Introduction
Weedy rices are paddy weeds congeneric to crop rice and collectively addressed as ‘red rice’ (Ziska et al., Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero, Vidotto, Johnson, Ferreira, Marchesan, Menezes, Cohn, Linscombe, Carmona, Tang and Merotto2015). These rices are indeed commonly characterized by a red caryopsis at maturity (Gianinetti and Vernieri, Reference Gianinetti and Vernieri2007; Ziska et al., Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero, Vidotto, Johnson, Ferreira, Marchesan, Menezes, Cohn, Linscombe, Carmona, Tang and Merotto2015; Gianinetti, Reference Gianinetti2016). In rice, the red pigmentation of the caryopsis is conferred by oxidative polymerization of colourless proanthocyanidins (PAs) to red phlobaphenes (Finocchiaro et al., Reference Finocchiaro, Ferrari, Gianinetti, Dall'Asta, Galaverna, Scazzina and Pellegrini2007). The red colour of the caryopsis is one of the wild-type traits against which human selection has had a major effect during domestication, and it is now most commonly associated with the weedy rice infesting paddies (Sweeney et al., Reference Sweeney, Thomson, Pfeil and McCouch2006).
Weedy rices are also characterized by a variable level of seed dormancy, i.e. a physiological state in which a seed does not germinate, or germinates slowly, even though the environmental conditions would otherwise allow quick germination. Seed dormancy can be gradually released by dry after-ripening, that is by keeping the dry seed at 30°C for several weeks (Gianinetti and Cohn, Reference Gianinetti and Cohn2008). As it tunes the timing of germination, seed dormancy is an important trait for seed survival and persistence, and it has a large ecological significance in the build-up of a weed seed bank in soils (Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015). However, seed persistence may be threatened by microbial activities that can drive seed mortality in the soil (Dalling et al., Reference Dalling, Davis, Schutte and Arnold2011; Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015). Resistance to ageing, predation and microbial decay is therefore essential for the survival of seeds in the soil and the persistence of a given seed population in the field (Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015). For this reason, seeds have physical, chemical and biological defence mechanisms that protect their food reserves from decay-inducing organisms and herbivores (Fuerst et al., Reference Fuerst, Okubara, Anderson and Morris2014). Seed dormancy and resistance to decay are fundamental persistence strategies, which, together, allow a population of seeds to germinate over long periods of time (Fuerst et al., Reference Fuerst, Okubara, Anderson and Morris2014; Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015). Dispersing seed germination over time reduces the risk of a generalized reproductive failure and increases the likelihood that some seeds of a plant's cohort will encounter favourable germination and establishment environments (Gianinetti and Cohn, Reference Gianinetti and Cohn2008; Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015).
Microbial invasions typically trigger the production of microbial elicitors, which are also referred to as pathogen or microbe-associated molecular patterns (PAMPs or MAMPs). In addition, because of tissue injury caused by micro-organisms, some molecules are generated upon plant cell damage and passively released as endogenous elicitors, which are called damage-associated molecular patterns (DAMPs) and serve as warning signals for infections (Wu et al., Reference Wu, Shan and He2014; Gust et al., Reference Gust, Pruitt and Nürnberger2017). DAMPs are perceived by host pattern-recognition receptors to activate pattern-triggered immunity responses (Yu et al., Reference Yu, Feng, He and Shan2017). Plant DAMPs include small peptides, nucleotides and cell-wall-derived oligosaccharides (Yu et al., Reference Yu, Feng, He and Shan2017).
The cellular responses induced by PAMPs and DAMPs involve: plasma membrane effluxes of anions and K+, cytosolic Ca2+ rise, and H+ influx that, together, cause membrane depolarization and pH changes (cytosolic acidification and extracellular alkalinization), reactive oxygen species (ROS) production, as well as activation of the mitogen-activated protein kinase (MAPK) cascade and of the ethylene and/or jasmonate pathways (Kärkönen and Kuchitsu, Reference Kärkönen and Kuchitsu2015; Yu et al., Reference Yu, Feng, He and Shan2017). Together, these mechanisms contribute to pattern-triggered immunity against a wide array of micro-organisms (Wu et al., Reference Wu, Shan and He2014). Specifically, transient and rapid generation of apoplastic ROS, referred to as a ROS burst, is a hallmark of the early response to elicitor treatment (Yu et al., Reference Yu, Feng, He and Shan2017). Thus, pathogen-related elicitors are commonly studied as external stimuli to induce ROS production (Vreeburg and Fry, Reference Vreeburg, Fry and Smirnoff2005). Both membrane-localized NADPH oxidases and cell wall-associated peroxidases can be involved in ROS burst, although NADPH oxidases are the key enzymes leading to the production of the superoxide anion radical, O2•–, which may be subsequently converted into hydrogen peroxide, H2O2 (Bolwell and Wojtaszek, Reference Bolwell and Wojtaszek1997; Wu et al., Reference Wu, Shan and He2014; Camejo et al., Reference Camejo, Guzmán-Cedeño and Moreno2016; Yu et al., Reference Yu, Feng, He and Shan2017). Release of ROS has been observed in a wide range of plant–pathogen interactions involving avirulent bacteria, fungi and viruses (Lamb and Dixon, Reference Lamb and Dixon1997; Wu et al., Reference Wu, Shan and He2014; Yu et al., Reference Yu, Feng, He and Shan2017).
Apart from interactions with microbes, seed imbibition, by itself, is accompanied by an immediate, transient burst of redox activity that involves superoxide (Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010; Leymarie et al., Reference Leymarie, Vitkauskaité, Hoang, Gendreau, Chazoule, Meimoun, Corbineau, El-Maarouf-Bouteau and Bailly2012). ROS production by germinating seeds represents an active, developmentally controlled physiological function, presumably for protecting the emerging seedling against attack by pathogens (Schopfer et al., Reference Schopfer, Plachy and Frahry2001). However, the involvement of superoxide in seed dormancy alleviation and the subsequent germination has also been suggested, in addition to the O2•− role in pathogen defence (Oracz et al., Reference Oracz, Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007; Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010).
This work was prompted by the observation that red rice caryopses treated with alkaline bicarbonate solution, to block growth of a blue-green mould, turned darker red in 1–2 days (Gianinetti, Reference Gianinetti2016). In addition, a preliminary trial indicated that when pronase (also known as proteinase type XIV) was added to the incubation solution, dehulled caryopses also became darker. Thus, the present experiments were set up to study whether pronase mimics a pathogen attack, thereby eliciting a seed defence response that modifies the colour of the caryopsis coat, and whether pH changes were involved. Both hypotheses were confirmed, and darkening of imbibed caryopses was shown to be associated with superoxide production, in turn induced by extracellular alkalinization.
