Introduction
The cultivation of genetically modified (GM) crops has increased constantly since they were first deployed in 1996, reaching 148 million hectares worldwide in 2010 (James, Reference James2010). Among them, insect resistant crops have been engineered to express insecticidal proteins of the Bacillus thuringiensis δ-endotoxin protein family (Bt proteins or Bt toxins) (Bravo et al., Reference Bravo, Gill and Soberon2007). Bt maize varieties expressing the Cry3Bb1 toxin, highly effective against the western corn rootworm (Diabrotica virgifera virgifera LeConte, WCR) were launched in 2003. This insect is one of the most destructive pests of corn in the USA (Meinke et al., Reference Meinke, Sappington, Onstad, Guillemaud, Miller, Komáromi, Levay, Furlan, Kiss and Toth2009), and it has been accidentally introduced in Europe on multiple occasions (Miller et al., Reference Miller, Estoup, Toepfer, Bourguet, Lapchin, Derridj, Kim, Reynaud, Furlan and Guillemaud2005; Rauschen et al., Reference Rauschen, Schaarschmidt and Gathmann2010).
The deployment of insect resistant transgenic crops into the environment represents a potential risk to other organisms present in the food web of an agro-ecosystem that should be assessed before and confirmed after commercialization. Exposure of non-target arthropods to Bt toxins could happen in nature by several ways: by direct feeding, by movement through the trophic chain, or by direct passage from the plant to the soil (Andow et al., Reference Andow, Lövei and Arpaia2006; Icoz & Stotzky, Reference Icoz and Stotzky2008; Miethling-Graff et al., Reference Miethling-Graff, Dockhorn and Tebbe2010). Among non-target arthropods potentially exposed to Bt toxins, predatory insects have received special attention mainly because they occur in large numbers in agricultural systems and because they contribute to regulation of herbivore populations. Furthermore, some species of this guild might be good indicators of potential ecological impacts of GM crops since they could ingest the toxin indirectly through the consumption of herbivore prey. As Bt maize expressing Cry3Bb1 protein targets the control of coleopteran pests, predatory beetles present in maize fields represent a key guild to be assessed for possible effects of prey-mediated exposure to this toxin. To date, some studies have addressed potential impacts of Cry3Bb1-expressing Bt maize on predatory beetles, such as ladybirds and carabids, without reporting adverse effects (Lundgren & Wiedenmann, Reference Lundgren and Wiedenmann2002; Ahmad et al., Reference Ahmad, Wilde, Whitworth and Zolnerowich2006; Duan et al., Reference Duan, Paradise, Lundgren, Bookout, Jiang and Wiedenmann2006; Li & Romeis, Reference Li and Romeis2010).
Staphylinids are important predatory beetles abundant and commonly present in farming systems, including maize fields (Bohac, Reference Bohac1999; Bhatti et al., Reference Bhatti, Duan, Head, Jiang, McKee, Nickson, Pilcher and Pilcher2005; de la Poza et al., Reference de la Poza, Pons, Farinós, López, Ortego, Eizaguirre, Castañera and Albajes2005; Lövei & Arpaia, Reference Lövei and Arpaia2005; Brunke et al., Reference Brunke, Bahlai, Sears and Hallett2009). The species richness of this group of predators in maize fields is similar to that of ground beetles (Coleoptera: Carabidae) and spiders (Farinós et al., Reference Farinós, de la Poza, Hernandez-Crespo, Ortego and Castañera2008). However, staphylinids have been largely ignored in non-target testing of GM plants because of the absence of dominant species and the difficulty associated with maintaining them in laboratory cultures. The implementation of risk assessment procedures to evaluate effects of GM crops on non-target arthropods entails the selection of surrogate species, i.e. species which act as representatives of other species in the community based on various criteria, such as ecological relevance, testability or exposure pathways (Caro & O'Doherty, Reference Caro and O'Doherty1999; Todd et al., Reference Todd, Ramankutty, Barraclough and Malone2008). Atheta coriaria Kraatz (Coleoptera: Staphylinidae) has recently been suggested as a suitable surrogate species for this aim, representative of other generalist predatory rove beetles (García et al., Reference García, Ortego, Castañera and Farinós2010; Porcar et al., Reference Porcar, García-Robles, Domínguez-Escribá and Latorre2010). Commonly used as a biological control agent for fungus gnats (Diptera: Sciaridae) in greenhouses, this cosmopolitan species belongs to the subfamily Aleocharinae, frequently found in maize fields (Farinós et al., Reference Farinós, de la Poza, Hernandez-Crespo, Ortego and Castañera2008; Balog et al., Reference Balog, Kiss, Szekeres, Szenasi and Marko2010). A study concerning toxicity of Cry3Aa endotoxin (which targets Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae)) on A. coriaria adults, reported no effects on mortality after 15 days of exposure through artificial diet-incorporation bioassay (Porcar et al., Reference Porcar, García-Robles, Domínguez-Escribá and Latorre2010). Similarly, negative effects were not found when this rove beetle was exposed to prey that had been fed a lepidopteran-specific toxin, Cry1Ab (García et al., Reference García, Ortego, Castañera and Farinós2010). However, to our knowledge, the potential effects of GM plants expressing the coleopteran-active insecticidal protein Cry3Bb1 have not been previously tested on staphylinids under laboratory conditions.
