Myocardial bridging is a congenital coronary anomaly in which the coronary artery has a partly “tunnelled” intramyocardial course. It is thought to be a benign variation of the norm caused by an aberrancy of the embryologic coronary development.Reference Lee and Chen 1 The tunnelling of the artery leads to compression of the “tunnelled” vessel segment during systole that can persist into diastole.Reference Yetman, McCrindle, MacDonald, Freedom and Gow 2 , Reference Corban, Hung and Eshtehardi 3 Complex and dynamically interacting biomechanical factors may influence coronary blood flow within and distal to the bridged coronary segments.Reference Corban, Hung and Eshtehardi 3 The clinical relevance of this systolic compression is controversial. In most cases, myocardial bridging is thought to be benign, but in a few patients it can cause severe cardiac issues including myocardial ischaemia, ventricular arrhythmia, and sudden cardiac death. Several studies could not find any systematic association between myocardial bridging in hypertrophic cardiomyopathy patients and sudden cardiac death.Reference Basso, Thiene, Mackey-Bojack, Frigo, Corrado and Maron 4 Despite the lack of association between myocardial bridging and symptoms, the authors speculate that there may be a potential increased risk in individual patients.
Myocardial bridging is found in about one-third of healthy adults. The number of affected people in different studies is highly variable; in autopsy series there is a reported mean of 25%, and in angiographic series it varies between 0.5 and 40% with provocation tests.Reference Lee and Chen 1 – Reference Corban, Hung and Eshtehardi 3 , Reference Möhlenkamp, Hort, Ge and Erbel 5 Typically, the ramus interventricularis anterior is affected in more than two-thirds of patients.Reference Corban, Hung and Eshtehardi 3
There are a few reports of myocardial bridging in children. In children with hypertrophic cardiomyopathy the frequency is stated to be about 28%,Reference Yetman, McCrindle, MacDonald, Freedom and Gow 2 , Reference Colan 6 but the condition seems to be rare in children without hypertrophic cardiomyopathy. A number of these children showed severe symptoms with chest pain, syncope, ventricular arrhythmia, palpitations, and cardiac arrest and required surgical treatment with division (“unroofing”).Reference Yetman, McCrindle, MacDonald, Freedom and Gow 2 , Reference Sharma, Hellenbrand, Kleinman and Mosca 7 In young patients without hypertrophic cardiomyopathy, there are rare single case reports regarding symptomatic myocardial bridging in otherwise healthy children.Reference Poryo, Khreish, Schäfers and Abdul-Khaliq 8 , Reference Daana, Wexler, Milgalter, Rein and Perles 9 Asymptomatic myocardial bridging has been reported in a child with familial subaortic stenosisReference Gokalp and Funda 10 and in a study of children with Williams syndrome – 3 out of 38 children.Reference Ergul, Nisli and Kayserili 11 These case reports related to children aged 5–16 years.
Materials and methods
We retrospectively analysed a series of six patients aged 0–16 years, who have been treated in our centre within the past years with symptomatic myocardial bridging and different underlying cardiac conditions. This retrospective analysis was approved by the head of our department. The report was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Results
In this retrospective single-centre study, we describe six patients who presented with signs of myocardial ischaemia and subsequently underwent coronary angiography, which revealed myocardial bridging of the ramus interventricularis anterior (Fig 1). All patients underwent echocardiographic function tests including two-dimensional echo and two-dimensional speckle tracking echocardiography, followed by invasive angiography. Our two adolescent patients additionally received magnetic resonance tomography and ergometric testing; one other patient received an additional CT-scan before being referred to our unit. In four out of six patients, an endomyocardial biopsy was performed (see Table 1). Clinical data for the six study patients are summarised in Table 1. The median age at diagnosis was 5.5 years – ranging from 12 days to 16 years. The median follow-up duration was 26 months – ranging from 4 to 33 months. During the follow-up period, we did not notice any major events with recurrent myocardial ischaemia, cardiac arrest, or death. The clinical manifestation varied from subclinical to severe. The subclinical presentation was an incidental finding of localised myocardial dysfunction demonstrated by two-dimensional speckle tracking echocardiography (Fig 2) and unusually long persistence of elevated myocardial markers of ischaemia following corrective surgery of CHD – closure of ventricular and atrial septal defects and correction of aortic coarctation – in a newborn girl. Two boys aged 1.5 and 2.5 years presented repeatedly with acute heart failure because of acute ischaemic events and dilative cardiomyopathy. Two children were admitted after successful resuscitation because of ventricular fibrillation. One of them suffered from unknown hypertrophic cardiomyopathy and had been admitted to our hospital at the age of 4 months. The other patient, a 13-year-old girl, had a known left ventricular non-compaction cardiomyopathy with a hypertrophic component, but was not on medication and did not attend regular follow-ups. Our oldest patient, a 16-year-old boy, was referred to our centre for further testing with newly diagnosed hypertrophic cardiomyopathy seen in a routine check-up. None of the patients had an obstructive component.