Materials and methods
Materials
Seed of a red rice genotype (Gianinetti et al., Reference Gianinetti, Laarhoven, Persijn, Harren and Petruzzelli2007; Gianinetti and Vernieri, Reference Gianinetti and Vernieri2007; Gianinetti, Reference Gianinetti2016) was used for this study. It is considered a weedy form of the crop species and has been referred to as Oryza sativa L. f. spontanea (Gianinetti et al., Reference Gianinetti, Laarhoven, Persijn, Harren and Petruzzelli2007; Gianinetti, Reference Gianinetti2016). Seeds (actually, the naked rice grain is a caryopsis, which is referred to as ‘seed’, in a broad sense, throughout this paper) were obtained by manually dehulling red rice spikelets that were either dormant (stored at –15°C soon after harvesting; Gianinetti, Reference Gianinetti2016) or non-dormant (dry after-ripened for 16 weeks at 30°C before storing at –15 °C; Gianinetti and Cohn, Reference Gianinetti and Cohn2008). Dehulled caryopses were pre-imbibed for 2–14 days in Petri dishes (preliminary work showed that superoxide production is negligible after 2 days of imbibition) on two filter paper discs with 5 ml of distilled water at 30°C; they were the transferred to capped 100 ml Erlenmeyer flasks for experiments (which were all conducted at 30°C). In some cases, a whole experiment was repeated twice with close results and the data were therefore merged. All the seeds used in a given experiment repeat were pre-imbibed for the same time; in different experiments, or in different repeats of a whole experiment, diverse durations of pre-imbibition (from 2 to 14 days, as mentioned above) may have been used. Each flask contained 10 seeds (30 seeds for the pH measurement test) and 2 ml of incubation solution. In tests where seeds were treated with a protease, incubation solution included either proteinase K (1 mg ml–1) or pronase (1 mg ml–1) and CaCl2 (20 mM). CaCl2 is commonly added to protect pronase from autolysis in prolonged incubations (Sweeney and Walker, Reference Sweeney and Walker1993). Preliminary controls showed no effect of this salt alone (at 20 mM) on superoxide production by red rice seeds; on the other hand, incubation with pronase was considerably less effective in promoting superoxide production if CaCl2 was not included.
To confirm the presence of superoxide, a superoxide dismutase (SOD) mimic, Mn-desferal (green complex), was used (Beyer and Fridovich, Reference Beyer and Fridovich1989). Mn-desferal was prepared from deferoxamine mesylate and MnO2. This scavenger was chosen because of its reported efficacy with respect to SOD itself, as SOD is less effective when used with seeds probably because of restricted permeability of the enzyme through the cell wall (Able et al., Reference Able, Guest and Sutherland1998; Schopfer et al., Reference Schopfer, Plachy and Frahry2001).
Superoxide assay
Extracellular release of O2•– in incubation solutions was estimated using two different colorimetric assays. In a first experiment, the oxidation of epinephrine (Misra and Fridovich, Reference Misra and Fridovich1972; Takeshige and Minakami, Reference Takeshige and Minakami1979) to adrenochrome was measured spectrophotometrically (A485) (Chen and Thakker, Reference Chen and Thakker2002). Epinephrine stock (10 mM) was prepared by dissolving epinephrine in 20 mM HCl, and epinephrine was included in the solution wherein seeds were incubated. This assay was used for semi-quantitative measure of superoxide, because the reaction between superoxide and epinephrine is self-amplifying as, once started, it generates further superoxide from air oxygen (Alhasan and Njus, Reference Alhasan and Njus2008). In the second assay, O2•– production was determined by reduction of XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt, sodium salt], followed spectrophotometrically (A470) (Sutherland and Learmonth, Reference Sutherland and Learmonth1997; Schopfer et al., Reference Schopfer, Plachy and Frahry2001). XTT was dissolved in warm water (37–50°C) to 2.5 mM. Differently from epinephrine, XTT reacts stoichiometrically with superoxide, without any self-amplifying effect; therefore, with this assay it was possible to quantify the amount of superoxide released in the incubation solution. The amount of superoxide production was expressed in nanomoles per seed and per hour using a molar extinction coefficient ε=21600 M–1 cm–1 (Sutherland and Learmonth, Reference Sutherland and Learmonth1997).
Extracellular O2•– production was detected as change in the absorbance of the solution containing epinephrine/XTT: epinephrine/XTT was added to the incubation solution either from the start of test (final concentration: 1 mM epinephrine or 0.5 mM XTT), and the change of absorbance measured after 8 h (and 20 h, for epinephrine), or epinephrine/XTT stock solution was added after 12 h and the change of absorbance was then measured at 20 h from the beginning of the test. Spontaneous oxidation of epinephrine and XTT were negligible during the experiments.
Hydrogen peroxide assay
Concentration of H2O2 in the incubation solution was measured with the Xylenol Orange assay, wherein hydroperoxides cause the oxidation of Fe2+ to Fe3+, which in acid solution forms an orange complex with Xylenol Orange that was detected spectrophotometrically at 560 nm (Gay and Gebicki, Reference Gay and Gebicki2000). To monitor the amount of hydrogen peroxide produced, 1.6 ml of the incubation solution was added to 400 µl of a mix of two assay reagents in equal proportions, which were mixed immediately prior to the assay (Gay and Gebicki, Reference Gay and Gebicki2000). The two reagents (Gay and Gebicki, Reference Gay and Gebicki2000, Reference Gay and Gebicki2002) were: reagent A, H2SO4 (360 mM), Xylenol Orange (2.5 mM) and ferrous iron (2.5 mM); and reagent B, sorbitol (1 M). The final reaction mixture (incubation solution plus reagents A and B) was incubated for 30 min at room temperature before measuring. The blank was prepared by adding 400 μl of the reagent mix to 1.6 ml of water. A calibration curve was obtained by preparing solutions with different concentrations of H2O2 (15.625, 7.812, 3.906, 1.953 and 0.976 µM) from a 30% (w/w) H2O2 stock (9.8 M). The assay was very sensitive when a series of diluted H2O2 solutions was tested (see Supplementary Fig. S1).
Phenolic content
To quantify total polyphenols in the solution used for incubating the seeds, the modified Prussian Blue assay (Graham, Reference Graham1992) was used; 100 µl of the seed incubation solution and 3 ml of distilled water were added to 15 ml Falcon tubes. Then, 1 ml of K3Fe(CN)6 (0.016 M) and 1 ml of FeCl3 (0.02 M) were added in rapid succession. After 15 min, 5 ml of distilled water was added to each sample. Absorbance was determined spectrophotometrically at 700 nm. The assay was standardized against 1 mM catechin and polyphenols were expressed as mg per ml of catechin equivalents (Finocchiaro et al., Reference Finocchiaro, Ferrari, Gianinetti, Dall'Asta, Galaverna, Scazzina and Pellegrini2007).
pH tests
To measure changes in the pH of seed incubation solution due to experimental treatment, 30 pre-imbibed seeds were incubated in a 100 ml Erlenmeyer flask with 2 ml of a solution containing pronase (1 mg ml–1) and 20 mM CaCl2; pH was then recorded at 0, 8 and 20 h by transferring most of the incubation solution to a 15 ml Falcon tube and measuring with a basic pH meter that was calibrated daily before measurements. Controls without either seeds or pronase (and CaCl2) were included in the experiment.
In order to study the effect of pH on O2•– production, a citrate/phosphate (25 mM/25 mM) buffer system was used. The pH of the buffer was adjusted to 5, 6, 6.5, 7 and 8 with NaOH before adjusting the final volume.
Superoxide generation test
To check any inhibitory effect of buffering on the capability of XTT to detect superoxide, a mixture of riboflavin (1.3 µM) and methionine (13 mM) (Giannopolitis and Ries, Reference Giannopolitis and Ries1977) was used to generate superoxide, by photochemical excitation (by Wood's lamps) for 15 min, in a solution (2 ml) including XTT (0.5 mM) and either MES [2-(N-morpholino)ethanesulfonate] or Bis-Tris [bis(2-hydroxyethyl)amino-tris(hydroxymethyl)methane] buffer (40 mM). All chemicals were added before photochemical excitation, and measurements were taken after a further 15 min. Superoxide generation was monitored spectrophotometrically (A470). Absorbance of the same complete solution immediately before it was given the photochemical excitation was used as reference blank in each case.