In this study, we carried out tritrophic bioassays to assess the potential prey-mediated effects of Cry3Bb1-expressing Bt maize on the performance of A. coriaria, using Tetranychus urticae Koch (Acari: Tetranychidae) as prey. Larvae and adults of A. coriaria were used to determine possible variations between different physiological stages. Specifically, we focused on: (i) the level of exposure and decay rate of Cry3Bb1 toxin in A. coriaria larvae and adults when ingested through their prey, and (ii) possible effects of Cry3Bb1-expressing maize on survival, growth, development, reproduction and predatory ability of A. coriaria larvae and adults.
Materials and methods
Plant and arthropod colonies
Transgenic maize (Zea mays L.) plants DKC5143Bt (event MON88017) from Monsanto Company (St. Louis, MO, USA) expressing a modified version of the Cry3Bb1 gene from B. thuringiensis (Bt maize) and its near-isogenic line DKC5143 (non-Bt maize) were used for feeding trials. Plants were grown in plastic pots (20 cm diameter) using Compo Sana® Universal as a substrate and maintained in a growth chamber (Sanyo MLR-350 H, Sanyo, Japan) at 25±0.3°C, 70±5% RH and L:D 16:8 h photoperiod. For all the experiments, plants were used when they had reached the five-leaf stage.
Tetranychus urticae were provided by Dr Vicente Marco (Universidad de La Rioja, Spain) in 2006 from a stable laboratory colony. Mites were reared on maize plants (Bt or non-Bt maize, depending on the treatment) at 25±0.3°C, 70±5% RH and L:D 16:8 h photoperiod for a minimum of two weeks before using them in the bioassays. All the stages (eggs, larvae, nymphs and adults) were used to feed rove beetles ad libitum.
Fungus gnat larvae of the species Bradysia ocellaris (Comstock) and B. difformis Frey (Diptera: Sciaridae) used in the feeding behaviour experiment were obtained from a laboratory-reared colony, established from fungus gnat larvae present in the substrate Compo Sana® Universal. The colony was maintained in the same moistened substrate at 20±0.3°C, 80±5% RH and L:D 16:8 h photoperiod in growth chambers (Sanyo MLR-350 H, Sanyo, Japan).
Atheta coriaria adults and larvae were obtained from an established laboratory colony originating in 2008 from adults and larvae purchased from Syngenta Bioline Ltd (Staphyline C®). The rearing substrate was a mix of peat (substrate Compo Sana Universal®, Compo Agricultura SL, Barcelona, Spain), coconut fiber and vermiculite (at a 4:2:1 volume ratio). The rearing food was a mixture of dog food (Brekkies Excel Tender & Delicious®, Affinity Petcare SA, Barcelona, Spain) and oatmeal (Kllön®, Peter Kllön KGaA, Elmshorn, Germany) (at a 4:1 weight ratio). The maintenance of the colony is described in García et al. (Reference García, Ortego, Castañera and Farinós2010). Sex of adults was determined according to Klimaszewski et al. (Reference Klimaszewski, Assing, Majka, Pelletier, Webster and Langor2007). Rearing and experiments were conducted at 20±0.3°C, 80±5% RH and L:D 16:8 h photoperiod in growth chambers (Sanyo MLR-350 H, Sanyo, Japan).
