INTRODUCTION
Steroid hormones such as testosterone are involved in the development and expression of physiological, morphological and behavioural traits. Testosterone seems in particular to play an important role in individuals’ risk of infection. A positive correlation between testosterone levels and parasite prevalence and/or infection intensity has been reported in a wide range of taxa (Zuk and McKean, Reference Zuk and McKean1996), including helminthes (e.g. Poulin, Reference Poulin1996), arthropods (e.g. Olsson et al. Reference Olsson, Wapstra, Madsen and Silverin2000; Cox and John-Adler, Reference Cox and John-Alder2007), protozoa (e.g. Hughes and Randolph, Reference Hughes and Randolph2001) and viruses (Easterbrook et al. Reference Easterbrook, Kaplan, Glass, Pletnikov and Klein2007). Despite the importance of this topic for parasitologists and the community of disease ecologists as a whole, the underlying mechanisms of this relationship remain relatively unclear.
Two types of mechanisms are usually invoked to explain the effect of testosterone on infection. It may have immunological effects, i.e., increase susceptibility to parasites by impairing the immune system, and/or behavioual effects, i.e., increase the exposition to parasites by modulating behaviours involved in parasite transmission. On the one hand, field and laboratory studies have linked sex differences in immune function with circulating steroid hormones (Zuk and McKean, Reference Zuk and McKean1996; Klein et al. Reference Klein, Bird and Glass2000; Roberts et al. Reference Roberts, Walker and Alexander2001) and several experimental approaches have shown that testosterone can suppress immune function (e.g., Hirota et al. Reference Hirota, Suzuki, Chazono and Bito1976; Grossman, Reference Grossman1985; Casto et al. Reference Casto, Nolan and Ketterson2001). The underlying physiological mechanisms remain nonetheless unclear. Sex hormones may inhibit the immune response directly by binding to steroid receptors on lymphocytes (Sullivan and Wira, Reference Sullivan and Wira1979; Tanriverdi et al. 2003) or indirectly (Owen-Ashley et al. Reference Owen-Ashley, Hasselquist and Wingfield2004) by redistributing energy reserves away from the costly immune response (Sheldon and Verhulst, Reference Sheldon and Verhulst1996) during stress (Apanius, Reference Apanius1998) or reproductive events (Wedekind and Folstad, 1994).
On the other hand, testosterone has been shown to have important effects on transmission-relevant behaviours. Male competitive behaviour during the breeding season has been linked to elevated testosterone concentrations across a wide variety of bird (Wingfield et al. Reference Wingfield, Jacobs, Tramontin, Perfito, Meddle, Maney, Soma, Wallen and Schneider2000; Smith et al. Reference Smith, Raouf, Brown, Wingfield and Brown2005), reptile (Klukowsi and Nelson, Reference Klukowski and Nelson1998) and mammal species (e.g. Mehlman et al. Reference Mehlman, Higley, Fernald, Sallee, Suomi and Linnoila1997; Brockman et al. Reference Brockman, Whitten, Richard and Schneider1998; Cavigelli and Pereira, Reference Cavigelli and Pereira2000; Buck and Barns, Reference Buck and Barnes2003). In particular, aggressiveness (Frank et al. Reference Frank, Davidson and Smith1985; Marler and Moore, Reference Marler and Moore1988; Wingfield et al. Reference Wingfield, Hegner, Dufty and Ball1990; Nelson, Reference Nelson2005) and territoriality (e.g., Negro et al. Reference Negro, Caudron, Dubois, Delahaut and Gemmell2010; Edler et al. Reference Edler, Goymann, Schwabl and Friedl2011) are known often to be testosterone-mediated and to increase individuals’ exposition to certain parasites (e.g. Salvador et al. Reference Salvador, Veiga, Martin, Lopez, Abelenda and Puertac1996; Easterbrook et al. Reference Easterbrook, Kaplan, Glass, Pletnikov and Klein2007; Negro et al. Reference Negro, Caudron, Dubois, Delahaut and Gemmell2010).
