INTRODUCTION
Mediterranean biodiversity is undergoing rapid alteration, driven by multiple stress factors, most due to anthropogenic activities (Bianchi et al., Reference Bianchi, Morri, Chiantore, Montefalcone, Parravicini, Rovere and Stambler2012). Among these factors, alien species are a major threat to the biodiversity of the basin, affecting communities synthesis, habitats and ecosystem functioning (Coll et al., Reference Coll, Piroddi, Steenbeek, Kaschner, Ben Rais Lasram, Aguzzi, Ballesteros, Bianchi, Corbera, Dailianis, Danovaro, Estrada, Froglia, Galil, Gasol, Gertwagen, Gil, Guilhaumon, Kesner-Reyes, Kitsos, Koukouras, Lampadariou, Laxamana, López-Fé de la Cuadra, Lotze, Martin, Mouillot, Oro, Raicevich, Rius-Barile, Saiz-Salinas, San Vicente, Somot, Templado, Turon, Vafidis, Villanueva and Voultsiadou2010; Katsanevakis et al., Reference Katsanevakis, Wallentinus, Zenetos, Leppäkoski, Çinar, Oztürk, Grabowski, Golani and Cardoso2014).
Rhodes Island is located in the south-eastern Aegean Sea, a geographically crucial region subjected to biological invasions (Corsini-Foka & Kondylatos, Reference Corsini-Foka and Kondylatos2015a; Corsini-Foka et al., Reference Corsini-Foka, Kondylatos and Louizidou2015a, Reference Corsini-Foka, Zenetos, Crocetta, Çinar, Koçak, Golani, Katsanevakis, Tsiamis, Cook, Froglia, Triandaphyllou, Lakkis, Kondylatos, Tricarico, Zuljevic, Almeida, Cardigos, Çağlar, Durucan, Fernandes, Ferrario, Haberle, Louizidou, Makris, Marić, Micu, Mifsud, Nall, Kytinou, Poursanidis, Spigoli, Stasolla, Yapici and Royb). The subtropical environment of the area is suitable for native thermophilic biota and also for tropical or subtropical alien biota colonization (Papaconstantinou, Reference Papaconstantinou2014), this latter phenomenon has been amplified in the last few decades by climate change and the warming of the sea (Pancucci-Papadopoulou et al., Reference Pancucci-Papadopoulou, Raitsos and Corsini-Foka2012; Bianchi et al., Reference Bianchi, Corsini-Foka, Morri and Zenetos2014). It represents a step for alien species establishment, important for their farther northward and westward dispersion into the Aegean waters and the remaining Mediterranean (Azzurro et al., Reference Azzurro, Moschella and Maynou2011; Kalogirou et al., Reference Kalogirou, Azzurro, Bariche and Lameed2012a).
The waters of the Dodecanese islands are oligotrophic, with a fishery production limited to 1–3% of the total Hellenic fishery production during the last 20 years (ELSTAT, 2015) and represented by few species of commercial interest (Labropoulou, Reference Labropoulou, Papaconstantinou, Zenetos, Vassilopoulou and Tserpes2007).
During that period, small-scale coastal fishery (trammel nets, gill nets, longlines), with a traditional family character, dominated by 96% (ELSTAT, 2015; Corsini-Foka et al., Reference Corsini-Foka, Kondylatos and Louizidou2015a). The distribution of the fleet and the use of the proper type of fishing gear in the Dodecanese depend largely on the morphology of the seabed, which is generally very deep and rugged. Nevertheless, north of the island of Cos, the seabed is shallower with a flatter relief. Apart from Rhodes, a considerable proportion of the fleet is concentrated in the northern islands of the Archipelago, mainly in Kalymnos, Leros and Cos (ELSTAT, 2015). Adapted infrastructures for fish landing and marketing are present only in Kalymnos Island, consequently catch data for all types of fishing gear for the island of Rhodes are scarce.