Bt-toxin uptake through the trophic chain
A bioassay was performed to know the fate of the toxin along the trophic chain. Newly emerged A. coriaria larvae and 0–3-day-old adults were individually placed in a plastic arena (38 mm diameter×19 mm height) containing maize leaf pieces of either Bt maize or non-Bt maize infested with enough T. urticae to feed A. coriaria ad libitum and one piece of moistened filter paper. Larvae were fed on mites during the whole immature period. Samples of first (L1), second (L2) and third (L3) larval instar of A. coriaria were collected after feeding ab libitum on T. urticae for 2, 4–5 and 6–8 days, respectively. Adult rove beetles were fed on T. urticae for four days. Final weights of each sample of L1, L2, L3 and adults were recorded (table 1). All samples were frozen at −20°C until determination of Cry3Bb1 levels by double-antibody sandwich enzyme-linked immunosorbent assays (DAS ELISA). To quantify the levels of the Cry3Bb1 toxin in Bt and non-Bt maize, leaf samples of both varieties were taken at the same phenological stage and time as leaves used in the experiments. Cry3Bb1 levels were also evaluated in samples of T. urticae adults taken from Bt and non-Bt maize plants. To rule out the presence of any contamination that could interfere with our bioassays, the absence of toxin in rearing food was verified by the analysis of three samples taken at random.
Table 1. ELISA screening protocols to measure Cry3Bb1 levels.

Cry3Bb1 levels of all samples were determined by ELISA using the Agdia Bt-Cry3Bb1 Microtiter Plate Kit (Elkhart, IN, USA). ELISA-screening protocols and sample size for each treatment are summarized in table 1. Bt toxin concentrations were expressed in μg Cry3Bb1 g−1 of fresh weight (FW). Samples were centrifuged for 5 min at 12,000×g and 100 ml of each sample was introduced into the ELISA plate following the manufacturer's instructions. Standard curves were made using different concentration solutions of the purified Cry3Bb1 protein that was provided by Monsanto Company (corrected for 87% purity). The limit of detection (LOD) was calculated using the following equation:
where σ is the standard deviation of 14 buffer-only controls and S is the slope of the calibration curve (ICH, 2005). The resulting LOD was 0.18 ng Cry3Bb1 ml−1 protein solution and measurements of all Bt samples revealed ODs above the LOD. Spectrophotometric measurements were conducted with a microtiter plate reader at 450 nm (VersaMax™ Microplate Reader, Molecular Devices Inc., Sunnyvale, CA, USA).
Cry3Bb1 protein detection time in A. coriaria larvae and adults
The detection time of Cry3Bb1 protein following consumption of Bt maize fed-prey was studied in A. coriaria larvae and adults. Single newly emerged first instar larvae (L1) and adults of 0–3 days old were placed in a test arena containing one piece of moistened filter paper and Bt maize infested with T. urticae. Larvae were allowed to feed for six to eight days and adults were fed for four days. Atheta coriaria larvae and adults were then separated from the Bt containing prey and placed into a new arena containing non-Bt maize infested with T. urticae. To assess the detection time of Bt toxin in the rove beetles, Cry3Bb1 concentration was determined at different post-exposure time points (0, 2, 6, 8, 12, 24 and 48 h). Thereafter, A. coriaria larvae and adults were weighed and immediately frozen at −20°C. Levels of Bt toxin at each time were determined by ELISA following the same protocol as above. Data were adjusted to an exponential decay equation model to calculate half-life time. The experiment was replicated five times with groups of five larvae and five adults at each time interval. Quantities of material and buffer volumes used are summarized in table 1.
Evaluation of predator fitness
Prey-mediated effects due to larval exposure to Cry3Bb1
To measure if there were effects on performance, first instar A. coriaria larvae from the laboratory colony were individually isolated into test arenas containing one piece of moistened filter paper and were assigned to one of three feeding categories: (i) regular rearing food (50 mg); (ii) leaf pieces of non-Bt maize infested with T. urticae; or (iii) leaf pieces of Bt maize infested with T. urticae. Larvae were fed ad libitum. The rearing food was renewed weekly and maize leaves were replaced every 2–3 days. All larvae were checked daily for moulting, death, pupation and adult emergence. Some sclerotized morphological structures were also monitored throughout development stages. The maximum width of the head-capsule (HC) was measured in each specimen at each larval instar to check if growth rate kept constant between the successive instars as stated by Dyar's rule (Dyar, Reference Dyar1890). According to it, the HC width of successive instars follows a geometrical progression expressed with the growth rate. Growth rate was computed as the ratio of HC width average estimations (m) for two consecutive instars i and i+1 using the following equation:
Weight, maximum width of the cephalic capsules, and elytra length were also measured in each newly emerged adult beetle. Adults were weighed in a Mettler-Toledo AX205 analytical balance (Mettler-Toledo International Inc., Madrid, Spain). All morphological measurements were done using a Leica M125 stereomicroscope equipped with a Leica DFC420 digital camera (Leica Microsystems S.A., Barcelona, Spain). Images were analysed with the image analysis program, tpsDig (Rohlf, Reference Rohlf2008). Treatments were replicated three times with 30 larvae per treatment.