However, most studies dealing with the effect of testosterone on parasitism only focus on a single parasite and are largely biased towards ectoparasites and intestinal worms. These have led to different patterns, ranging from an absence of association (e.g., blood parasites of honey-eaters, Buttemer and Astheimer, Reference Buttemer and Astheimer2000), to positive (e.g. helminthes, Poulin, Reference Poulin1996) or even negative correlations (e.g. ecto- and hemoparasites in wall lizards, Oppliger et al. Reference Oppliger, Giorgi, Conelli, Nembrini and John-Alder2004) between testosterone and parasite load. Such discrepancies appear both between and within parasite taxa (e.g. positive effect on worm burden in rats, Kamis et al. Reference Kamis, Ahmad and Badrul-Munir1992 but a negative effect in mice, Nakazawa et al. Reference Nakazawa, Fantappie, Freeman, Eloi-Santos, Olsen, Kovacs, Secor and Colley1997; more references in Klein, Reference Klein2004). It is important to consider that testosterone may differentially affect different types of parasites (e.g. Roberts et al. Reference Roberts, Satoskar and Alexander1996; Veiga et al. 1998; Fuxjager et al. Reference Fuxjager, Foufopoulos, Diaz-Uriarte and Marler2011). A previous meta-analysis suggested, for instance, that testosterone was more likely to increase the abundance of ectoparasites compared to that of endoparasites (Roberts et al. Reference Roberts, Buchanan and Evans2004). Considering several parasites in the same host population, Fuxjager et al. (Reference Fuxjager, Foufopoulos, Diaz-Uriarte and Marler2011) revealed a positive effect of testosterone on mite loads, but negative or null effects on nematodes and little on blood-borne endoparasites in free-living mountain spiny lizards (Sceloporus jarrovi). Considering the parasites one by one may also lead to misinterpretations because numerous parasites co-circulate within natural populations. As an example, studies focusing on ectoparasites do not take into account the fact that they may contribute to the transmission of bacteria or viruses they harbour, which can introduce important confounding factors. More generally, host species, populations and/or individuals experiencing different life conditions, diverse multi-infections and exhibiting different social and mating systems may show different abilities to resist parasite infection (Sheldon and Verhulst, Reference Sheldon and Verhulst1996) and are likely to express differently trade-offs between immunocompetence and reproduction (Norris and Evans, Reference Norris and Evans2000; Schmid-Hempel, Reference Schmid-Hempel2003; Lee, Reference Lee2006). To understand the discrepancies between studies, it is therefore necessary to investigate the testosterone effect on different types of parasites in a given socio-environmental context. Although experimental protocols may help to tease apart physiological and behavioural effects in certain cases (Mougeot et al. Reference Mougeot, Redpath, Piertney and Hudson2005), it cannot be applied in all natural populations. Investigating the relationship between testosterone and several parasites inducing close immune responses (e.g. several viruses, several nematode species or several blood parasites), but with different transmission modes (e.g. the exposition of the host involving different behaviours), and circulating in the same host population(s) may help, in a less invasive way, to better understand the nature of the relationship between testosterone and parasitism.