The continuous influx of species of Red Sea origin is one of the factors that contribute to alter the fish communities in the eastern Mediterranean (Turan et al., Reference Turan, Erguden, Uygur, Gurlek, Erdogan, Sonmez, Uyan, Karan and Dogdu2015), including the Hellenic coasts of the south-eastern Aegean (Kalogirou et al., Reference Kalogirou, Corsini-Foka, Sioulas, Wennhage and Pihl2010; Corsini et al., Reference Corsini-Foka, Zenetos, Crocetta, Çinar, Koçak, Golani, Katsanevakis, Tsiamis, Cook, Froglia, Triandaphyllou, Lakkis, Kondylatos, Tricarico, Zuljevic, Almeida, Cardigos, Çağlar, Durucan, Fernandes, Ferrario, Haberle, Louizidou, Makris, Marić, Micu, Mifsud, Nall, Kytinou, Poursanidis, Spigoli, Stasolla, Yapici and Roy2015b). The non-indigenous fish species (in the present work the term ‘alien’ species is used as a synonym of non-indigenous species) reported in the Dodecanese complex consists of 32 species (30 recorded at Rhodes), after the addition to the reviewed list in Corsini-Foka et al. (Reference Corsini-Foka, Zenetos, Crocetta, Çinar, Koçak, Golani, Katsanevakis, Tsiamis, Cook, Froglia, Triandaphyllou, Lakkis, Kondylatos, Tricarico, Zuljevic, Almeida, Cardigos, Çağlar, Durucan, Fernandes, Ferrario, Haberle, Louizidou, Makris, Marić, Micu, Mifsud, Nall, Kytinou, Poursanidis, Spigoli, Stasolla, Yapici and Roy2015b) of Scarus ghobban Forsskål, 1775 and Oxyurichthys petersi (Klunzinger, 1871) (Apostolopoulos & Karachlè, Reference Apostolopoulos and Karachlè2016), Pterois miles (Bennett, 1828) (Corsini-Foka & Kondylatos, Reference Corsini-Foka and Kondylatos2015b) and Synchiropus sechellensis Regan, 1908 (Kondylatos et al., Reference Kondylatos, Corsini-Foka, Apostolopoulos and Zenetos2016). All these alien species are Lessepsian migrants (Golani, Reference Golani, Golani and Appelbaum-Golani2010); they belong to 22 families, eight of which are new to the Hellenic ichthyofauna, namely Champsodontidae, Fistulariidae, Hemiramphidae, Holocentridae, Leiognathidae, Monacanthidae, Pempheridae and Siganidae. Following Papaconstantinou (Reference Papaconstantinou2014) and updating with the above records, the ichthyofauna diversity known in the wider area of the Dodecanese counts ~274 bony fish species and 51 cartilaginous fish species. Therefore, Lessepsian fish in the area correspond to about 12% of total bony fish species known. Furthermore, the Lessepsian fish species recorded in the Dodecanese are about 31% of the total 102 Lessepsian fish introduced to date into the Mediterranean (Fricke et al., Reference Fricke, Golani and Appelbaum-Golani2015; Hata & Motomura, Reference Hata and Motomura2016; Rothman et al., Reference Rothman, Stern and Goren2016; Gurlek et al., Reference Gurlek, Erguden, Dogdu and Turan2016; Seyhan et al., Reference Seyhan, Irmak and Fricke2017).
In the region of Rhodes, a few alien species are of commercial value while the majority are discarded for their small size or unpalatability or danger for human health, such as the highly toxic Lagocephalus sceleratus (Gmelin, 1789), an invasive species that causes serious ecological and socio-economic negative effects in the entire eastern Mediterranean, with incursions in the western part of the basin (Nader et al., Reference Nader, Indary and Boustany2012; Ünal et al., Reference Ünal, Göncüoğlu, Durgun, Tosunoğlu, Deval and Turan2015).