Superoxide production during imbibition of dormant and non-dormant seeds
Superoxide production was monitored during imbibition of dormant and non-dormant seeds by using XTT. To do this, 10 dry seeds were placed in a 100 ml Erlenmeyer flask containing 2 ml of a 0.5 mM XTT solution.
Statistical analysis
Tukey's test was used to evaluate the significance of the differences between means only after one-way ANOVA had identified a significant effect of treatment (fixed factor). In performing ANOVA on absorbance data (and superoxide release), values were transformed as X1/2+(X + 1)1/2 to compensate for the unequal variances that were proportional to the magnitude of the means (Zar, Reference Zar1999).
Results
Epinephrine assay
Table 1 shows that by 20 h, but not at 8 h, seeds treated with pronase (plus 20 mM CaCl2) had produced a significant amount of superoxide. It is worth noting that when epinephrine was added to the solution after 12 h rather than at the start of the incubation with pronase (0 h), the increase of absorbance at 20 h was even greater, and the absorbance of the control without seeds was lower (Table 1). The latter effect (lower epinephrine oxidation in the control with pronase and calcium but without seeds, if epinephrine was added at 12 h rather than at 0 h) might be due to the zinc contained in metalloproteases present in pronase (Sweeney and Walker, Reference Sweeney and Walker1993), as epinephrine can be rapidly oxidized by zinc and other easily reducible metals. The increase of absorbance in the presence of pronase was indeed more erratic when epinephrine was added at 0 h, suggesting an interference from some random effect, and it required more than 8 h of incubation to become relevant, probably because of the self-amplifying reaction mechanism for epinephrine oxidation (Alhasan and Njus, Reference Alhasan and Njus2008). For this reason, we preferred not to keep epinephrine in the incubation solution for more than 8 h, and, to assay superoxide production up to 20 h, we preferred to use separate tests wherein epinephrine was added at 12 h. In this way, results were much more consistent, and formation of adrenochrome from epinephrine in the incubation solution of seeds treated with pronase for 20 h resulted in 51.5 and 21.5 times higher levels than in controls without pronase and without seeds, respectively.
Table 1. Epinephrine assay: absorbance of incubation solutions of dormant seeds after 0, 8 and 20 h at 30°C

Values are means ± s.e.m.; averages of 5–7 replicates. Epinephrine, when present, was added either at the start of the experiment (0 h) or at 12 h, as indicated. Values with the same letter are not significantly different (P ≤ 0.05; Tukey's test).
A modest increase of the absorption in solutions with untreated seeds and, especially, seeds treated with pronase (plus 20 mM CaCl2) was noted at 8 h even in controls without epinephrine (Table 1). In fact, the caryopsis coat of red-grained rice contains PAs, and low molecular weight PAs are colourless and slightly soluble in water (Pourcel et al., Reference Pourcel, Routaboul, Cheynier, Lepiniec and Debeaujon2007; Finocchiaro et al., Reference Finocchiaro, Ferrari, Gianinetti, Dall'Asta, Galaverna, Scazzina and Pellegrini2007). PAs, however, are phenolics, like epinephrine, and act as scavengers of free radicals such as ROS (Pourcel et al., Reference Pourcel, Routaboul, Cheynier, Lepiniec and Debeaujon2007). Thereby, they undergo oxidative polymerization to a reddish pigment (Finocchiaro et al., Reference Finocchiaro, Ferrari, Gianinetti, Dall'Asta, Galaverna, Scazzina and Pellegrini2007). Oxidative browning of PAs is, indeed, a widespread phenomenon, especially in seeds (Pourcel et al., Reference Pourcel, Routaboul, Cheynier, Lepiniec and Debeaujon2007). Accordingly, during incubation of dormant red rice dehulled caryopses, a gradual, dim browning of the solution is commonly observed, and it was systematically detected in our experiments. This is probably due to low amounts of PAs that pass into solution (Table 2) and undergo oxidative polymerization. Thus, PAs oxidation produces coloured compounds that absorb at the wavelength used for adrenochrome detection (485 nm). In the presence of pronase, superoxide production was induced, and PAs oxidative browning was, evidently, enhanced. Given that seeds were pre-imbibed, this experiment demonstrates that an extracellular superoxide burst is induced in a few hours when imbibed dormant caryopses are challenged with pronase.
Table 2. Release of total phenolics by red rice dormant dehulled caryopses into the incubation solution for 24 h at 30°C

Values are means ± s.e.m.; 8–9 replications for each tested condition. Values with the same letter are not significantly different (P = 0.841, ANOVA).
The quantity of PAs released into solution did not depend upon the presence of pronase, since, as measured in terms of total phenolic content (because, in the caryopsis of red-pigmented rices, PAs are the main phenolics; Finocchiaro et al. (Reference Finocchiaro, Ferrari, Gianinetti, Dall'Asta, Galaverna, Scazzina and Pellegrini2007), it was the same whether pronase was present or not (Table 2).
XTT assay
Determination of superoxide by the XTT assay (Supplementary Table S1) closely matched results obtained with the epinephrine test (Table 1). To quantify the amount of superoxide released in the incubation solution, the effect of PAs on absorbance had to be removed; this was done by subtracting the absorbance of the corresponding control without XTT. The change in absorbance between the start of the experiment and either 8 h or 20 h was then used to estimate the amount of superoxide produced during the 8 h corresponding to the 0–8 h and 12–20 h time periods. In calculating superoxide produced during the latter time period, it was assumed that any superoxide produced prior to 12 h was not persisting in the incubation solution. This should be a reasonable assumption, as superoxide is a highly reactive species and is therefore short-lived. The amount of superoxide production (Table 3) was calculated assuming a relatively constant production over the 8 h time periods.
Table 3. XTT assay: calculated average amounts of superoxide released by red rice dormant dehulled caryopses into the incubation solution at 30°C

Values are means ± s.e.m.; five replications for each treatment. Reagent blanks (without XTT) run in parallel were used to correct for unspecific absorbance changes. Values with the same letter are not significantly different (P ≤ 0.05; Tukey's test).
Following incubation with pronase, production of superoxide approached 1.15 nmol seed–1 h–1 between 12 and 20 h, whereas it was half that value in the first 8 h, probably because of a lag time required for pronase action and seed response. The extracellular superoxide burst is induced within 8 h when imbibed dormant caryopses are challenged with pronase, but the burst is stronger if the time of exposure to pronase is longer, indicating that the response of the red rice kernel intensifies as a consequence of pronase activity rather than being due to pronase presence per se.
Hydrogen peroxide
No significant levels of H2O2 were detected in the incubation solution of seeds treated with pronase, and this finding was clear even though seed incubation solution (without pronase) and pronase (+CaCl2) solution (without seeds) showed that unspecific interferences from PAs released by seeds and from pronase (+CaCl2) increased the absorbance values observed at 20 h (Supplementary Table S2). It is therefore inferred that, in red rice dormant seeds challenged with pronase, H2O2 is neither produced concomitantly with superoxide, nor it is produced from superoxide.
Mn-desferal
In our conditions, 300 µM Mn-desferal caused a 62% inhibition of XTT reduction when added at the start of incubation (Table 4). It is thus confirmed that a large part, if not all, of the oxidative burst observed in the previous experiments is indeed superoxide.