Prey-mediated effects due to adult exposure to Cry3Bb1
To measure if there were effects on survival, fecundity and fertility, one female and one male of 0–3 days old were randomly selected from the laboratory colony. Each pair was placed in the test arena containing rearing substrate (500 mg) moistened with 0.5 ml of water and assigned to one of the following treatments: (i) rearing food (100 mg); (ii) leaf pieces of non-Bt maize infested with T. urticae; or (iii) leaf pieces of Bt maize infested with T. urticae. Adults were fed ad libitum by adding weekly rearing food and replacing maize leaf pieces every 2–3 days. Mortality, preoviposition period and fecundity were daily recorded. To assess fertility, eggs from each treatment were collected daily and individually placed in the test arena containing a piece of moistened filter paper. Egg hatching was checked daily. Fecundity and fertility were evaluated during the first 30 days and adult survival was recorded during 60 days. Ten replicates per treatment were performed.
Effects on predatory ability of A. coriaria larvae and adults
To assess possible sublethal effects on feeding behaviour, we evaluated whether exposure to Cry3Bb1 toxin could affect normal predation capacity of A. coriaria L3 larvae and adults. B. ocellaris and B. difformis were used as prey indistinctly. Single newly emerged A. coriaria larvae fed during 7–8 days on each of the three treatments (rearing food and T. urticae raised on Bt or non-Bt maize) and single adults (<24 h old) reared from each of the diet treatments were individually placed into a plastic test arena (25 mm diameter×15 height) containing five L2–L3 fungus gnat larvae and one piece of moistened filter paper. After 24 h, the number of fungus gnat larvae killed by each A. coriaria larva or adult was recorded and the proportion of consumed larvae was visually estimated under a stereomicroscope. Twenty and 30 replicates per treatment were performed with larvae and adults, respectively.
Data analysis
Homogeneity of variances (Levene test) and normal distribution (Kolmogorov–Smirnov test) were tested in all variables before statistical analysis. When these requirements were not fulfilled, data were transformed using logarithmic transformation (if variables were continuous) or square root transformation (if variables were counts) to normalize distributions and stabilize variances. Those data that were unable to improve distribution with transformations and showed major departures from normality were analyzed using non-parametric tests. Differences in larval and pupal development times, survival to adults, adult weight and fertility were analyzed by Kruskal-Wallis test, followed by Dunn's multiple comparisons tests. Larvae HC width measurements were analyzed using repeated-measurements (RM) one way-analysis of variance (ANOVA), followed by Tukey's multiple comparisons tests. Differences among levels of toxin concentration in Bt maize, T. urticae and A. coriaria and immature development time from L1–adult, differences in preoviposition period, fecundity and among treatments in fungus gnat larvae predation and consumption, were compared by one way-ANOVA, followed by Tukey's multiple comparisons tests. The Kaplan-Meier survival analysis was applied to compare survival of the test insects on the different diets and their survival distributions were compared by Mantel log-rank test.
Results
Bt-toxin uptake through the trophic chain
Atheta coriaria larvae and adults were exposed to the Cry3Bb1 toxin through prey raised on Bt maize. Cry3Bb1 measurements in the tritrophic assay (plant-herbivore-predator) gave the following mean concentration for each level: 21.7 (Bt maize); 5.6 (T. urticae raised on Bt maize); 4.6 (A. coriaria L1); 4.1 (A. coriaria L2); 4.5 (A. coriaria L3) and 1.4 (A. coriaria adults) μg Cry3Bb1 g−1 FW (fig. 1). Concentration among samples presented significant differences. Mean toxin level of Bt maize was significantly higher than the toxin level presented by T. urticae and A. coriaria (larvae and adults). However, no statistical differences were found among T. urticae and the three A. coriaria larval instars. Atheta coriaria adults presented lower mean toxin concentration than larvae after four days eating mites raised on Bt maize. No Cry3Bb1 protein was detected in rearing food, non-Bt maize, T. urticae raised on non-Bt maize, and A. coriaria larvae or adults fed with T. urticae raised on non-Bt maize (data not shown).

Fig. 1. Mean Cry3Bb1 toxin concentration in Bt maize (N=10), Tetranychus urticae fed with Bt maize (N=10) and Atheta coriaria larvae (L1, L2 and L3) and adults fed on T. urticae raised on Bt maize (N=5 for each development stage). Error bars represent±SE. Columns with the same letters are not significantly different.
