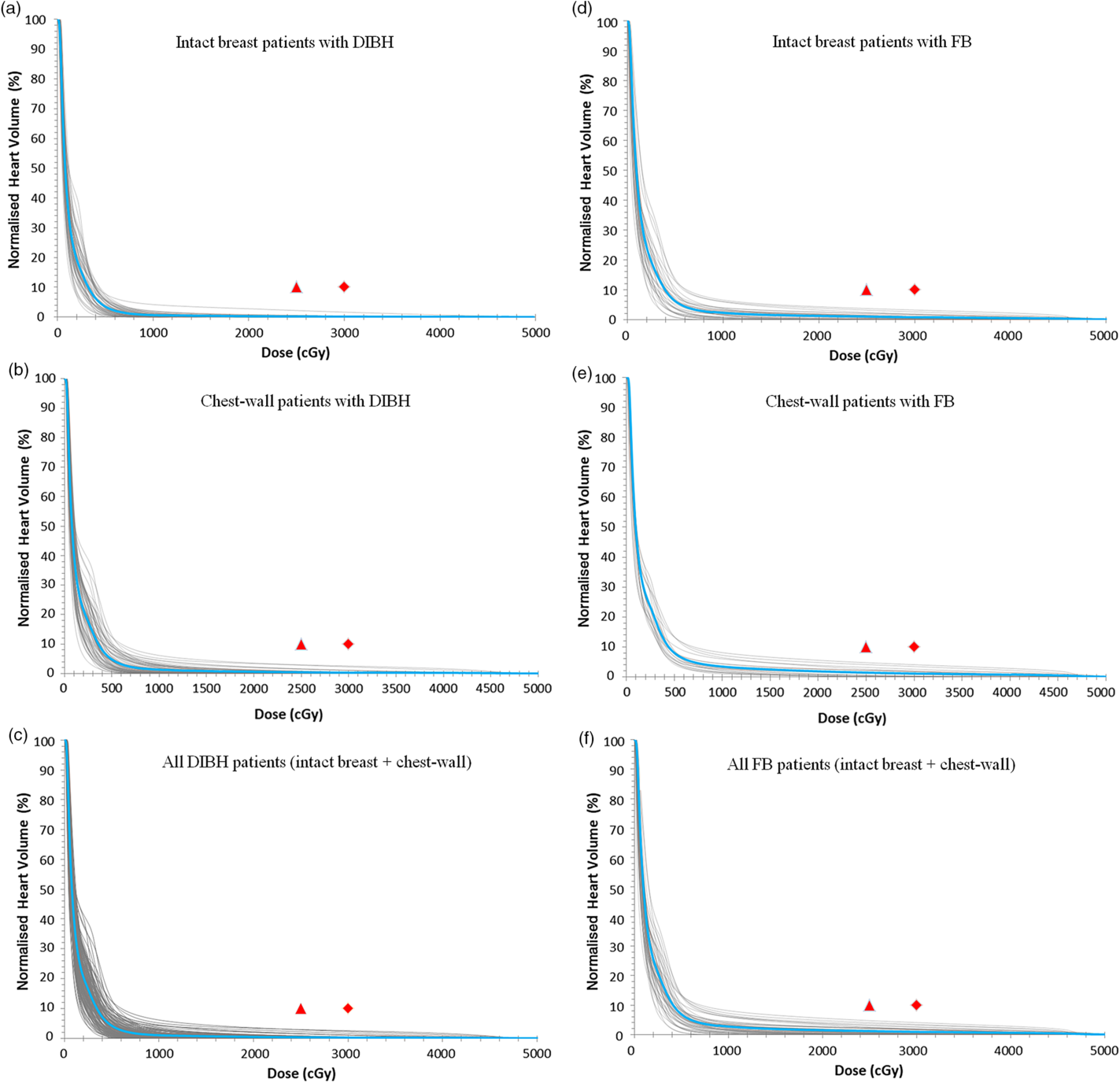
Figure 5. A plot of the dose-volume histogram (DVH) of the heart for patients treated using either the DIBH technique (a, b and c) or the FB technique (d, e and f). (c) All patients treated with the DIBH and (f) all patients treated with the FB techniques. The grey lines represent individual patients DVH and the blue lines in each plot are the mean DVHs. The red data points are the planning dose objectives of V25Gy ≤ 10 (ideal: triangles) or V30Gy ≤ 10% (acceptable: diamonds).