Table 4. Superoxide-scavenging effect of Mn-desferal: absorbance of incubation solutions of dormant caryopses

Values are means ± s.e.m.; three replications for each treatment. As in previous experiments, XTT was added after 12 h of incubation and absorbance was measured after 20 h of incubation at 30°C. Values with the same letter are not significantly different (P ≤ 0.05; Tukey's test).
pH measurements
To ascertain if the effect of pronase involved a change of extracellular pH, the pH of solutions was measured during incubation of 30 seeds (Fig. 1). Whereas the pH of the solution with seeds alone slightly decreased, probably because of seed respiration, the pH of the solution wherein seeds were incubated with pronase increased within 8 h, indicating that pronase elicited an increase of pH. As a plateau appears to be reached by 8 h, it can be argued that extracellular alkalinization is a quickly completed process following pronase action.
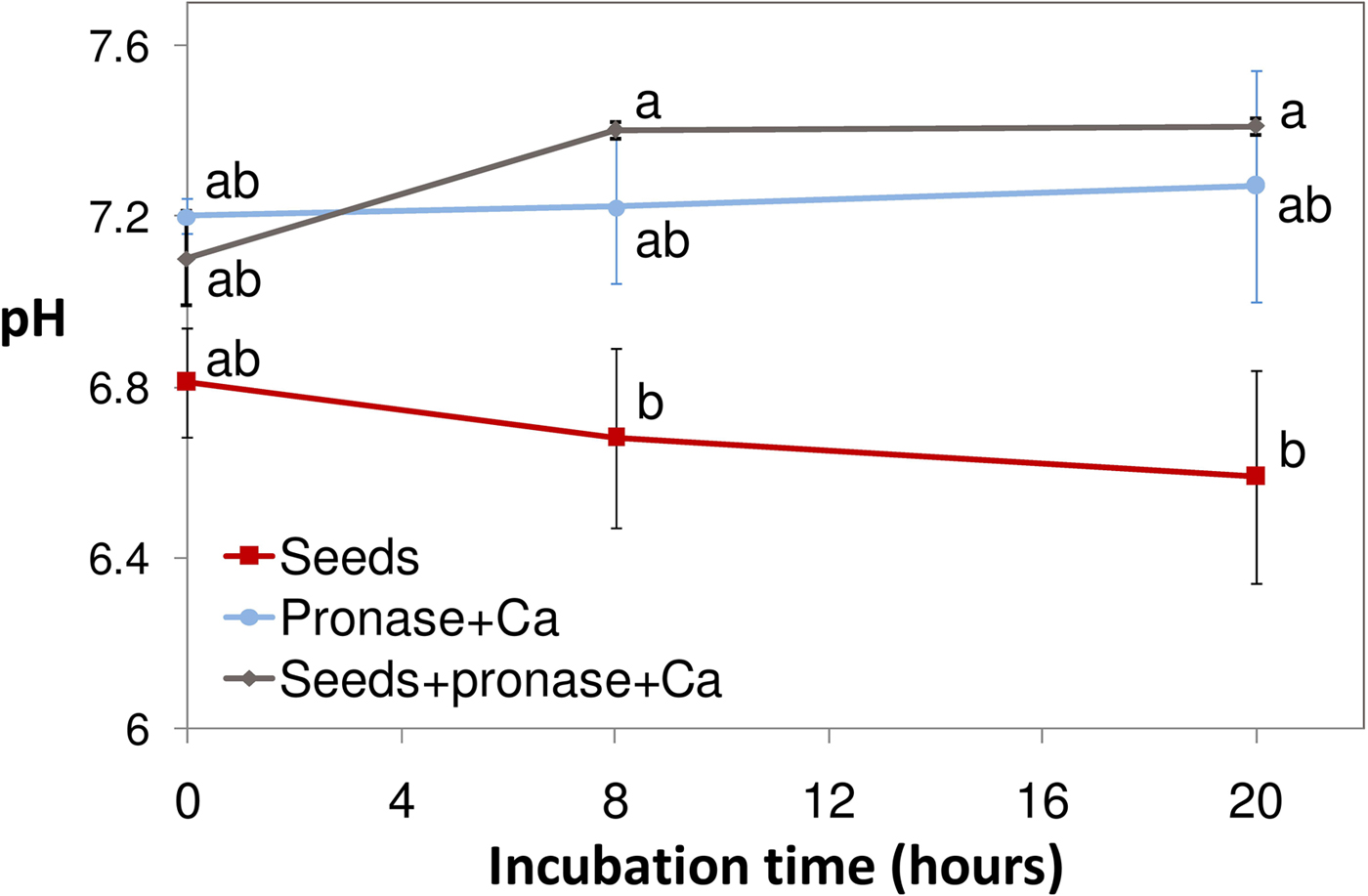
Figure 1. Effect of pronase on the pH of the solutions wherein dormant caryopses were incubated at 30°C: pH was measured at 0, 8 and 20 h (mean ± s.e.m; 4–5 replications for each treatment). Values with the same letter are not significantly different (P ≤ 0.05; Tukey's test). Error bars indicate standard errors.
The effect of pH
To establish if an increase of pH could, by itself, induce superoxide production by the seeds, seeds were incubated in solutions with citrate/phosphate buffer at different pH values. It was thus shown that XTT reduction increased with pH in the 5–8 range, with a stronger production of superoxide at pH 8 (Fig. 2). A relevant production of superoxide also occurred by incubation with Tris buffer pH 7.8, confirming that increased superoxide production at high pH was not due to citrate or phosphate. Figure 3 shows that, by 2 days, the colour of seeds became darker at higher pH, as enhanced oxidative browning of PAs took place along with superoxide production.