Cry3Bb1 protein detection time in A. coriaria larvae and adults
Cry3Bb1 toxin was detected in rove beetles (L3 larvae and adults) at different post exposure times after consumption of T. urticae raised on Bt maize (fig. 2). The level of the protein decreased at an exponential rate over time, but the pattern was different in A. coriaria larvae and adults (fig. 2). In larvae, the toxin half-life was 6.9 h, and it was detected at the longest post-exposure time tested, 48 h, whereas the toxin half-life was shorter in adults (5.4 h), being detectable after 12 h at the most.

Fig. 2. Detection of Cry3Bb1 toxin at different post-exposure times in Atheta coriaria third instar larvae and adults fed during four days on Tetranychus urticae raised on Bt maize. Symbols represent mean values±S.E. Larval detection time: Y(t)=3.55(−0.10t); r2=0.85. Adult detection time: Y(t)=1.43(−0.10t); r2=0.60. Y0 is the initial concentration of Cry3Bb1 (μg g−1 fresh weight). Data are means of five measurements for each time period. Error bars represent±SE (![]() , A. Coriaria L3;
, A. Coriaria L3; ![]() , A. Coriaria adults).
, A. Coriaria adults).
Effects on predator fitness
Prey-mediated effects due to larval exposure to Cry3Bb1
Atheta coriaria completed its life cycle when feeding on any of the three diets evaluated herein. The development time of the first two instars presented no difference among diets. Duration of L3 fed with rearing food (5.2 days) was significantly shorter than larvae fed on T. urticae raised on non-Bt maize (5.6 days), but similar to larvae that preyed on T. urticae raised on Bt maize (5.4 days) (table 2). The extent of total larval development (L1–L3) showed no differences among treatments. Likewise, no difference in the duration of the pupal stage was found among the diets. When total immature developmental time (L1–adult) was analyzed, no differences were found among treatments. Survival to adults presented no differences related with the three diets. Sex ratio of adults emerged were also alike among treatments.
Table 2. Pre-imaginal development time, survival and sex ratio of A. coriaria when larvae were reared on different diets.
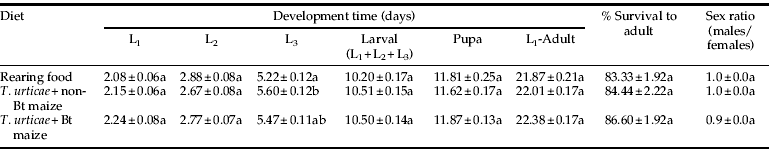
Data are means±SE of 30 samples per treatment.
Means followed by the same letter within a column are not significantly different from each other.
When larval HC width was compared among feeding treatments, no significant differences were found in the two first instars (table 3). In the last instar stage, larvae fed on regular rearing diet presented significant higher values of HC width than those fed on mites reared on Bt maize, but there were not differences between larvae fed on mites raised on Bt maize and non-Bt maize. The increment of HC width between successive instars presented almost the same geometrical progression when fed on the three diets, having a growth rate between 1.21 and 1.22. Only in the case of HC width, values in females fed on rearing food were significantly lower than in females reared on T. urticae raised on non-Bt maize, although there were no differences between females reared on mites raised on non-Bt maize or Bt maize. The adult weight and elytra length of males or females emerged from larvae reared under three food regimes did not display any significant difference (table 3).
Table 3. Larval and adult morphometric measurements and adult body weight of A. coriaria when larvae were reared on different diets.

HC (head capsule) width was measured across the widest part of the head.
Data are means±SE of 30 samples per treatment.
Means followed by the same letter within a column are not significantly different from each other.
Prey-mediated effects due to adult exposure to Cry3Bb1
Individualized mating pairs of A. coriaria exposed to different diets through 60 days presented no differences in reproductive parameters associated with the treatments (table 4). Rove beetle pairs presented similar preoviposition period in each of the three treatments. When fecundity was assessed during the first 30 days (42–46 eggs), no significant effect related with the diet was observed. Likewise, egg hatching ranged from 91% to 93% in all treatments, and there were no significant differences among them (table 4). After 60 days, adults exposed to the three diets did not present statistical differences on survival for females (log-rank test; X 2=0.87, P=0.64) and males (log-rank test; X 2=2.10, P=0.35) (fig. 3). At the end of the assay, females presented values of survival ranging between 30% and 50% and males between 10% and 30%.

Fig. 3. Survival probability of Atheta coriaria (A) females (N=10) and (B) males (N=10) when they fed on three different diets during 60 days (![]() , rearing food;
, rearing food; ![]() , non-Bt maize; ——, Bt maize). Adults were examined daily.