Abbreviations: DIBH, deep inspiration breathe hold; FB, free breathing.
Table 5. A summary of the measures of central tendency and dispersion of the heart volume dose parameters for both intact breast and chest-wall patients stratified into patients who were treated with the free-breathing (FB) technique and those treated with the deep inspiration breath hold (DIBH) technique. The p value analysis of the tabulated results for the various doses when comparing the DIBH and the FB techniques is also shown in the table
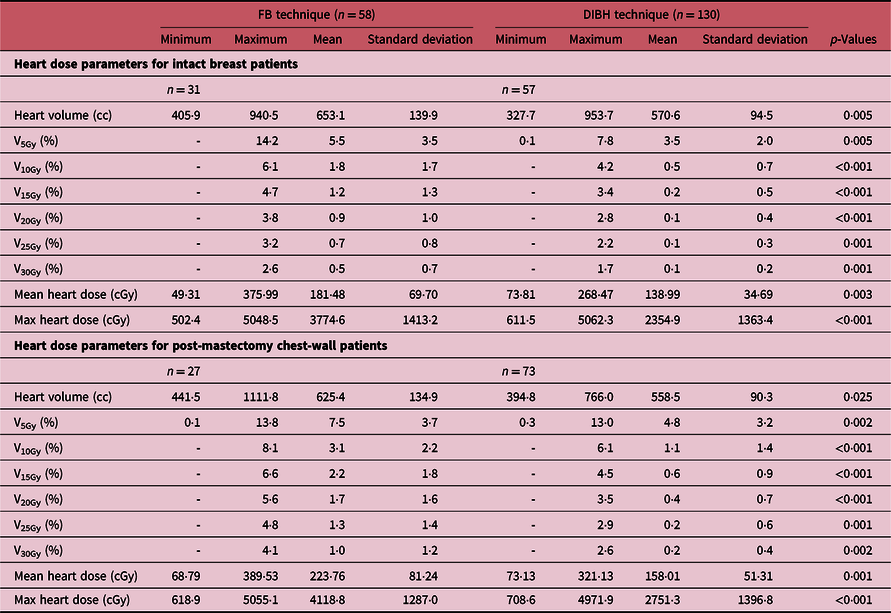
‘-’ Indicates the value is less than 0.1%.
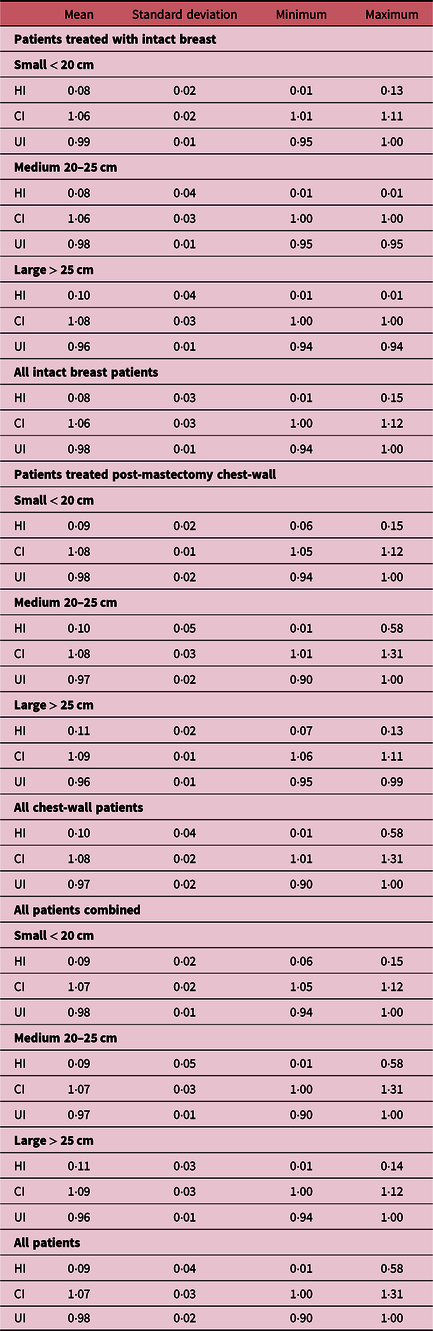
Evaluation of plan quality
The quality of each plan was quantitatively assessed by calculating the HI, UI and the CI for the PTV_eval for all patients. Table 6 shows a summary of the measures of central tendency and dispersion of the HI, UI and CI calculated for the PTV_eval and stratified into patients treated for intact breast and chest-wall and further grouped by breast separation. The mean HI, CI and UI for all PTV_eval of all patients are 0·09 ± 0·04, 1·07 ± 0·03, 0·98 ± 0·02, respectively (Tables 6). There were similar values of the mean HI, CI and UI values when stratified into patients treated with intact breast and post-mastectomy chest-wall patients and also into the three groups (small, medium and large) by breast separation (Table 6). When values of CI and UI are close to unity, it indicates greater conformity and uniformity and values of HI close to zero indicate greater homogeneity; therefore, our data show superior dose conformity, uniformity and homogeneous to the PTV_eval for breast 3 and/or 4 field treatment plans irrespective of target (i.e., intact breast or post-mastectomy chest-wall) or the size of the intact breast or chest-wall (Table 6). Current data show that using the hybrid IMRT technique for tangential intact breast radiation therapy plus a supraclavicular field to treat the lymph nodes can result in smaller ‘hotspots’ while maintaining improved dose coverage and dose uniformity throughout the whole breast or chest-wall. Hotspots and dose inhomogeneity can lead to poor cosmetic outcomes and more skin