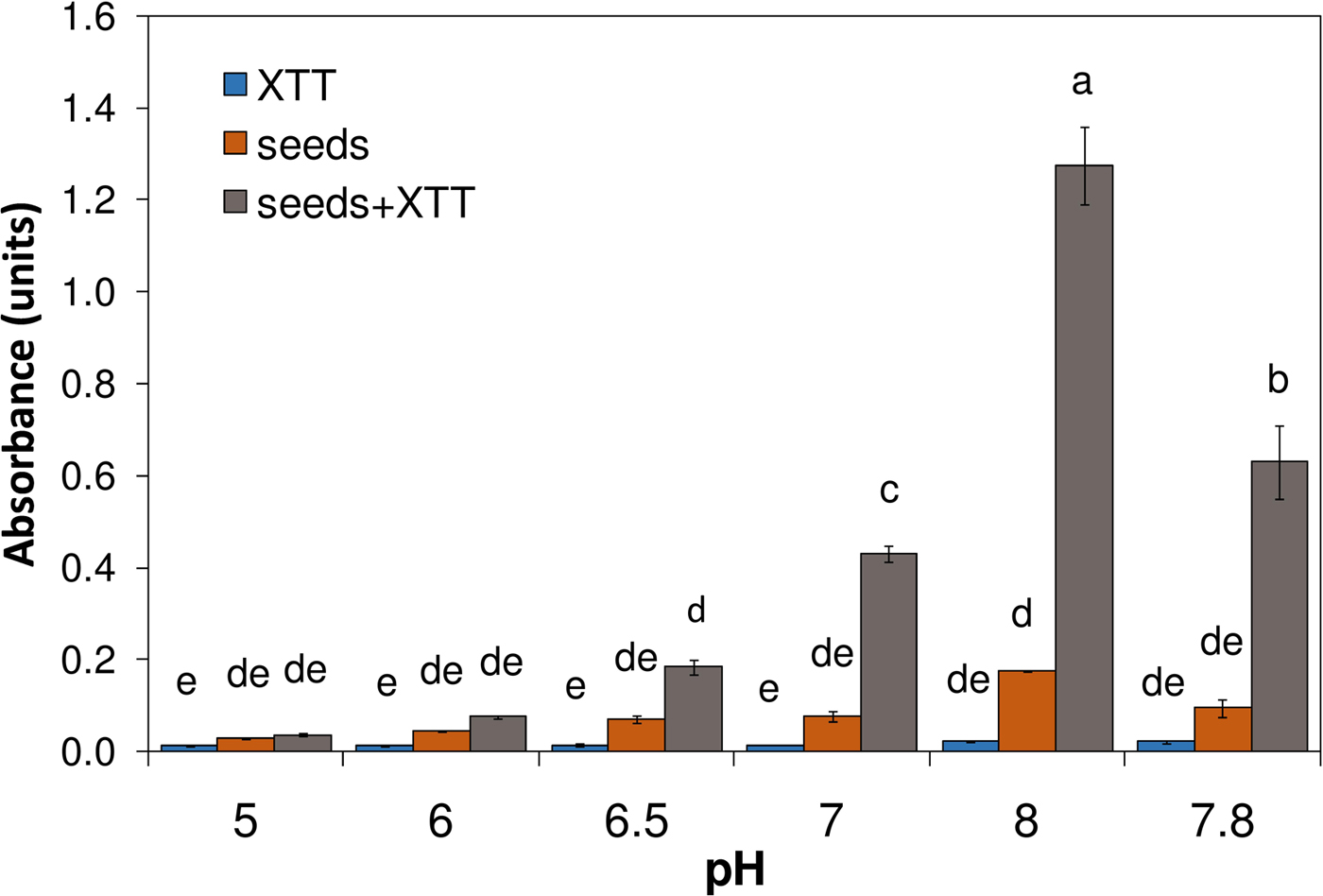
Figure 2. Effect of pH on superoxide production (XTT assay) by dormant caryopses (at 30°C): absorbance at different incubation pH values (two replications for seeds alone and four for other tests) of incubation solutions buffered either at pH 5, 6, 6.5, 7 and 8 with 25 mM citrate/phosphate buffer or at pH 7.8 with Tris buffer. As in previous experiments, XTT was added after 12 h of incubation and absorbance was measured at 470 nm after 20 h of incubation. Bars labelled with the same letter represent means not significantly different from each other (P ≤ 0.05; Tukey's test). Error bars indicate standard errors.

Figure 3. The effect of pH on the colour of dormant caryopses after 2 days of incubation in 25 mM citrate/phosphate buffer solutions at 30°C.
Superoxide production in response to pronase was additionally tested by buffering the incubation solution with two other buffers that have good buffering capability at pH 6, namely MES and Bis-Tris. Our results (Fig. 4) clearly show that buffering at pH 6 inhibited the production of superoxide in response to pronase.

Figure 4. Effect of buffering at pH 6 (with either 40 mM MES or 40 mM Bis-Tris): solution absorbance (mean ± s.e.m.; five replications for each treatment) after 20 h of incubation at 30°C (either epinephrine or XTT was added at 12 h). Seeds correspond to dormant caryopses. For either the epinephrine test (A485) or XTT test (A470), values with the same letter are not significantly different (P ≤ 0.05; Tukey's test). The dashed lines above the bar plots represent reference absorbance values obtained without buffering for the epinephrine test (violet, value as from Table 1) and XTT test (green, value as from Supplementary Table S1), respectively. Error bars indicate standard errors.
To confirm that buffering at pH 6 with either MES or Bis-Tris inhibits superoxide production rather than the reaction of superoxide with either epinephrine or XTT, the photochemical reduction of riboflavin was used to generate superoxide (Giannopolitis and Ries, Reference Giannopolitis and Ries1977). Table 5 shows that reduction of XTT by superoxide is possible in solutions buffered at pH 6 with either MES or Bis-Tris. Although pH 6 is suitable for the XTT assay (Schopfer et al., Reference Schopfer, Plachy and Frahry2001), these controls support findings obtained with our own system. It is therefore concluded that an extracellular increase of pH is necessary and sufficient to induce the superoxide burst caused by pronase activity.
Table 5. XTT assay for superoxide detection in solutions buffered at pH 6

Absorbance of buffered solutions after photochemical generation of superoxide (at RT, about 23°C) is shown (mean ± s.e.m.; six replications for each treatment). Absorbance reading was set to 0 (blank) before photochemical excitation.
Superoxide production during imbibition of dry seeds (XTT assay)
Superoxide production by dormant and non-dormant seeds was compared during imbibition (dry seeds were put into solution incubation at time 0). Absorption increased quite linearly for about 2 days in dormant seeds, whereas in non-dormant seeds it increased much less after about 1 day of incubation (Fig. 5). Non-dormant seeds showed some pericarp splitting (first morphological marker of germination; Gianinetti, Reference Gianinetti2016) at 12 h of incubation, and reached 100% pericarp splitting within 26 h of incubation (Fig. 5). There is, therefore, no evidence of a greater production of extracellular superoxide in germinating seeds with respect to dormant seeds.

Figure 5. Superoxide production during imbibition of dry seeds at 30°C (XTT assay). The absorbance (A470) of the incubation solution is shown for: dormant seeds with XTT, non-dormant seeds with XTT, dormant seeds (control), non-dormant seeds (control) and XTT control solution. Bars represent standard errors (n = 4). The arrow indicates the time point by which 100% pericarp splitting (first morphological marker of germination) was attained by non-dormant seeds.
Proteinase K
To ascertain if the same response triggered by pronase could be also elicited by another generic protease, proteinase K (from cosmopolitan, saprophytic fungus Engyodontium album, opportunistic on animals and humans) was tested, by the XTT assay, for its capability to induce superoxide production by the seeds. Table 6 shows that proteinase K had a much lower effect than pronase on superoxide release in the incubation solution. It is therefore reasoned that it is not proteolysis in general that is responsible for the effect of pronase, but some enzymatic activity (or joint activities), specific to pronase, elicits the response of the red rice dormant kernel.
Table 6. Effect of proteinase K: absorbance of incubation solutions of dormant seeds (XTT assay) after 0, 8 and 20 h at 30°C

Values are means ± s.e.m.; four replications for each treatment. Values with the same letter are not significantly different (P ≤ 0.05; Tukey's test).
Discussion
Our study showed that the imbibed dormant red rice caryopsis recognizes pronase activity as a potential microbial attack, presumably by sensing some proteolysis product. In red rice caryopses, the defence reaction included extracellular alkalinization and superoxide production, and the former was necessary to activate the latter. No H2O2 production was detected, but a sustained release of PAs was observed, either with or without pronase. In addition, time profiles of superoxide production by dormant and non-dormant red rice caryopses during imbibition did not support a direct relationship between extracellular superoxide levels and dormancy breaking or germination.