Figure 1 Angiography of the left coronary system in the diastolic ( a ) and systolic ( b ) phase in left anterior oblique view (patient 1). Ramus interventricularis anterior compression in the middle aspect with reduced flow as the ventricle contracts.

Figure 2 Two-dimensional speckle tracking echocardiography reveals localised myocardial deformation abnormalities in anterior septal left ventricular segments in patient 5 (Epiq 7G, QLab 10.4, aCMQ module; Philips Healthcare North America, Andover, MA, United States of America). ANT=anterior; AP2=apical two chamber view; AP3=apical three chamber view; AP4=apical four chamber view; ApS=apicoseptal; ApL=apicolateral; BAL=basal anterolateral; BIS=basal inferoseptal; EDV=enddiastolic volume; ESV=endsystolic volume; EF=ejection fraction; HR=heart rate; INF=inferior; LAT=lateral; MAL=mid anterolateral; MIS=mid inferoseptal; SEPT=septal.
Table 1 Clinical characteristics of six children with symptomatic myocardial bridging.

2D-E=two-dimensional echocardiography; 2D-STE=two-dimensional speckle tracking echocardiography; AICD=automatic implantable cardioverter defibrillator; Angio=angiography; ASS=acetylsalicylic acid; DCM=dilatative cardiomyopathy; EMB=endomyocardial biopsy; Ergo=ergometric testing; HCM=hypertrophic cardiomyopathy; LVNC=left ventricular cardiomyopathy; MRT=magnetic resonance tomography; RIVA=ramus interventricularis anterior
In all patients, therapy with β blocker was started to reduce heart rate and myocardial contractility. Although there is no clear evidence for β blocker treatment, it is still referred to as first-line therapy.Reference Lee and Chen 1 , Reference Corban, Hung and Eshtehardi 3 In theory, β blockers also prolong diastole and improve coronary artery blood flow.Reference Corban, Hung and Eshtehardi 3 Four of our patients were discharged in improved clinical condition under β blocker treatment. A long-term β blocker therapy is planned contingent upon the future clinical course of the patients and the emerging evidence.
In two patients, clinical signs remained with recurrent myocardial ischaemia, severe reduction of general health, and depression in ST-segments, respectively, echocardiography stress testing positive for ischaemia and ST-elevations in ergometric testing (Fig 3). Both were referred for surgery with exposure of the involved coronary segment on cardiopulmonary bypass (Fig 4). Surgical exposure was successful and recovery was complete and uneventful (Supplementary video 1). Post-surgery speckle tracking echocardiography revealed a normalised deformation pattern (Fig 5). The mean follow-up period after surgery has been 22 months with no major events.