, non-Bt maize; ——, Bt maize). Adults were examined daily.
Table 4. Reproductive measurements of single mating pairs of A. coriaria after feeding on different diets for 30 days.
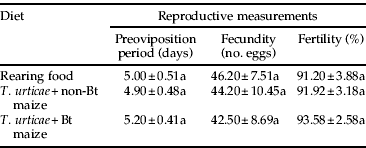
Data are means±SE of ten samples per treatment.
Means followed by the same letter within a column are not significantly different from each other.
Effects on predatory ability of A. coriaria larvae and adults
Both A. coriaria L3 instar larvae and adults that had been reared on the three diets preyed actively on sciarid fly larvae. A. coriaria larvae were more voracious than adults, having killed and consumed more fungus gnat larvae at the end of the assay (table 5). When compared among treatments, no differences were found in the number of fungus gnat larvae killed and consumed by A. coriaria L3 after 24 h. Likewise, the exposure to Cry3Bb1 during the entire immature development did not affect the predatory ability of newly emerged A. coriaria adults. The number of fungus gnat larvae killed after 24 h presented no statistical differences among treatments in females and in males. Prey consumption in adults was also not affected by diet treatment, as no differences were detected in the number of fungus gnat larvae consumed by females and by males (table 5).
Table 5. Predation and consumption of fungus gnats larvae by single A. coriaria L3 larvae and adults during 24 h.
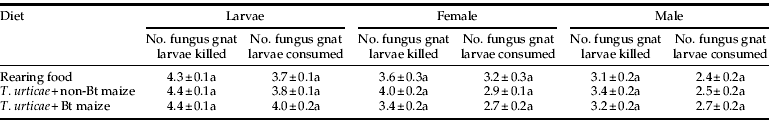
Data are means±SE.
Means followed by the same letter within a column are not significantly different from each other.
Discussion
The Cry3Bb1 protein is known to be biologically active against several species within the coleopteran family Chrysomelidae (Vaughn et al., Reference Vaughn, Cavato, Brar, Coombe, DeGooyer, Ford, Groth, Howe, Johnson, Kolacz, Pilcher, Purcell, Romano, English and Pershing2005) but innocuous for other insects, including predatory beetles from the families Carabidae and Coccinellidae (Lundgren & Wiedenmann, Reference Lundgren and Wiedenmann2002; Duan et al., Reference Duan, Paradise, Lundgren, Bookout, Jiang and Wiedenmann2006; Álvarez-Alfageme et al., Reference Álvarez-Alfageme, Bigler and Romeis2011). However, laboratory studies evaluating Cry3Bb1 for potential toxicity on staphylinids are scarce even though they are important predators of agronomic pests and have been used as biological control agents (Jandricic et al., Reference Jandricic, Scott-Dupree, Broadbent, Harris and Murphy2006). The staphylinid A. coriaria is an agronomically important species for pest control in greenhouses that may feed on a wide range of arthropods, including mites (Helyer et al., Reference Helyer, Brown and Cattlin2003). It has recently been proposed as a suitable surrogate species for risk assessment protocols, as representative of other generalist predatory rove beetles (García et al., Reference García, Ortego, Castañera and Farinós2010; Porcar et al., Reference Porcar, García-Robles, Domínguez-Escribá and Latorre2010). In the present study, we performed tritrophic bioassays to assess the potential prey-mediated effects of Cry3Bb1-expressing maize on the performance of A. coriaria using T. urticae as prey, which has been proved to be a good vehicle for Bt toxins.