In this paper, we propose to investigate the effect of circulating testosterone levels on the infection risk by 3 feline viruses with contrasted transmission modes in natural populations of domestic cats (Felis silvestris catus). The cat-viruses system is particularly appropriate to investigate such questions. First, cat natural populations have been extensively studied from a behavioural point of view (Liberg, Reference Liberg1980; Pontier et al. Reference Pontier, Fromont, Courchamp, Artois and Yoccoz1998, Reference Pontier, Fouchet, Bahi-Jaber, Poulet, Guiserix, Natoli and Sauvage2009; Say et al. Reference Say, Pontier and Natoli1999; Natoli et al. Reference Natoli, Say, Cafazzo, Bonanni, Schmid and Pontier2005; Hellard et al. Reference Hellard, Fouchet, Santin-Janin, Tarin, Badol, Coupier, Leblanc, Poulet and Pontier2011). Second, its main viruses are well known due to their veterinary implications and because they are easy to survey in the field. It enables the study of the effect of testosterone on viruses, a type of parasite that is largely under studied up to now (but see Friedman et al. Reference Friedman, Glasgow and Grota1972; Lindstrom et al. Reference Lindstrom, Krakower, Lundstrom and Silverin2001; Easterbrook et al. Reference Easterbrook, Kaplan, Glass, Pletnikov and Klein2007). Interestingly, their transmission also implies different behaviours. The feline immunodeficiency virus (FIV) is transmitted through a direct horizontal mode (Sparger, Reference Sparger1993) by bites during aggressive or sexual contacts and induces a life-long chronic disease (Courchamp and Pontier, Reference Courchamp and Pontier1994; Bendinelli et al. Reference Bendinelli, Pistello, Lombardi, Poli, Garzelli, Matteucci, Ceccherininelli, Malvaldi and Tozzini1995). By contrast, the feline herpesvirus (FHV) and calicivirus (FCV) are transmitted through ‘amicable’ close and prolonged contacts, by oral, nasal and ocular secretions, causing acute infections often followed by latent infections (Povey and Johnson, Reference Povey and Johnson1970; Gaskell and Povey, Reference Gaskell and Povey1982). During latency, FHV can be reactivated by a stress (Gaskell and Povey, Reference Gaskell and Povey1977). Such a context offers the opportunity to investigate the effect of testosterone in a natural system and to determine whether it acts similarly on different viruses. By studying multiple parasites in a same host population, we avoid potential confounding effects linked to host and context diversity. In addition, thanks to the contrasted characteristics of the studied viruses, we can test whether data are coherent with a behavioural-mediated effect of testosterone.
MATERIALS AND METHODS
Cat populations and sampling
Three rural cat populations were sampled in April and May 2007 (i.e. during cats’ breeding season), in North-Eastern France (Fouchet et al. Reference Fouchet, Leblanc, Sauvage, Guiserix, Poulet and Pontier2010; Hellard et al. Reference Hellard, Fouchet, Santin-Janin, Tarin, Badol, Coupier, Leblanc, Poulet and Pontier2011). The populations are located in a mixed habitat of deciduous forests and pasturelands. Cat densities are low (≈200 cats/km2) as in other rural areas (Liberg et al. Reference Liberg, Sandell, Pontier, Natoli, Turner and Bateson2000). Males live in large home ranges overlapping those of females (Corbett, Reference Corbett1979; Pontier and Natoli, Reference Pontier and Natoli1996; Liberg et al. Reference Liberg, Sandell, Pontier, Natoli, Turner and Bateson2000). The mating system has been shown to be polygynous in our area (Say et al. Reference Say, Pontier and Natoli1999). Males enter into intense agonistic interactions with other males during the reproductive period to monopolize the access to receptive females (Liberg, Reference Liberg1981, Reference Liberg1983; Davies, Reference Davies, Krebs and Davies1991; Yamane et al. Reference Yamane, Doi and Ono1996).
Cats were captured using baited traps or directly caught by the owner and anaesthetized. For each captured cat, sex, age and body mass were recorded. Age was given by the owner or estimated according to Pascal and Castanet (Reference Pascal and Castanet1978) for un-owned cats. They all received an electronic passive integrative transponder (pig-tag) to allow individuals to be identified in case of re-capture during the same session. Blood samples were taken from the jugular vein before cats were released. An exhaustive survey was carried out to determine whether cats were vaccinated, neutered and had an owner. This enabled us to remove any neutered or vaccinated cat from the study as this would interact with testosterone levels and serological status. On the whole, 41 males (34 feral and 7 free-to-roam owned males) were kept in the analysis.
Circulating testosterone assay
After each capture, the serum was extracted from blood samples by centrifugation (5000 g for 5 min) and stored at −20 °C until assayed. The circulating testosterone levels were then determined spectrophotometrically (Xenius MP96, SAFAS, Monaco), using an Enzyme Immuno-Assay commercial kit (Testosterone EIA kit, Cayman Chemical). The test is based on the competition between testosterone contained in the sample and a T-acetylcholinesterase (AChE) conjugate for a limited amount of testosterone antiserum. Concentrations of testosterone (expressed in pg/ml) were calculated against a standard curve. Cross-reactivity with the other main steroid hormones was considered negligible (Androsterone: 0·05%, Estradiol: <0·01%).