The role of proteases in eliciting plant defence responses
Defence against pathogens relies on a complex surveillance system for signs of danger. DAMPs are danger signals originated from the host because of deleterious activities of microbial hydrolytic enzymes (Gust et al., Reference Gust, Pruitt and Nürnberger2017). As bacterial proteases PrpL, secreted by Pseudomonas aeruginosa, and ArgC, by Xanthomonas campestris, can activate an immune response in Arabidopsis that includes induction of an oxidative burst (Cheng et al., Reference Cheng, Li, Niu, Zhang, Woody, Xiong, Djonović, Millet, Bush, McConkey, Sheen and Ausubel2015), it appears that plants have evolved a mechanism to detect the proteolytic activity of pathogen-encoded proteases (Cheng et al., Reference Cheng, Li, Niu, Zhang, Woody, Xiong, Djonović, Millet, Bush, McConkey, Sheen and Ausubel2015; Cheng, Reference Cheng2016). Although it remains to be determined how the protease triggers the activation of the immune pathway (Cheng, Reference Cheng2016), it has been suggested that pathogen-secreted proteases could release host polypeptides that function as DAMPs and are subsequently recognized by immune receptors (Cheng et al., Reference Cheng, Li, Niu, Zhang, Woody, Xiong, Djonović, Millet, Bush, McConkey, Sheen and Ausubel2015). This mechanism, or a similar one (Cheng, Reference Cheng2016), appears to occur in red rice seeds too. As P. aeruginosa is an opportunistic bacterial pathogen and X. campestris is a true pathogen, whereas S. griseus is non-pathogenic, the mechanism to detect the proteolytic activity of damaging microbial proteases might be a rather generic one. Indeed, the cellular responses induced by PAMPs and DAMPs, including medium alkalinization and ROS production, play an important role in plant basal defence against a broad spectrum of microbial infections (Wu et al., Reference Wu, Shan and He2014). In red rice, pronase activity mimics a potential microbial attack, presumably through DAMPs, which convey a damaged-self warning.
The dormant caryopsis/pronase system
Streptomyces griseus is a cosmopolitan, non-pathogenic species of mycelial bacteria commonly found in soil (Antony-Babu and Goodfellow, Reference Antony-Babu and Goodfellow2008; Bignell et al., Reference Bignell, Huguet-Tapia, Joshi, Pettis and Loria2010), and pronase is a commercially available mixture of several non-specific exo- and endoproteases, isolated from the extracellular fluid of S. griseus, that digests virtually all proteins, which are extensively broken down into single amino acids (Sweeney and Walker, Reference Sweeney and Walker1993). Extracellular proteases are involved in assimilating extracellular proteinaceous nitrogen sources (Chater et al., Reference Chater, Biró, Lee, Palmer and Schrempf2010). Thus, S. griseus represents a typical microbe that tries to obtain its nutritional supply from sources available in the surrounding soil, whereas a seed is a potential nutritional source that strives not to be used as such. As the presence of a living micro-organism can complicate the study of the seed response, in the present work we showed that challenging imbibed red rice caryopses with pronase provides a simplified, in vitro system suitable to study the response of dormant seeds (actually, dehulled caryopses) to microbe attack (see ‘Auxiliary considerations’ in the Supplementary Material).
As a defence response is triggered in red rice seeds, it provides protection against invaders that are therefore prevented from infecting the plant tissue (Wu et al., Reference Wu, Shan and He2014; Gust et al., Reference Gust, Pruitt and Nürnberger2017). This is a common non-pathogenic interaction, wherein the healthy seed usually succeeds (even because, in natural conditions, micro-organisms also have to trespass the hull). Actually, in nature, plant resistance to microbial infections is the rule rather than the exception (Wu et al., Reference Wu, Shan and He2014). In fact, besides the preformed physical barriers, plants have evolved an innate immune system to recognize microbial invasion and launch effective defence responses to fend off pathogen attacks (Wu et al., Reference Wu, Shan and He2014). Although through a mechanism that is not fully elucidated, even non-pathogenic micro-organisms, including several soil Streptomyces, can elicit a non-specific defence response, known as induced systemic resistance (Senthilraja, Reference Senthilraja, Subramaniam, Arumugam and Rajendran2016). Specifically, non-pathogenic Streptomyces sp. OE7 strain (having 99–100% sequence identity with Streptomyces griseus) can be sensed by plant cells and triggers an oxidative burst leading to defence responses in BY2 tobacco cell suspensions (Baz et al., Reference Baz, Tran, Kettani-Halabi, Samri, Jamjari, Biligui, Meimoun, El-Maarouf-Bouteau, Garmier, Saindrenan, Ennaji, Barakate and Bouteau2012). As culture filtrates of Streptomyces sp. OE7 attenuated disease development in plants treated with a pathogen, it was suggested that OE7-secreted molecules could indeed reinforce a pathway related to the induced systemic resistance (Baz et al., Reference Baz, Tran, Kettani-Halabi, Samri, Jamjari, Biligui, Meimoun, El-Maarouf-Bouteau, Garmier, Saindrenan, Ennaji, Barakate and Bouteau2012). However, our findings differ from results obtained by Baz et al. (Reference Baz, Tran, Kettani-Halabi, Samri, Jamjari, Biligui, Meimoun, El-Maarouf-Bouteau, Garmier, Saindrenan, Ennaji, Barakate and Bouteau2012) with non-pathogenic Streptomyces sp. OE7 (closely related to S. griseus), because culture filtrates elicited H2O2 production in BY2 tobacco cell suspensions. The dormant caryopsis/pronase system, therefore, evidences a distinct defence response of red rice seeds to challenges by non-pathogenic microbes.
The connection between extracellular alkalinization and superoxide production
Our findings indicate that, notwithstanding its generic action, pronase produces one or more elicitors (not produced by proteinase K) that trigger(s) a defence response by the red rice caryopsis. The present work also demonstrates that this response includes sustained superoxide accumulation and that this extracellular oxidative burst is induced by an extracellular alkalinization. In fact, it can be elicited just by incubating the seeds in sub-alkaline buffer solutions. Incubation in neutral or sub-acidic buffer solutions, on the other hand, prevents extracellular superoxide production even in presence of pronase, thereby indicating that extracellular alkalinization is a necessary mediating step for the seed to trigger the oxidative burst in response to this challenging agent. Accordingly, Bolwell et al. (Reference Bolwell, Butt, Davies and Zimmerlin1995) found that an essential factor in eliciting an oxidative burst by suspension-cultured cells of French bean on exposure to an elicitor preparation from a fungal pathogen was a transient alkalinization of the apoplast to pH 7.0–7.2. Dissipation of this pH change with a number of treatments, including ionophores and strong buffers, substantially inhibited the oxidative burst (Bolwell et al., Reference Bolwell, Butt, Davies and Zimmerlin1995). In that study, an oxidative burst was rapidly elicited simply by transferring bean cells into a medium buffered at higher pH (Bolwell et al., Reference Bolwell, Butt, Davies and Zimmerlin1995). This is fully consistent with our present results, yet it is evidently different from bacteria-inoculated tobacco cell suspensions, wherein increases of extracellular pH and ROS production were correlated and concomitant, but an effect of the former on the latter was ruled out (Keppler et al., Reference Keppler, Baker and Atkinson1989). It has therefore been proposed that different mechanisms are used to generate the oxidative burst in diverse plant–pathogen interactions and elicitor-treated model systems, but such mechanisms are hard to differentiate (Bolwell and Wojtaszek, Reference Bolwell and Wojtaszek1997). Although the pH-dependent generation of ROS was supposed to be associated with cell wall peroxidases, which directly produce H2O2 (Bolwell and Wojtaszek, Reference Bolwell and Wojtaszek1997), even production of superoxide by membrane-bound enzymes shows a pH optimum around 7.5 in bean roots, and the rate of superoxide production is much reduced at pH values less than 6 (Pinton et al., Reference Pinton, Cakmak and Marschner1994). Correspondingly, in tomato fruit tissue, ROS generation (in the presence of ammonium, which elicits ROS accumulation) was strongly enhanced by an increase in extracellular pH from 4 to 8, and the source of this ROS production, as established by the use of antisense lines, was the membrane-bound NADPH oxidase, which produces superoxide (Alkan et al., Reference Alkan, Davydov, Sagi, Fluhr and Prusky2009). Even though the enzyme mechanisms responsible for pH-dependent extracellular oxidative burst are still controversial, it is clear that the role of extracellular alkalinization in red rice seeds is to trigger superoxide production, whereas a direct protective effect of extracellular alkalinization seems to be excluded (see ‘Auxiliary considerations’ in the Supplementary Material).