The primary aim of this study is to make preliminary estimations of diversity, abundance and biomass of alien vs native fish species caught by small-scale fisheries, in order to provide data useful as descriptor of the environmental status of a region heavily affected by the influx of alien species and also useful to monitor eventual trends of changes in the diversity of coastal communities in a ‘biopolluted’ Mediterranean marine ecosystem, such as that of Rhodes Island (Pancucci-Papadopoulou et al., Reference Pancucci-Papadopoulou, Corsini-Foka and Raitsos2011, Reference Pancucci-Papadopoulou, Raitsos and Corsini-Foka2012). This is required in the MSFD Annex I 2008/56/EC descriptors of Good Environmental Status and the associated criteria and indicators established by MSFD Commission Decision 2010/477/EU of relevance to the achievement of Good Environmental Status in terms of non-indigenous species.
Furthermore, the results can be used for the design of future fishery policies and regulations and of course as the basis for an extensive study on the fisheries with gill and trammel nets of the area for which the existing qualitative and quantitative information is scarce.
MATERIALS AND METHODS
Fishing operations were carried out within half a year, between December 2014 and May 2015, at two stations, Faliraki (F) (36°19′43″N 28°13′21″E), and Kolimbia (K) (36°14′42″N 28°10′43″E), in the Afandou Bay, north-east coast of Rhodes (Figure 1), over soft, sandy bottom, interrupted by hard substratum. Cystoseira sp. and Posidonia oceanica meadows were present.
Fig. 1. Locations of fishery operations at Afandou Bay, Rhodes Island. (F: Faliraki, K: Kolimbia.)
The fishing method involved the traditional gill nets, using a professional fishing vessel of 9 m in length. Knot to knot mesh sizes of nets were 28–36 mm. Approximately 2000–2100 m of nets, with a height of 2.4 m, were deployed for each operation at 5–30 m depth in late afternoon and retrieved in the early morning.
Fish were identified to species level, and their total length (0.1 cm accuracy) and weight (0.1 g accuracy) were measured on board.
RESULTS
A total of 21 gill net experimental samplings took place at stations F and K, the majority concentrated in spring 2015, depending on the suitability of weather conditions for fishing operations and vessel availability. More exactly, two samplings took place in December 2014, one in January, eight in March, two in April and eight in May 2015. Seawater temperature at the surface ranged approximately from 20.5°C in December to 17.5°C in March and it reached 21–22.5°C in May, while salinity ranged from 39.5‰ in December to 39.1 and 39.2‰ in March and May respectively.
Overall 49 fish species were identified, belonging to 25 families, the richest being Sparidae with 14 species and Serranidae with four species (Figure 2). There were 43 native species (41 bony fish, two cartilaginous), and six alien species (12% of total), namely Siganus luridus (Rüppell, 1828), Siganus rivulatus Forsskål, 1775, Sargocentron rubrum (Forsskål, 1775), Fistularia commersonii (Rüppell, 1838), Lagocephalus sceleratus and Sphyraena flavicauda Rüppell, 1838, with an overall ratio alien to native species of 0.14.

Fig. 2. Number of fish species per family in gill nets.
Concerning the frequency of occurrence in the hauls, four of the six above-mentioned alien species were most frequent, with S. luridus showing the highest value (95%), followed by S. rubrum and S. rivulatus (71%) and F. commersonii (52%), while L. sceleratus and S. flavicauda were present only in 10 and 5% of samplings respectively (Figure 3). Within the native species, Sparisoma cretense (Linnaeus, 1758) showed the highest frequency of occurrence in nets (81%), followed by Scorpaena scrofa Linnaeus, 1758 (76%), Mullus surmuletus Linnaeus, 1758 (67%), Serranus cabrilla (Linnaeus, 1758) (57%) and Pagrus pagrus (Linnaeus, 1758) (52%).