Serological data
The serological statuses for the feline immunodeficiency virus (FIV), herpesvirus (FHV) and calicivirus (FCV) were determined from blood samples. FIV-antibodies were immediately searched for with a commercial kit using the ELISA method (SNAP Combo +, Idexx), whereas specific antibodies against FHV or FCV were measured by a specific blocking ELISA (Poulet, Reference Poulet2007).
Statistical analyses
Determination of the most appropriate model to describe T levels
First, a model selection procedure was applied to determine the most appropriate model describing the observed testosterone levels in males. The aim was to identify factors being potentially confounding to the association between testosterone levels and serological statuses. Four factors were considered; 2 continuous variables: age (AGE) and body mass (MASS) and 2 factors with 2 modalities: way of life (WoL; feral or domestic) and orange phenotype (PHENO; orange or non-orange). This last factor was included because cats with an orange fur colour are sometimes suspected to be more aggressive than other fur coat colours (Pontier et al. Reference Pontier, Rioux and Heizmann1995, Reference Pontier, Fromont, Courchamp, Artois and Yoccoz1998). Testosterone level, Mass and Age were standardized to make their effects dimension-free and comparable to those of the other factors. All possible models with simple effects and double interactions were generated. A square transformation was applied to ensure the normality of the residuals.
The most appropriate model was selected using the Akaike Information Criterion adjusted for small sample size (AICc, Anderson et al. Reference Anderson, Burnham and White1994). The models were ranked according to the smallest AICc differences (denoted ΔAICc) between the focal model and the lowest AICc model. When ΔAICc was smaller than 2 for more than one model, those models were combined to ensure the inclusion of a maximum of potential confounding factors in the second part of the analysis.
The parameters of the selected linear model were estimated using Bayesian inference. Flat priors were used (in practice independent Gaussian laws with very large variances for each parameter). Samples from the posterior distribution of the model parameters were derived from a MCMC algorithm using JAGS and the package rjags in R software (R Development Core Team, 2009). To get rid of the effect of initial condition, the 10 000 first elements of the chain were burnt up. To avoid auto-correlation only 1 element of the chain out of 100 was kept.
Testosterone effect on serological statuses
Second, selected variables were included in logistic regression models to describe each serological status. The testosterone level was added as a continuous variable in each model. Since quantitative variables were standardized, the odds-ratios now represent the effect of an increase of testosterone level, mass or age of 1 standard deviation on the probability of infection. For each virus, model parameters were estimated using Bayesian inference as described previously.
A fundamental question is to determine whether testosterone affects the different viruses similarly. Answering this question requires to test the plausibility of the hypothesis according to which the coefficient of the testosterone effect is the same for the different viruses. In mathematical terms, let us call b V the coefficient of testosterone for virus V and δ = b V1−bV2 the differential effect of testosterone on 2 viruses. Testing whether two viruses are similarly affected by testosterone is made by comparing H0: δ = 0 against H1: δ≠0. Now, if we assume that the serological statuses of the two viruses are independent, then sampling from the posterior distribution of δ is easily obtained by subtracting the chains obtained for b V1 and b V2. Hypothesis H0 is then simply tested by looking at whether zero is a plausible value for δ. P-values testing coefficients (i.e., regression coefficients or δ) are estimated by P = 2min(p, 1 − p) where p is the ratio between the number of simulated values below zero plus one and the total chain length plus one.
RESULTS
The most appropriate model to describe T levels
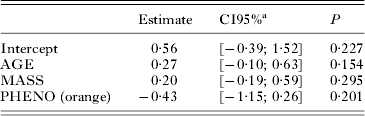
According to the AICc, we selected the model explaining the testosterone levels by the age, body mass and phenotype of the cats (Table 1). The effects of these explanatory variables were not significant (Table 2) but all were kept to remove potential confounding factors when investigating the relationship between testosterone and the serological statuses of the 3 viruses. Similarly, although age and body mass were correlated (ρ = 0·56), meaning that it is likely that only one of the two variables was biologically relevant (i.e., the other being linked to T through a confounding effect), both were kept in the analysis.