reactions, especially in women with larger breasts. One of such reaction, moist desquamation, has been correlated with increased pain and reduction in quality of life.Reference Pignol, Olivotto and Rakovitch37 Zheng et al.Reference Zheng, Lai and Zhou27 in their study reported CI of 0·59 ± 0·10, HI of 1·08 ± 0·01 using the hybrid planning technique and CI of 0·42 ± 0·06 and HI of 1·12 ± 0·03 when the FIF-IMRT technique is used. Ma et al.Reference Ma, Zhang and Lu14 on the other hand reported HI of 0·24 ± 0·02 and CI of 0·27 ± 0·07. Hjelstuen et al.Reference Hjelstuen, Mjaaland and Vikström20 also reported HI values of 0·10 ± 0·01 and 0·11 ± 0·01 for treatment based on patient FB and DIBH, respectively, and corresponding CI values 0·99 ± 0·00 and 0·99 ± 0·00 for FB and DIBH, respectively.
Table 6. Summary of the measures of central tendency and dispersion of the homogeneity index (HI), uniformity index (UI) and conformity index (CI) for the PTV_eval for all patients and stratified by breast separation of small (< 20 cm), medium (20 ≤ x ≥ 25) and large (> 25 cm) and by intact breast or chest-wall treatment
Dosimetric Comparison of 2 field and 3 and/or 4 field Techniques
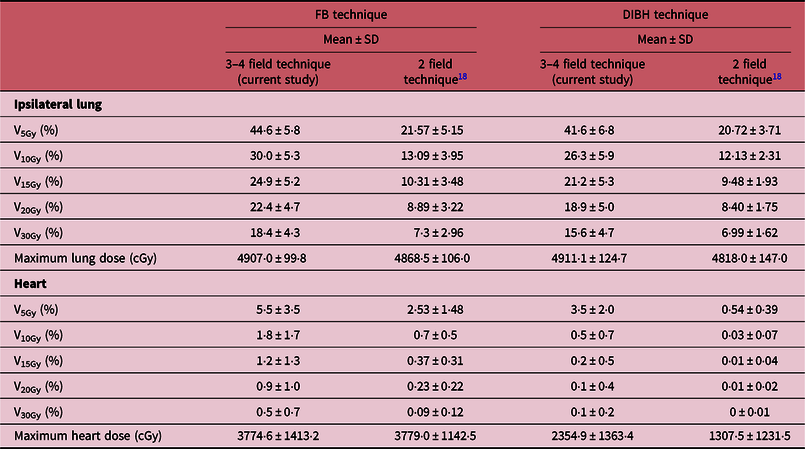
In a previous study,Reference Osei, Darko and Fleck18 we retrospectively evaluated the treatment plans of 623 low-risk breast cancer patients and reported the mean DVHs for PTV_eval, ipsilateral lung and heart for all patients. In Figure 6, we have plotted a comparison of the mean DVHs in the current study for treatment of high-risk breast cancer patients with nodes positive or risk of nodal involvement using 3 and/or 4 field technique with the mean DVHs derived from the previous studyReference Osei, Darko and Fleck18 for low-risk patients with node negative using a 2-field tangential treatment technique. In Table 7, we have presented a comparison of the mean volume dose parameters for ipsilateral lung and heart for the 2-field tangential techniqueReference Osei, Darko and Fleck18 and 3 and/or 4 field technique (current study) stratified into patients who were treated with FB technique and those treated with DIBH technique. We observed similar target coverage based on the mean