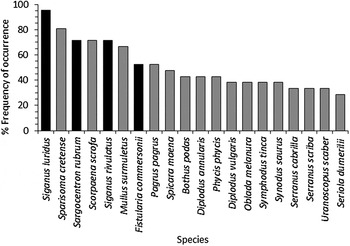
Fig. 3. The 20 fish species showing the higher frequency of occurrence in gill nets (Alien species: black).
Catches were relatively poor and produced a total fish biomass of 183 kg with an average per sampling of 8.7 ± 4.3 kg (range 2.5–17.7 kg), with an average of 4.4 kg per 1000 m of net (range 1.2–8.9 kg). Samplings comprised 1072 individuals, with an average of 50 ± 23 specimens per sampling (range 16–92 specimens). Also 17.6 kg of molluscs, the native Sepia officinalis Linnaeus, 1758 and the alien Conomurex persicus (Swainson, 1821) and Sepioteuthis lessoniana Lesson, 1830 as well as 4.1 kg of crustacean decapods, the native Scyllarides latus (Latreille, 1802) and Dardanus calidus (Risso, 1827) and the alien Gonioinfradens paucidentatus (A. Milne-Edwards, 1861), were collected.
The native S. cretense prevailed in terms of abundance (30% of total specimens) and biomass (27.4% of the total catch), followed by S. luridus, S. scrofa and S. rivulatus (Figure 4). The abundance of alien fish was 320 specimens (29.9% of total abundance) (overall ratio alien/native abundance 0.43), their biomass 48.3 kg (26.4% of total biomass) (overall ratio alien/native biomass 0.36), with the two siganids dominant among aliens (72.8% of total alien specimens and 71.8% of their total biomass). The two siganids considered together reached 22% of total specimens and 19% of total biomass. The abundance of S. rubrum and F. commersonii was 5.6 and 2.2%, while their biomass was 3.1 and 2.7%, respectively. The contribution of L. sceleratus and S. flavicauda to abundance and biomass was negligible, since only two and one specimens respectively were captured.
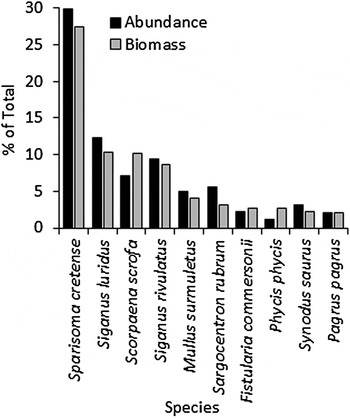
Fig. 4. Species showing higher abundance and biomass in gill nets.
At least one alien species was present in each haul, nevertheless, in terms of number of species, abundance and biomass, native overcame aliens in 20 of the 21 samplings. Since results greatly varied between hauls, also the range of ratios varied: the ratio alien species to native species ranged from 0.13 to 1.50, the ratio alien to native abundance ranged from 0.06 to 3.88 and the ratio alien to native biomass ranged from 0.08 to 3.34.
DISCUSSION
The gill net and trammel net techniques are widely spread in Hellenic seas and their use is more frequent in recent years, after the application, since June 2010, of the European Council Regulation (EC) No. 1967/2006 across Hellenic territorial waters, prohibiting the use of trawlers (including boat seines and bottom trawls) ‘within 3 nautical miles of the coast or within the 50 m isobaths, where that depth is reached at a shorter distance from the coast’.
The results of the present work revealed great difference between hauls, while the overview of all samplings allows a better appraisal of the situation in fishery, concerning both alien and native species.
Among the six Lessepsian fish participating in the catches, four species, Siganus luridus, Siganus rivulatus, Sargocentron rubrum and Fistularia commersonii showed high frequency of occurrence. The two herbivorous siganids, considered together, made up a significantly high proportion of the total abundance and biomass, in second place after the native herbivorous Sparisoma cretense, while S. rubrum and F. commersonii made up a relatively lower proportion. The two invasive siganids are well-known commercially valuable species (10–15 € per kg) since their first appearance and spreading in the area (Corsini-Foka, Reference Corsini-Foka, Golani and Appelbaum-Golani2010), while the also invasive F. commersonii, discarded during the 2010s, is now locally appreciated (Corsini-Foka & Kondylatos, Reference Corsini-Foka and Kondylatos2015a) and has acquired a value of 5–10 € per kg. Other valuable species such as Sphyraena chrysotaenia Klunzinger, 1884 and Etrumeus golanii DiBattista, Randall & Bowen, 2012 are caught in the Rhodes region mainly with the boat seine and the purse seine method, respectively.