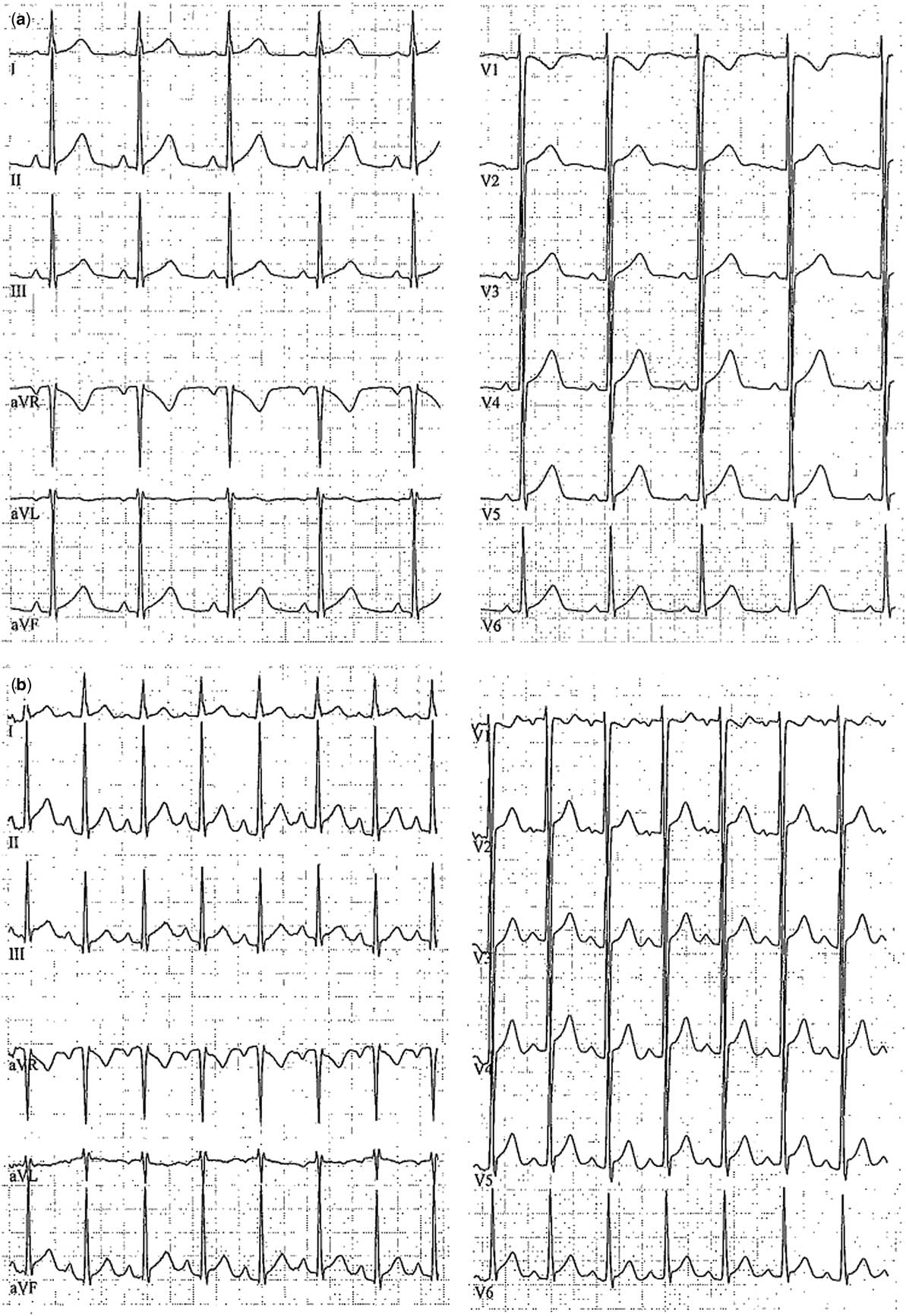
Figure 3 Ergometric testing of patient 5 with signs of myocardial ischaemia with ST-segment elevations in II, aVF, V2-6 at rest ( a ), and under physical stress ( b ). aVF=augmented voltage foot; aVL=augmented voltage left; aVR=augmented voltage right. Limb leads given by I–III and precordial leads given by V1–V6.