DVH plots for the PTV_eval for both treatment techniques; however, there is significant difference in the mean DVHs for the ipsilateral lung and the heart DVHs also vary slightly in the low dose region (2–6 Gy) for both FB and DIBH techniques (Figure 6). The volume dose parameters (V5Gy, V10Gy, V15Gy, V20Gy and V30Gy) for both ipsilateral lung and heart are significantly different between the 2-field and 3 and/or 4 field treatment techniques (Table 7). For a 2-field treatment of low-risk patients, the prescription dose is 50 Gy in 25 fractions to treat the intact breast or chest-wall, whereas for high-risk patients the prescription dose is 50 Gy in 25 fractions to treat the intact breast or chest-wall and a 50 Gy in 25 fractions to treat the supraclavicular region. The increased lung and heart doses observed with the 3 and/or 4 field technique (approximately a factor of 2) could potential be due to scatter radiation dose contributions from the 3rd and/or 4th fields treating the supraclavicular region. Therefore, whereas a plan acceptability criteria used for the PTV_eval (i.e., intact breast or chest-wall) can be used for both the 2-field and 3 and/or 4 field hybrid IMRT treatment techniques as the target coverages are very similar, the OARs should have different plan acceptability criteria for the two techniques due to the differences in the mean volumetric doses. It is possible to develop 3 and/or 4 field hybrid IMRT treatment plan for high-risk patients with nodal involvement or risk of nodal involvement that aim to achieve the mean volumetric doses presented in this study for both the ipsilateral lung and the heart with no imposition on resources and time constraints. Predefined dose-volume constraints and objectives can be achieved and can result in improved dose coverage of target breast tissue, reduction in breast volume receiving high doses and dose to adjacent normal tissue, and therefore with the potential to reduce the rate of acute skin reaction, decrease pain and improve quality of life. The results of this study would enable the development of local criteria for treatment plans acceptability for this technique based on available resources and technology.