In the present work, the two siganids prevailed in abundance and biomass over the other four Lessepsian species caught. A slight dominance of S. luridus compared with S. rivulatus was observed, as reported during the 1960s, in trammel nets or gill nets (Kavalakis, Reference Kavalakis1968; Papaconstantinou, Reference Papaconstantinou1990) and during the 1990s, in trammel nets (Peristeraki et al., Reference Peristeraki, Lazarakis, Skarvelis, Georgiadis and Tserpes2006). In the present study, alien exceeded native species in only one sampling in terms of diversity, abundance and biomass, similarly to a single sampling performed at Rhodes in 2009 with trammel nets where alien fish (S. luridus, S. rivulatus, F. commersonii, Lagocephalus sceleratus) prevailed over native, with S. luridus dominating over all the remaining species (Corsini-Foka et al., Reference Corsini-Foka, Pancucci-Papadopoulou and Kalogirou2010). In comprehensive studies conducted along the Libyan coasts, central Mediterranean, using the trammel nets technique, the two siganids largely prevailed in abundance and biomass over native and alien species, with S. luridus dominant in the western and middle coastal regions, and S. rivulatus in the eastern region (Shakman & Kinzelbach, Reference Shakman and Kinzelbach2007a, Reference Shakman and Kinzelbachb). The same authors observed that S. rivulatus was found in several different overgrown habitats whereas S. luridus was found in one specific vegetation habitat (rocks with algae). This is in agreement with Bariche et al. (Reference Bariche, Letourneur and Harmelin-Viviena2004), who stated that in the eastern Mediterranean, S. rivulatus has a wider settlement range of substrates and habitats than that of S. luridus. In Rhodes, in addition to rocky habitat, both species occur also on Posidonia oceanica meadows, where the abundance of S. luridus was found to be lower compared with S. rivulatus, in boat seine operations (Kalogirou et al., Reference Kalogirou, Corsini-Foka, Sioulas, Wennhage and Pihl2010).
The two herbivorous siganids of tropical origin that massively colonized the Levantine basin, were probably aided by the absence of many herbivorous competitors and exploited presumably unsaturated niches (Golani, Reference Golani, Golani and Appelbaum-Golani2010). To date, S. luridus has invaded the whole south Aegean Sea and has entered its northern sector, while S. rivulatus is distributed in the south of the basin (ELNAIS, 2016). Both siganids spread to the Central Mediterranean, reaching Tunisian and southern Italian waters (Azzurro & Andaloro, Reference Azzurro and Andaloro2004; Corsini-Foka, Reference Corsini-Foka, Golani and Appelbaum-Golani2010; Insacco & Zava, Reference Insacco and Zava2016; Ounifi–Ben Amor et al., Reference Ounifi-Ben Amor, Rafrafi-Nouira, El Kamel-Moutalibi and Ben Amor2016) and the Adriatic Sea (Dulčić & Pallaoro, Reference Dulčić and Pallaoro2004; Poloniato et al., Reference Poloniato, Ciriaco, Odorico, Dulčić and Lipej2010). Siganus luridus also extended northwards as far as the Tyrrhenian Sea and the French coast, near Marseilles (Castriota & Andaloro, Reference Castriota and Andaloro2008; Daniel et al., Reference Daniel, Piro, Charbonnel, Francour and Letourneur2009).