Figure 4 Intraoperative situs of patient 4 after exposure (“unroofing”) of the Ramus interventricularis anterior on cardiopulmonary bypass.

Figure 5 Two-dimensional speckle tracking echocardiography reveals harmonised myocardial deformation after surgery in patient 5 (Epiq 7G, QLab 10.4, aCMQ module). ANT=anterior; AP2=apical two chamber view; AP3=apical three chamber view; AP4=apical four chamber view; ApS=apicoseptal; ApL=apicolateral; BAL=basal anterolateral; BIS=basal inferoseptal; EDV=enddiastolic volume; ESV=endsystolic volume; EF=ejection fraction; HR=heart rate; INF=inferior; LAT=lateral; MAL=mid anterolateral; MIS=mid inferoseptal; SEPT=septal.
Discussion
Clinical relevance of myocardial bridging is controversial because of the high prevalence found in angiography and autopsy compared with the few symptomatic clinical courses. Basso et al analysed the association between hypertrophic cardiomyopathy-related sudden deaths with myocardial bridging and did not find any significant linkage. Nevertheless, the authors included the possibility of increased risk in individual patients.Reference Basso, Thiene, Mackey-Bojack, Frigo, Corrado and Maron 4 Most data on myocardial bridging in paediatrics are on children with hypertrophic cardiomyopathy.Reference Yetman, McCrindle, MacDonald, Freedom and Gow 2 There are few case reports on symptomatic myocardial bridging in otherwise healthy children.Reference Poryo, Khreish, Schäfers and Abdul-Khaliq 8 , Reference Daana, Wexler, Milgalter, Rein and Perles 9 Three of our patients showed the typical constellation of hypertrophic cardiomyopathy and myocardial bridging. The other three patients suffered from different underlying cardiac conditions. Two patients had known dilative cardiomyopathy of unknown aetiology and presented with acute ischaemic events. The coronary angiography revealed myocardial bridging as a feasible cause of these acute episodes and a possible cause for the presence of dilative cardiomyopathy.
Another useful tool to detect the localised myocardial dysfunction secondary to irregular coronary perfusion is speckle tracking strain echocardiography at rest and on physical stress testing. Its high sensitivity and specificity in detection of myocardial injury has been extensively reviewed.Reference Geyer, Caracciolo and Abe 12 Although speckle tracking echocardiography at rest was performed in all patients, stress echo testing with strain analysis was only performed in patient number 5, a 13-year-old girl, because there is no appropriate non-invasive test protocol available for young children in our setting. Patient number 6, a 16-year-old boy, was also referred for stress echo testing. However, this patient had initially been suspected to have myocarditis and thus stress testing was thought to be contraindicated.
Adult interventional cardiologists perform intravascular ultrasound and measurement of functional flow reserve on a more regular base.Reference Corban, Hung and Eshtehardi 3 , Reference Agrawal, Molossi and Alam 13 Agrawal et alReference Agrawal, Molossi and Alam 13 suggested the use of these techniques for risk stratification in children with anomalous coronary arteries and myocardial bridges. These novel techniques could have completed our diagnostic procedures in our teenage patients. A widespread use of these techniques in the future seems possible.
Conclusion
To the authors’ knowledge, the presented series is the largest so far to illustrate varying symptomatic courses of myocardial bridging even in early childhood in children with different underlying cardiac conditions. Myocardial bridging seems to be a rare, but a relevant differential diagnosis of acute ischaemic events in early childhood, especially if combined not only with hypertrophic cardiomyopathy but also with other cardiac diseases. This rare entity should be considered even in young children because it may require urgent treatment and in rare cases surgical intervention.
Acknowledgements
The authors thank J. Schindler for critical revision of the manuscript for language content.
Financial Support
This research did not receive any specific grant from funding agencies or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the local head of the department.
Supplemental material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951118000409