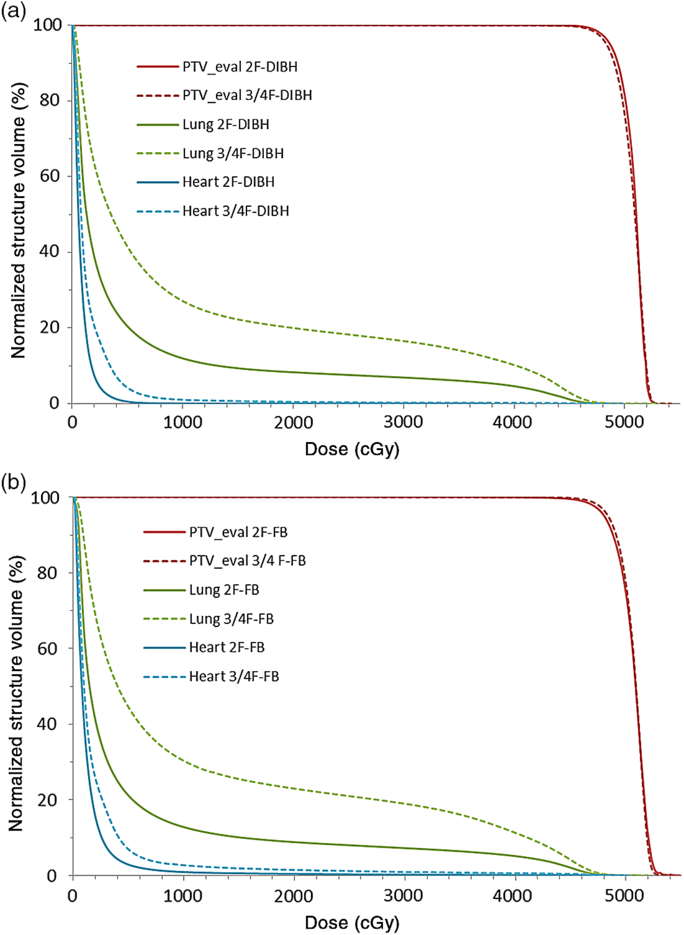
Figure 6. Comparison of dose-volume histograms (DVHs) for 3 or 4 fields (2 tangential fields plus supraclavicular fields) radiotherapy (this work) with 2-field tangential radiotherapy of the breastReference Osei, Darko and Fleck18 for patients treated at 50 Gy in 25 fractions using the deep inspiration breathe hold technique (a) and free breathing technique (b).
Table 7. A comparison of the mean volume dose parameters for ipsilateral lung and heart for 2-field tangential techniqueReference Osei, Darko and Fleck18 and 3 and/or 4 field technique: 2 tangential fields plus supraclavicular field(s) (current study) stratified into patients who were treated with free-breathing (FB) technique and those treated with deep inspiration breath hold (DIBH) technique
Conclusions
The use of 3 and/or 4 field hybrid IMRT for radiation therapy of high-risk node positive breast cancer patients provides an efficient and reliable method for achieving superior dose uniformity, conformity and homogeneity throughout the whole breast or post-mastectomy chest-wall volume and the nodal regions with minimal doses to the OAR. Based on current data, it is possible to develop breast treatment plans that aim to achieve dose coverage for the PTV_eval volume of 92% (V92%) and 95% (V95%) of the prescribed dose to at least 99 and 97% of the normalised volume, respectively, while at the same time, restricting the normalised volume of the PTV_eval receiving a hotspot of 105% (V105%) of the prescribed dose to less than 5%. Treatment planning for intact breast or post-mastectomy chest-wall with lymph nodes involvement should also aim to achieve ipsilateral lung V5Gy ≤ 50, V10Gy ≤ 35, V15Gy ≤ 30, V20Gy ≤ 25, V30Gy ≤ 20% for FB technique and V5Gy ≤ 45, V10Gy ≤ 30, V15Gy ≤ 25, V20Gy ≤ 20, V30Gy ≤ 15% for DIBH technique. Similarly, the criteria for the heart should aim to achieve V5Gy ≤ 10, V10Gy ≤ 4, V15Gy ≤ 3, V20Gy ≤ 2, V30Gy ≤ 1% and mean heart dose of 5 Gy for FB technique and V5Gy ≤ 5, V10Gy ≤ 2, V15Gy ≤ 1·5, V20Gy ≤ 1, V30Gy ≤ 0·5% and mean heart dose of 3 Gy for DIBH. The clinical implementation of this technique for high-risk breast cancer patients with nodal involvement can be achieved with minimal or no imposition on resources and time constraints.
Acknowledgements
The authors would like to acknowledge with much gratitude the financial support from the Kitchener-Waterloo Chapter of the TELUS Ride For Dad and the Prostate Cancer Fight Foundation for this study. The authors would also like to acknowledge with much gratitude the support from Abraham Park who developed the code for extracting the patients’ data and all the staff in the oncology programme.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
