In the eastern Mediterranean Sea, the herbivores S. luridus and S. rivulatus compete with the native herbivores S. cretense and Sarpa salpa (Linnaeus, 1758) for food resources and habitat, leading to the alteration of the community structure and the native food web along the eastern Levantine rocky coasts (Giakoumi, Reference Giakoumi2014, and references therein). The siganids constituted one third of the fish biomass over hard bottoms along the Israeli coast and reached 80% of the abundance of the herbivorous fish in shallow coastal areas in Lebanon (Galil, Reference Galil, Gollasch, Galil and Cohen2006). Similarly, in the central Mediterranean, along the coasts of Libya, the most abundant herbivorous fish were S. rivulatus and S. luridus, which were more numerous than the native S. cretense and S. salpa (Shakman & Kinzelbach, Reference Shakman and Kinzelbach2007b). More westward in the central Mediterranean, at Linosa Island, Italy, the abundance of S. luridus is increasing, but still lower than the coexisting native herbivore fish S. salpa and S. cretense, as shown by underwater visual census (UVC) and surface snorkelling (Azzurro et al., Reference Azzurro, Franzitta, Milazzo, Bariche and Fanelli2016). In the present study S. luridus and S. rivulatus ranked lower in abundance and biomass than the native herbivorous species S. cretense, but higher than S. salpa, which was only 0.5% of total abundance and 0.6% of total catch. Over Posidonia beds of Rhodes Island, S. rivulatus and S. luridus ranked lower in biomass than S. cretense, although higher than the native S. salpa (Kalogirou et al., Reference Kalogirou, Corsini-Foka, Sioulas, Wennhage and Pihl2010). On the basis of the authors’ personal observations and interviews with local fishermen, S. salpa was common in the 1980s and 1990s in Rhodes. In terms of abundance and biomass, the low values for S. salpa recently found in small-scale coastal fishery of the island may indicate that some changes concerning this herbivore are taking place in the shallow waters, but a quantitative comparison with the past is not easy, due to the lack of older data for the specific area. Pennington et al. (Reference Pennington, Shepperson, Jeffery, Miliou and Anagnostou2013) assessed that S. luridus has clearly replaced the native S. salpa in Fournoi Island, northern to the Dodecanese complex, in both the UVC and fisheries surveys. On the other hand the most abundant herbivorous fish observed in UVC surveys in the Cyclades Archipelago was the native species S. salpa followed by S. luridus and S. cretense (Giakoumi, Reference Giakoumi2014).
The frequency of capture, abundance and biomass of the remaining two Lessepsian fish, Sphyraena flavicauda and the highly toxic L. sceleratus, were negligible in this study. The yellowtail barracuda S. flavicauda is not abundant in the area compared with the alien yellowstripe barracuda S. chrysotaenia and to the native barracudas, but sometimes it appears in fishing gears (Kalogirou et al., Reference Kalogirou, Wennhage and Pihl2012b). Despite the inconsistent contribution in the present study, the invasive L. sceleratus persists dramatically in the area, appearing abundant in certain cases and causing damage to fishing gears and commercial catches. This has been ascertained through recent interviews with local fishermen and through single boat seine and trammel net operations carried out at Rhodes in January and November 2015 respectively, for research and educational purposes (unpublished data). Trammel nets and boat seine nets might be more effective in the entanglement of L. sceleratus over gill nets, because the species has a slippery skin surface and a torpedo-like shape that facilitates its escape, especially when the size of the individuals present in the area is smaller or quite larger than the mesh size. Nevertheless, the presence of the species in a particular area is not consistent either because the continuous use of nets reduces their numbers locally and for short periods or because the different groups of this puffer fish tend to move around in search for food. Additionally in the same location and almost every time, there is more than one set of fishing gear operating, as the fishing grounds are limited and there are many professional fishermen along with their gear.