Table 1. Best linear regression models according to their AICc to describe testosterone levels

a Sample size.
b Number of parameters.
Table 2. Selected linear regression model: testosterone levels in relation to age, body mass and orange phenotype

a Credibility interval.
T levels and risks of infection
After correction by age, mass and phenotype, the testosterone levels of rural cats were significantly linked to their FIV serological status (P-value = 0·18 × 10−3, Table 3). The risk of being seropositive to FIV strongly increased with testosterone level (Fig. 1). When the testosterone level increased of one standard deviation, the risk to be infected increased 105 times (Odds-ratio = 104·90, CI95% = [8·14; 10259·92], Table 3). This value is not plausible from a biological point of view; however, it may be due to the small sample size. What is important is to look at the lower bound of the credibility interval; as it is 8·14, we are confident in saying that testosterone effect is marked. On the contrary, no significant relationship was evidenced between testosterone levels and the other serological statuses (FHV: P-value = 0·052; FCV: P-value = 0·174, Table 3, Fig. 1).

Fig. 1. Infection risk by each virus according to males’ testosterone levels. Predicted (line) and observed (dots) infection risks for FIV (A), FHV (B) and FCV (C).
Table 3. Logistic regression models to test for an effect of testosterone level on each serological status

a Credibility interval.
b Odds-ratio.
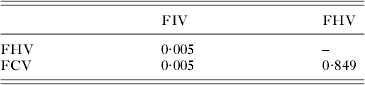
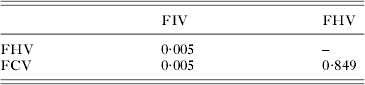
Furthermore, the comparison of the coefficients of testosterone revealed that the effect of the circulating hormone was different on the status to FIV than on the status to the other viruses (Table 4, after Bonferroni correction: P-value = 0·014 for FIV-FHV, P-value = 0·015 for FIV-FCV, but P-value = 0·996 for FHV-FCV).
Table 4. Test of a differential effect of testosterone level on the serological statuses
DISCUSSION
This work investigated the relationship between testosterone level in male cats and their risk of being seropositive to 3 feline viruses with different transmission modes. Contrary to most studies, which focus on a single parasite and a single mechanism (i.e. immunological or behavioural) we argue that considering several parasites in the same host population (i.e. in a given socio-environmental context), may help deciphering some of the underlying mechanisms.
Applied here to 3 viruses infecting cats, this approach revealed different relationships between testosterone and males’ serological status, depending on the considered virus. High levels of testosterone were significantly associated with high infection risk by FIV but not with that of the other viruses. It does not mean that testosterone has no effect at all on FHV and FCV but indicates that the plasma hormone is much more associated with FIV.
Understanding the differential effects of testosterone
Behaviours exposing cats to infection by the studied viruses, and the effects testosterone has on these behaviours are well known. FHV and FCV are transmitted by close and prolonged contacts (Povey and Johnson, Reference Povey and Johnson1970; Gaskell and Povey, Reference Gaskell and Povey1982), which are not known to be testosterone-dependent behaviours. Conversely, FIV is transmitted by bites, between males during fights (females do not bite each other) and from male to female during coitus (when the male bites the female at the neck) (Yamamoto et al. Reference Yamamoto, Hansen, Ho, Morishita, Okuda, Sawa, Nakamura and Pedersen1989; Sparger, Reference Sparger1993; Bendinelli et al. Reference Bendinelli, Pistello, Lombardi, Poli, Garzelli, Matteucci, Ceccherininelli, Malvaldi and Tozzini1995; Courchamp et al. Reference Courchamp, Artois, Yoccoz and Pontier1998). Aggressive males are more exposed to this virus than non-aggressive ones, as evidenced by the higher vulnerability to FIV of bold (pro-active) individuals (Courchamp et al. Reference Courchamp, Artois, Yoccoz and Pontier1998, Reference Courchamp, Say and Pontier2000; Natoli et al. Reference Natoli, Say, Cafazzo, Bonanni, Schmid and Pontier2005). Since aggressiveness is known to be testosterone -mediated in cats (Rosenblatt and Aronjon, Reference Rosenblatt and Aronjon1958; Hart and Barrett, Reference Hart and Barrett1973; Brown and Bradshaw, Reference Brown and Bradshaw1996) as in many other species (e.g., Frank et al. Reference Frank, Davidson and Smith1985; Marler and Moore, Reference Marler and Moore1988; Wingfield et al. Reference Wingfield, Hegner, Dufty and Ball1990; Nelson, Reference Nelson2005), the higher FIV-infection of males with high testosterone levels found in this study is consistent with a higher exposition to FIV through increased aggressiveness.