Our data show that larvae and adults of A. coriaria were exposed to the Cry3Bb1 toxin throughout the trophic chain. No feeding activity of A. coriaria was observed when they were exposed to maize leaves without mites, excluding the possibility of direct uptake of the toxin from leaf tissues (data not shown). The passage of the toxin from the Bt maize to the next trophic level (T. urticae) presented a 4-fold reduction. However, depletion of the toxin from the herbivore to the predator varied depending on the predator developmental stage. Cry3Bb1 levels in L1, L2 and L3 larvae of A. coriaria and the herbivore were similar, whereas passage of the toxin from mites to A. coriaria adults showed a 4-fold reduction. A previous study carried out in our laboratory with Cry1Ab-expressing Bt maize demonstrated that the depletion of the toxin from T. urticae to A. coriaria was 6-fold lower for L1 and adults and 2- to 3-fold lower for L2 and L3 larvae fed on Bt raised mites (García et al., Reference García, Ortego, Castañera and Farinós2010). Other studies using similar tritrophic bioassays with predatory beetles showed that predators contained about one order of magnitude lower Bt protein concentrations than prey (Álvarez-Alfageme et al., Reference Álvarez-Alfageme, Ferry, Castañera, Ortego and Gatehouse2008, Reference Álvarez-Alfageme, Bigler and Romeis2011; Li & Romeis, Reference Li and Romeis2010). It has been suggested that this decline might be explained because the prey's gut represents a fraction of the consumable prey and because a part of the Bt protein ingested could be digested and excreted (Meissle & Romeis, Reference Meissle and Romeis2009). Nevertheless, the high levels recorded in A. coriaria larvae indicate that there might be other factors influencing the amount of toxin found in these predatory larvae. The maximum time of detection of Cry3Bb1 in A. coriaria adults was 12 h after exposure to the toxin, similar to that found in an analogous bioassay using Cry1Ab-expressing maize, whereas the half-life of Cry3Bb1 (5.4 h) was higher than the one found in adults exposed to Cry1Ab toxin (3.3 h) (García et al., Reference García, Ortego, Castañera and Farinós2010). For third instar larvae of A. coriaria, the maximum time of detection of the Cry3Bb1 toxin was the upper limit tested (48 h) and its half-life was 6.9 h; while maximum time of detection was 24 h for the toxin Cry1Ab and its half-life was 5.7 h (unpublished results). These findings suggest that digestion/excretion of Cry3Bb1 is slower in larvae than in adults of A. coriaria and that larvae excrete/digest Cry3Bb1 slower than Cry1Ab protein. The methodology employed to expose A. coriaria to T. urticae raised on Bt maize expressing Cry1Ab and Cry3Bb1 toxin was the same, so differences could be related with the physiological interactions of each protein inside A. coriaria. Interestingly, the toxin level detected in A. coriaria larvae (about 4.5 μg Cry3Bb1 g−1 FW) represents more than 20% of the concentration found in the plant. This is the highest Cry3Bb1 level reported in any predatory beetle. The high levels of toxin found in L3 larvae and its detection 48 h after exposure highlights the selection of A. coriaria as a surrogate species for tritrophic studies. These results also indicate that this stage could be an intermediary for the passage of the Cry3Bb1 toxin to other predators in a higher trophic level or to the environment via excretion or organism death.
The exposure of A. coriaria to Cry3Bb1 toxin expressed in Bt maize through the trophic chain did not have negative effects on survival and development. These results are consistent with other studies that assessed the impact of Cry3Bb1 on coleopteran predators. The ingestion of pollen expressing Cry3Bb1 produced no detrimental effects on the fitness of Coleomegilla maculata DeGeer (Coleoptera: Coccinellidae) (Duan et al., Reference Duan, Head, McKee, Nickson, Martin and Sayegh2002; Lundgren & Wiedenmann, Reference Lundgren and Wiedenmann2002). Similarly, no effects were found when a tritrophic experimental design was used with larvae of the ladybirds Adalia bipunctata L. (Álvarez-Alfageme et al., Reference Álvarez-Alfageme, Bigler and Romeis2011) and Stethorus punctillum (Weise) (Coleoptera: Coccinellidae) (Li & Romeis, Reference Li and Romeis2010). Other laboratory studies with the ground beetles Harpalus caliginosus F., Harpalus pensylvanicus De Geer and Poecilus chalcites (Say) (Coleoptera: Carabidae) exposed to Cry3Bb1-expressing Bt maize revealed no negative effects on the fitness parameters (Ahmad et al., Reference Ahmad, Wilde, Whitworth and Zolnerowich2006; Duan et al., Reference Duan, Paradise, Lundgren, Bookout, Jiang and Wiedenmann2006). However, the lack of a detrimental effect on fitness parameters does not necessarily indicate a lack of negative effects on other parameters. Growth of immature insects is strongly influenced by food quality (Delbac et al., Reference Delbac, Lecharpentier and Thiery2010) and morphometrics of larvae at the same instar may differ if reared on different foods or different qualities of food (Daly, Reference Daly1985; Chege et al., Reference Chege, Clark and Hibbard2005). Our study demonstrates that the HC width of successive instars of A. coriaria larvae fed on mites raised on Bt maize follows a geometrical progression, in accordance to Dyar´s rule. Moreover, the growth rate was constant in larvae reared in the three treatments, confirming that the larval growth is not being affected by the ingestion of Cry3Bb1. Similarly, adults emerged from larvae exposed to Cry3Bb1 toxin during their entire immature development, presented no significant changes related with morphological measurements of sclerotized structures (HC width and elytra length) and with body weight.