The importance of aliens in fishery might be significantly influenced by fishing technique, time of operation, substrate, habitat, physicochemical characteristics of the water, sampling season, depth, species composition, fish habits and other factors. For example, in Rhodes, the importance of aliens in gill nets appears more evident than in boat seine nets, particularly concerning biomass. In the gill nets of the present work aliens constituted 26.4% of total biomass. In 77 boat seine hauls carried out randomly between 2004 and 2010 at the north-west coast of Rhodes, the alien fish biomass was significantly lower, 2.3% of total biomass (unpublished data), similar to the value obtained using the same fishing technique in 2008–2009 in Cos Island, north to Rhodes (Lefkaditou & Petrakis, Reference Lefkaditou and Petrakis2010), while it was slightly lower than 4% obtained in Rhodes in 2008 (Kalogirou et al., Reference Kalogirou, Corsini-Foka, Sioulas, Wennhage and Pihl2010).
Alien fish constituted 55% of the total catch biomass in the north-eastern Levantine Sea, Iskenderun Bay, using bottom trawling from 30 to 110 m depth (Yemisken et al., Reference Yemisken, Dalyan and Eryilmaz2014). Alien fish biomass in the same area reached 69% of total catch using the same technique, at 9–46 m depth, and decreased to ~30% at the north-western Levant/Aegean Sea border (Bilecenoğlu, Reference Bilecenoğlu2016). Lessepsian fish biomass ranged between 50 and 90% of total biomass in the South-eastern Levantine Sea (Goren & Galil, Reference Goren and Galil2005; Edelist et al., Reference Edelist, Rilov, Golani, Carlton and Spanier2013). Goren et al. (Reference Goren, Galil, Diamant, Stern and Levitt2015) showed a rapid increase of alien biomass up to 60% in the 20–60 m layer in recent years. Also the biomass of alien fish in trammel nets along the Libyan coasts was at a high level, between 44 and 55% of total catch, mostly due to the large abundance of siganids (Shakman & Kinzelbach, Reference Shakman and Kinzelbach2007b).
The proportion of 12% of the alien fish species collected during the gill net operations of this study was also obtained through boat seine operations in the same area (Kalogirou et al., Reference Kalogirou, Corsini-Foka, Sioulas, Wennhage and Pihl2010; Corsini-Foka & Kondylatos, Reference Corsini-Foka and Kondylatos2015a). In the north-eastern Levantine waters (Iskenderun Gulf), using the bottom trawl technique between 30 and 110 m depth, among a total of 97 fish species, 27 were Lessepsian (27.8% of total number of fish species), of which 13 species are commercial (Yemisken et al., Reference Yemisken, Dalyan and Eryilmaz2014). In bottom trawls carried out in the south-eastern Levantine waters, alien fish species made up 35% of total species at 20–60 m depth and 30% at 80–120 m depth (Goren et al., Reference Goren, Galil, Diamant, Stern and Levitt2015). In the eastern coastal region of Libya, among a total of 42 species collected with trammel nets, 16 were Lessepsian fish (38%) (six commercial species), in the middle region Lessepsian species made up 45% (three commercial species) while in the western region they were 29% (two commercial species) (Shakman & Kinzelbach, Reference Shakman and Kinzelbach2007a, Reference Shakman and Kinzelbachb).
Native demersal species encountered in recent coastal fishery studies, including the present work, are comparable to those listed in the past (Maldura, Reference Maldura1938; Tortonese, Reference Tortonese1947; Kavalakis, Reference Kavalakis1964; Papaconstantinou et al., Reference Papaconstantinou, Caragitsou, Vasilopoulou, Petrakis and Stergiou1988) and there is no evidence that certain species have disappeared from the specific area or have been displaced.