In addition, mean levels of circulating testosterone measured in our polygynous rural populations (1085·34 ± 892·42 pg/ml) were significantly higher (F = 22·43, d.f. = 1, P = 1·10 × 10−5) than in a much less aggressive and promiscuous urban population (335·67 ± 204·91 pg/ml; La Croix-Rousse, Lyon, France, unpublished data; see also Say et al. Reference Say, Pontier and Natoli1999; Say and Pontier, Reference Say and Pontier2004). Those results are consistent with unpublished data suggesting that the type of mating system significantly affects testosterone levels and cats’ aggressiveness (D. Pontier, O. Hubert, L. Say and C. Duchamp, personal communication). Although more data are necessary to generalize, this would be in accordance with the challenge hypothesis (Wingfield et al. Reference Wingfield, Hegner, Dufty and Ball1990) that states that increases in testosterone levels of male animals during the breeding season are directly related to the extent of intrasexual competition for resources or mates that they experience. Accordingly, the prevalence of FIV in natural cat populations is higher in polygynous cat populations than in promiscuous urban ones where FIV can be absent (Xemar, Reference Xemar1997; Courchamp et al. Reference Courchamp, Artois, Yoccoz and Pontier1998, Reference Courchamp, Say and Pontier2000; Hellard et al. Reference Hellard, Fouchet, Santin-Janin, Tarin, Badol, Coupier, Leblanc, Poulet and Pontier2011).
Furthermore, we cannot exclude subtle differences in the immune responses induced by the studied viruses and/or a differential effect of testosterone on such responses. As we work with serological data, we are looking at a possible link between testosterone levels and the establishment of the infection, i.e., on the immune response occurring before seroconversion. FHV and FCV are transmitted through body secretions and first need to pass epithelial barriers to infect a new host. By contrast, FIV is transmitted by bite and thus bypasses epithelial barriers more easily and more rapidly ends up in the circulatory system. The relative importance of the different components of the immune system may not be the same for these two types of virus entry. The innate and cell-mediated immune responses are likely to play an important role in the infection success of FHV and FCV, as supported by the existence of some cats protected against FHV and FCV infection but with no detectable antibody levels (Knowles et al. Reference Knowles, McArdle, Dawson, Carter, Gaskell and Gaskell1991; Radford et al. Reference Radford, Coyne, Dawson, Porter and Gaskell2007), whereas FIV may be quickly exposed to the cellular actors of the immune system. Such differences in the immune responses remain nonetheless hypothetical.
It is also interesting to note that cats’ status to FHV tends to be influenced by testosterone levels as well (P = 0·052), even if this link is much weaker than for FIV (P = 0·005). Considering that contrary to FCV this virus can be reactivated, it raises the question of a potential link between testosterone and the reactivation of latent infections.
Taken together, our results support the hypothesis that the strong relationship between testosterone levels and FIV status suggests a behavioural action of testosterone. Knowing the effect testosterone has on cats’ aggressiveness (Rosenblatt and Aronjon, Reference Rosenblatt and Aronjon1958; Hart and Barrett, Reference Hart and Barrett1973; Brown and Bradshaw, Reference Brown and Bradshaw1996), this hormone is likely to increase the exposition to this aggressively transmitted microparasite. We cannot exclude an additional immunological action of testosterone, but this would need further (experimental) investigations. A better knowledge of testosterone's impact on the different components of the immune system, as well as of cats’ immune response following infection by each of the studied viruses (i.e. key molecular or cellular actors and if they are influenced by testosterone) would be valuable. Besides, the immunosuppressive effects of the hormone were investigated on birds and small mammals (i.e. rabbits, rodents) and we cannot exclude Felids/domestic cats’ particularities in terms of sensitivity to testosterone effects on the immune system.