The most prominent trait of red rice seeds is the accumulation of PAs and PA-derived red pigment
In red-grained rices, PAs accumulate during the development of the caryopsis and oxidize and polymerize to form the pigment that confers a red hue to the mature caryopsis (Finocchiaro et al., Reference Finocchiaro, Ferrari, Gianinetti, Dall'Asta, Galaverna, Scazzina and Pellegrini2007; Ferrari et al., Reference Ferrari, Gianinetti, Finocchiaro and Terzi2015). Although in the mature caryopsis the reddish pigment is only present in the fused seed coat/pericarp, which is a dead tissue (Krishnan and Dayanandan, Reference Krishnan and Dayanandan2003; Han et al., Reference Han, Dong, Yang, Huang, Wang and Wu2009), colourless oligomeric PAs, and/or leucoanthocyanidins (PAs precursors) can be synthesized and accumulated in the living aleurone layer too (Han et al., Reference Han, Dong, Yang, Huang, Wang and Wu2009), and, based on present findings, they are released into the apoplast during incubation in water. In accordance with these observations, dormant red rice seeds show high expression of genes for PA synthesis during imbibition (Gianinetti et al., Reference Gianinetti, Finocchiaro, Bagnaresi, Zechini, Faccioli, Cattivelli, Valè and Biselli2018).
As, in the rice dispersal unit, the hull tightly wraps the caryopsis, this release presumably brings about a high concentration of soluble, oligomeric PAs in the interstitial space between the caryopsis and the hull. PAs play an important role in the defence against pathogens and predators (fungi, bacteria and insects), specifically in the seed (Pourcel et al., Reference Pourcel, Routaboul, Cheynier, Lepiniec and Debeaujon2007). Besides, mechanical resistance of the caryopsis coats could be enforced, as PAs polymerize to large, poorly soluble phlobaphenes (Finocchiaro et al., Reference Finocchiaro, Ferrari, Gianinetti, Dall'Asta, Galaverna, Scazzina and Pellegrini2007). PAs release therefore appears to represent a pre-emptive strategy that dormant seeds adopt to protect themselves from intrusive assaults by other organisms (Gianinetti et al., Reference Gianinetti, Finocchiaro, Bagnaresi, Zechini, Faccioli, Cattivelli, Valè and Biselli2018). Remarkably, pronase-induced defence response favours polymerization of colourless PAs to red phlobaphenes, as indicated by the colour change of the caryopsis (Fig. 3). This is probably consequent to enhanced extracellular superoxide production. In fact, oligomeric PAs quickly react with superoxide (Taubert et al., Reference Taubert, Breitenbach, Lazar, Censarek, Harlfinger, Berkels, Klaus and Roesen2003), and seed coat PAs effectively scavenge superoxide in arabidopsis (Jia et al., Reference Jia, Sheng, Xu, Li, Liu, Xia and Zhang2012). PAs also show higher radical scavenging effect and stronger polymerization reactions with increasing pH values (Altunkaya et al., Reference Altunkaya, Gökmen and Skibsted2016). Interaction of PAs and superoxide is then unavoidable when they come to co-exist in the extracellular space of the seed. The presence of PAs is therefore a prominent feature of red rice seeds that is directly involved in seed protection.
A distinct defence response of seeds
Rapid generation of O2•– and H2O2 has been reported in many hypersensitive responses following inoculation with avirulent fungal, bacterial or viral pathogens (Lamb and Dixon, Reference Lamb and Dixon1997). In general, a much stronger superoxide production takes place in incompatible plant–microbe relationships (wherein disease development is typically blocked by the plant defence reaction) with respect to compatible plant–microbe relationships (wherein disease development is the typical outcome). This sustained ROS accumulation correlates with disease resistance to avirulent and non-host pathogens, which are successfully recognized by the plant immune system, and it is specifically involved in the plant immune response to these incompatible interactions (Doke, Reference Doke1983, Reference Doke1985; Sekizawa et al., Reference Sekizawa, Haga, Hirabayashi, Takeuchi and Takino1987; Lamb and Dixon, Reference Lamb and Dixon1997; Torres et al., Reference Torres, Jones and Dangl2006). In fact, in a compatible, successful infection, pathogens must be able to counteract plant pattern-triggered immunity and thus they synthesize and secrete virulence effectors that are capable of suppressing plant defence responses and orchestrate the reprogramming of the infected tissue so that it becomes a source of nutrients for the pathogen (Koeck et al., Reference Koeck, Hardham and Dodds2011; Selin et al., Reference Selin, de Kievit, Belmonte and Fernando2016). It is therefore argued that, as observed in other plant–microbe interactions, a surge of extracellular superoxide is a key component of a seed's reaction for blocking infection by incompatible micro-organisms, that is, micro-organisms from either a pathogenic or non-pathogenic species, which are not able to establish a pathogenic relationships when they challenge healthy seeds.