More than half of the 32 Lessepsian fish known in the area were reported in the last 25 years. Considering an approximate number of 242 native bony fish species known today in the area (Papaconstantinou, Reference Papaconstantinou2014) and assuming this number to be valid since the first decades of the previous century, the ratio between the number of alien fish species (on the basis of their first record in the area, cf. Corsini-Foka, Reference Corsini-Foka, Golani and Appelbaum-Golani2010; Papaconstantinou, Reference Papaconstantinou2014) and the number of native species appears now 11 times greater than in the far past (0.132 vs 0.012) (Figure 5). Considering only the bony fish species, the ratios alien : native obtained through various fishery studies since the 1930s (Maldura, Reference Maldura1938; Tortonese, Reference Tortonese1947; Kavalakis, Reference Kavalakis1964; Papaconstantinou et al., Reference Papaconstantinou, Caragitsou, Vasilopoulou, Petrakis and Stergiou1988; Spanou, Reference Spanou2007; Kalogirou et al., Reference Kalogirou, Corsini-Foka, Sioulas, Wennhage and Pihl2010; Corsini-Foka & Kondylatos, Reference Corsini-Foka and Kondylatos2015a) and including the results of the present work, appear to reflect the increasing trend obtained when all the alien and the native fish species known in the area are considered (Figure 5).
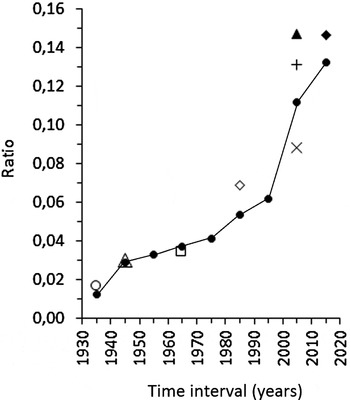
Fig. 5. Temporal trend of the ratio alien to native bony fish species in the Dodecanese region: ratio between all alien and native bony fish species known (![]() ) and ratio alien to native bony fish species obtained from coastal fishery studies (○, Maldura, Reference Maldura1938; △, Tortonese, Reference Tortonese1947; □, Kavalakis, Reference Kavalakis1968; ◊, Papaconstantinou et al., Reference Papaconstantinou, Caragitsou, Vasilopoulou, Petrakis and Stergiou1988;
) and ratio alien to native bony fish species obtained from coastal fishery studies (○, Maldura, Reference Maldura1938; △, Tortonese, Reference Tortonese1947; □, Kavalakis, Reference Kavalakis1968; ◊, Papaconstantinou et al., Reference Papaconstantinou, Caragitsou, Vasilopoulou, Petrakis and Stergiou1988; ![]() , Spanou, Reference Spanou2007; +, Corsini-Foka & Kondylatos, Reference Corsini-Foka and Kondylatos2015a;
, Spanou, Reference Spanou2007; +, Corsini-Foka & Kondylatos, Reference Corsini-Foka and Kondylatos2015a; ![]() , Kalogirou et al., Reference Kalogirou, Corsini-Foka, Sioulas, Wennhage and Pihl2010; ♦, This study). Detail in text.
, Kalogirou et al., Reference Kalogirou, Corsini-Foka, Sioulas, Wennhage and Pihl2010; ♦, This study). Detail in text.
In conclusion, despite the oligotrophy and the low fish productivity of the study area, the fisheries data collected in this study enrich the knowledge on current diversity and composition of the coastal ichthyofaunal community, providing a quantified description of the environmental status in a Mediterranean area impacted by biological invasion, following the MSFD. These data may also be useful for the management of Hellenic inshore fisheries resources and also constitute a tool for estimating the economic status of the local fishermen who use artisanal fishery as their only source of income.
Due to the ongoing changes of the marine biodiversity in the Mediterranean Sea, intensification of surveys of coastal waters assemblages in a region particularly prone to biological invasions is imperative.
ACKNOWLEDGEMENTS
This study was carried out in the frame of the Academic Degree Thesis in Marine Sciences, University of the Aegean, School of the Environment, of one of the authors, S. Mastis. The authors are grateful to S. Vagianos, the Captain of the fishing vessel ‘Saratoga’, and the crew for providing help and information during fieldwork. The authors acknowledge furthermore two anonymous referees for providing useful suggestions and constructive comments that improved the first version of the manuscript.