Towards epidemiological and evolutionary consequences
This study supports the idea of a crucial role of behaviour and behavioural variability in the dynamics and evolution of host-parasite systems (Bahi-Jaber et al. Reference Bahi-Jaber, Fouchet and Pontier2008; Barber and Dingemanse, Reference Barber and Dingemanse2010; Hawley et al. Reference Hawley, Etienne, Ezenwa and Jolles2011). If testosterone increases hosts’ aggressiveness, it may fuel the spread of any aggressively transmitted parasite, and individuals with high testosterone levels may accumulate such parasites. This is an important point as aggressive encounters are hypothesized to be a mode of transmission for several pathogens, such as, for example, the simian immunodeficiency virus in non-human primates (Nerrientet et al. Reference Nerrientet, Amouretti, MuÈller-Trutwin, Poaty-Mavoungou, Bedjebaga, Nguyen, Dubreuil, Corbet, Wickings, Barre-Sinoussi, Georges and Georges-Courbot1998; Fouchet et al. Reference Fouchet, Verrier, Ngoubangoye, Souquière, Makuwa, Kazanji, Gonzalez and Pontier2012) or hantaviruses in rodents (Glass et al. Reference Glass, Childs, Korch and LeDuc1988; Klein, Reference Klein2000). If immunological and behavioural effects co-occur, testosterone may also serve as a common driver of superspreading phenomena for aggressively transmitted parasites.
More generally, the question of the role of testosterone in infection risk and intensity is of great interest for many human and animal pathogens (e.g. Toxoplasma gondii, Flegr et al. Reference Flegr, Lindová and Kodym2008; Kankova et al. Reference Kankova, Kodem and Flegr2011; Leishmania donovani, Zhang et al. Reference Zhang, Zhao, Wang and Qiao2001). It is particularly true for those whose transmission depends on behaviours that can be influenced by circulating testosterone. In certain cases, determining whether a correlation between testosterone and infection is due to a testosterone-mediated increase in host exposition or to a manipulation of host hormone level by the parasite is also an important challenge (Flegr et al. Reference Flegr, Lindová and Kodym2008; Kankova et al. Reference Kankova, Kodem and Flegr2011).
Finally, our approach is general and could be applied to any type of parasite. While more studies are still needed to define (i) the precise effects of testosterone on the immune system, (ii) the specific immune response developed by hosts toward each parasite and parasite type, and (iii) the interactions between testosterone and other hormones (e.g. corticosterone, glucocorticoïdes, see for example Fuxjager et al. Reference Fuxjager, Foufopoulos, Diaz-Uriarte and Marler2011), this study suggests an important role for testosterone in the circulation of an aggressively transmitted parasite. The search for the possible underlying mechanisms also confirms the complexity of the interactions existing between immunity, behaviour and physiology. If, as suggested here, the relationship between testosterone and infection risk differs when only 3 viruses are considered – 3 closely related viruses studied within the same host population – important variations are certainly expected at a larger scale, when more parasite types and/or host species are involved. Finally, our work highlights the need for interdisciplinary and integrative approaches if one wants to understand the complex interplays that exist both within and between the endocrine and immune systems, and their consequences for the evolution of parasite-host interactions, mating systems and epidemiological patterns in a world with multiple parasites.
ACKNOWLEDGEMENTS
The authors would like to thank Guillaume Leblanc for serological data collection in the field (Accreditation number 692660703 delivered to the UMR-CNRS 5558 for the program); Béatrice Tarin, Vincent Badol and Christine Coupier for laboratory analyses. They are also grateful to Professor Patrice Debré for fruitful discussions.
FINANCIAL SUPPORT
This work was supported by Lyon-Biopôle (FIV-VAX program).