Interestingly, we did not find a significant production of hydrogen peroxide in the incubation solution of dormant seeds, although such ROS is commonly involved in cell-wall polymer cross-linking and lignification as a component of the response to pathogens (Levine et al., Reference Levine, Tenhaken, Dixon and Lamb1994; Torres et al., Reference Torres, Jones and Dangl2006). As seen, however, PAs can act as superoxide scavengers, and thereby they might contribute to prevent H2O2 formation in red rice seeds. Perhaps, as PAs are peculiar to seeds and caryopses, they could be part of a seed-specific defence mechanism that requires superoxide alone. Analogously, extracellular alkalinization and O2•– accumulation, but not extracellular H2O2 accumulation, were elicited in alfalfa seedlings treated with oligogalacturonides (Camejo et al., Reference Camejo, Martí, Jiménez, Cabrera, Olmos and Sevilla2011). In typical hypersensitive responses, recognition of an avirulent pathogen stimulates an oxidative burst generating O2•–, and subsequent accumulation of H2O2 is then brought about (Lamb and Dixon, Reference Lamb and Dixon1997; Camejo et al., Reference Camejo, Guzmán-Cedeño and Moreno2016). Both these ROS are direct anti-microbial agents, but H2O2 is also a substrate for oxidative cross-linking in the cell wall. Furthermore, accumulation of H2O2 functions as a threshold trigger in the induction of the hypersensitive response and, then, programmed cell death, which involves localized necrotic lesion on host plant upon infection caused by a virulent pathogen in order to arrest further growth of the pathogen (Levine et al., Reference Levine, Tenhaken, Dixon and Lamb1994; Lamb and Dixon, Reference Lamb and Dixon1997). Although hypersensitive cell death is a milestone of defence against biotrophic pathogens, it can increase host susceptibility to necrotrophic pathogens (which invade and kill plant tissue rapidly and then live saprotrophically on the dead remains) by providing dead tissue for pathogen nutrition (Moore et al., Reference Moore, Robson and Trinci2011; Barna et al., Reference Barna, Fodor, Harrach, Pogány and Király2012; Lightfoot et al., Reference Lightfoot, Mcgrann and Able2017). It is worth noting that whereas biotrophs are specialized pathogens that cause little direct damage to the host plant, and produce few, if any, lytic enzymes, necrotrophs secrete copious cell-wall-degrading enzymes and toxins (Moore et al., Reference Moore, Robson and Trinci2011). In fact, microbial infection-induced plant damage is often due to deleterious activities of microbial hydrolytic enzymes or toxins (Gust et al., Reference Gust, Pruitt and Nürnberger2017). It can be supposed that tissue death resulting from ROS-induced hypersensitive response would not be a good strategy for dormant seeds to endure in the soil, especially following an attempted lytic challenge by a saprophytic microbe. It might therefore be speculated that a non-specific defence response to non-pathogenic micro-organisms occurs in red rice dormant seeds challenged with pronase, and programmed cell death does not take place in consequence of superoxide production in this interaction. If so, a basic defensive response, with superoxide acting as a direct anti-microbial agent and promoting oxidative cross-linking of PAs in the cell wall, would take place in these seeds. A seed-specific defence mechanism can then be hypothesized, wherein oxidized PAs constitute a preformed physical barrier and new oligomeric PAs are released to gradually reinforce it. Superoxide production is triggered, to complement and potentiate this process, when a potential microbial invasion is recognized.
The immune response of dormant seeds
Not much is presently known about how dormant seeds, which can persist in the soil for one or more years (Dalling et al., Reference Dalling, Davis, Schutte and Arnold2011; Ziska et al., Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero, Vidotto, Johnson, Ferreira, Marchesan, Menezes, Cohn, Linscombe, Carmona, Tang and Merotto2015), defend themselves from the attack of micro-organisms. Dalling et al. (Reference Dalling, Davis, Schutte and Arnold2011) proposed some mechanisms that seeds can adopt to defend themselves from damaging organisms. Among them, a continuum of physical and chemical defences can be most effective for seeds with physiological dormancy and is expected to be arrayed on the exterior of the seed (Dalling et al., Reference Dalling, Davis, Schutte and Arnold2011). Chemical defences like phenolics are indeed frequently associated with seed coats (Pourcel et al., Reference Pourcel, Routaboul, Cheynier, Lepiniec and Debeaujon2007; Gallagher et al., Reference Gallagher, Burnham, Fuerst and Gallagher2014). Active biochemical defence mechanisms also contribute to seed longevity and survival in the soil (Pourcel et al., Reference Pourcel, Routaboul, Cheynier, Lepiniec and Debeaujon2007; Fuerst et al., Reference Fuerst, Okubara, Anderson and Morris2014), demonstrating that dormant seeds are responsive and capable of activating and releasing defence enzymes in response to a pathogen (Fuerst et al., Reference Fuerst, James, Pollard and Okubara2018). The potential immune response of seeds to microbial infection, however, has been somewhat neglected as a defence mechanism. Our work provides an insight into this particular plant–microbe interaction. Although our study represents only an initial characterization of the immune response by which seeds defend themselves from microbial attacks, it highlights the immune reaction as a strategy by which dormant seeds, just like other plant organs, fend off micro-organisms through an active and coordinated response to ensure their own survival.
Dormant vs non-dormant seeds
Finally, comparison of XTT reduction in the incubation solution of dormant and non-dormant seeds during imbibition (without pronase), showed that, up to 100% pericarp splitting (that is, all non-dormant seeds had germinated), superoxide production intensity was very close in the two conditions. Thus, superoxide release is a common feature of dormant and non-dormant seeds during the first day of imbibition. Thereafter, absorbance increase was much lower in the incubation solution of non-dormant seeds. So, in red rice seeds, extracellular superoxide accumulation is triggered by microbial elicitors and also occurs during imbibition, but superoxide production is decreased during germination. Whereas the former results match those obtained in other studies (Schopfer et al., Reference Schopfer, Plachy and Frahry2001; Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010), the latter finding is not in agreement with some studies (Oracz et al., Reference Oracz, Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007, Reference Oracz, El-Maarouf-Bouteau, Kranner, Bogatek, Corbineau and Bailly2009; Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010; Leymarie et al., Reference Leymarie, Vitkauskaité, Hoang, Gendreau, Chazoule, Meimoun, Corbineau, El-Maarouf-Bouteau and Bailly2012). Actually, some authors (Oracz et al., Reference Oracz, Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007, Reference Oracz, El-Maarouf-Bouteau, Kranner, Bogatek, Corbineau and Bailly2009) measured the superoxide content of ground tissues, rather than extracellular superoxide accumulation. Other studies were done in dicotyledonous species (Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010; Leymarie et al., Reference Leymarie, Vitkauskaité, Hoang, Gendreau, Chazoule, Meimoun, Corbineau, El-Maarouf-Bouteau and Bailly2012), and the intensity of superoxide production during germination might be a species-specific feature. It should, however, be noted that red rice non-dormant seeds were obtained by dry after-ripening dormant seeds; as this process consisted of storing the low-moisture seed for 16 weeks at 30°C, and since seed ageing has been linked to impaired ability to generate O2•– during germination (Kranner et al., Reference Kranner, Roach, Beckett, Whitaker and Minibayeva2010), it could well be that, in red rice, dry after-ripening improves germination because of dormancy breaking, but also impairs seed's ability to generate extracellular O2•–, at least after pericarp splitting. In any case, at least as for what happens in the red rice apoplast, enhanced superoxide production does not appear to be associated with dormancy alleviation and germination. Thus, the role of this ROS in red rice imbibed caryopses appears to be essentially aimed at defence against attacks by challenging micro-organisms.
Future advances
Recognition of an important role of proteases in eliciting plant defence responses (Cheng et al., Reference Cheng, Li, Niu, Zhang, Woody, Xiong, Djonović, Millet, Bush, McConkey, Sheen and Ausubel2015) will propel identification of specific microbial elicitors produced by the proteolytic activity of microbe-encoded proteases. This, in turn, could have a significant impact on our understanding of what generates the host–pathogen specificity commonly observed in plants. We are confident that the red rice dormant caryopsis/pronase system identified here will help identifying microbial elicitors of the seed immune response. Dormant red rice caryopses, and dormant seeds in general, could also be used to test the seed-specific response to different elicitors.
Ultimately, our work has established some peculiarities of the defence reaction occurring in dormant red rice seeds. Intriguingly, PAs appear to be a specific component of the seed protective strategy, but their presence, together with seed dormancy, has been selected against in grain crops, which therefore cannot be considered to display a complete, wild-type defence system in their seeds. Further studies will show whether the features displayed by weedy (red) rice seeds are common to other species and represent a general mechanism of seed persistence.
Financial support
S.G. was supported by a fellowship from the Iranian Ministry of Science.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0960258518000260