It has been reported that the ingestion of potato plants expressing Cry3B protein stopped the development of ovaries in females of the target beetle, L. decemlineata (Arpaia et al., Reference Arpaia, De Marzo, Di Leo, Santoro, Mennella and van Loon2000). This could be considered an unexpected sublethal effect (and probably indirect), since Cry3 proteins target the insect midgut. However, no effects on relative fecundity and on egg viability were found when the non-target ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) was exposed to toxin Cry3A (Ferry et al., Reference Ferry, Mulligan, Majerus and Gatehouse2007). When females and males of A. coriaria adults were exposed to the toxin during an extended period of time, no effects on reproductive parameters were seen. Female nutrition is critical during oogenesis, affecting both the level and quality of egg production (Wheeler, Reference Wheeler1996). A. coriaria females exposed to different diets presented similar values of fertility and fecundity during the first 30 days after emergence, showing that T. urticae containing the Bt toxin is a food source nourishing enough to allow reproduction in this species. These results are consistent with another study that assessed the impact of mCry3A toxin on the fecundity of the parasitic rove beetle Aleochara bilineata (Gyll.) (Coleoptera: Staphylinidae) (Stacey et al., Reference Stacey, Graser, Mead-Briggs and Raybould2006). Survival rates of A. coriaria adults exposed to Cry3Bb1 toxin for 60 days were similar among treatments, presenting females lower mortality than males across all treatments. Similarly, when A. coriaria adults were exposed to artificial diets treated with and without Cry3A toxin for 15 days no differential mortality was found (Porcar et al., Reference Porcar, García-Robles, Domínguez-Escribá and Latorre2010).
Relatively less effort in GM risk assessments has been devoted to behavioural studies, such as alterations on feeding behaviour. Search ability and prey consumption capacity are considered some of the most important attributes of effective natural enemies. Therefore, possible effects on feeding behaviour of predatory beetle could represent important sublethal effects that should be assessed. Interestingly, we have found that the ingestion of a Bt maize-fed prey during the entire immature stage produced no negative effects on the predatory ability and prey consumption of A. coriaria (L3 and adults). Generally, when larvae and adults of A. coriaria were placed in the test arena containing fungus gnat larvae, both immediately attacked one of the larvae and started to feed on them. All the staphylinids (larvae and adults) killed more fungus gnat larvae than consumed; this kind of behaviour (surplus killing) was already described for A. coriaria adults and larvae (Miller & Williams, Reference Miller and Williams1983; Carney et al., Reference Carney, Diamond, Murphy and Marshall2002). Likewise, bioassays with adults of Nebria brevicollis (Fabricius) (Coleoptera: Carabidae) indicated that beetle activity, prey location time, prey handling time and number of contacts resulting in feeding were not affected after eating Cry3A-fed prey (Ferry et al., Reference Ferry, Mulligan, Majerus and Gatehouse2007). Some studies on beneficial non-target beetles have shown no detrimental effects of Cry3-toxins on other behavioural responses. When C. maculata was fed on transgenic pollen expressing Cry3Bb toxin (event MON863) during its complete immature development, no effects on some movement behaviours (walking speed and flip time) were found in adults (Lundgren & Wiedenmann, Reference Lundgren and Wiedenmann2002). In the same way, consumption of anthers and pollen of Cry3A expressing potato did not affect locomotor behaviour of H. axyridis adults (Ferry et al., Reference Ferry, Mulligan, Majerus and Gatehouse2007).
In summary, this study shows that the Cry3Bb1 protein ingested through prey raised on MON88017 Bt maize has no adverse effects on the survival, growth, development, reproduction and predatory behaviour of A. coriaria. The significance of our findings is supported by the ‘worst-case scenario’ used in this study, where A. coriaria was subjected to continuous exposure of high levels of Cry3Bb1 toxin throughout the immature and adult stages. This study provides insights for the risk assessment of other GM crops expressing Cry toxins.
Acknowledgements
We thank Dr Vicente Marco (Universidad de la Rioja, Spain) for providing the laboratory colony of T. urticae, Dr Kai Heller (Heikendorf, Germany) for the taxonomic classification of the Sciaridae specimens and Monsanto for providing seeds of the two maize varieties used and the toxin used to prepare standards for ELISA tests. This work received financial support from the Spanish Ministry of Environment (MMA) and CICYT (AGL2009-08813). Matías García's research was supported by a contract from the Science and Innovation Ministry (JAE-Doc).










