Introduction
It is well established that intrauterine growth restriction results in infants whose birth weight is below the 10th percentile and generally are predisposed to cardiovascular and metabolic diseases later in life.Reference Barker 1 , Reference Martyn, Barker and Jespersen 2 Studies have suggested a link between birth weight and adult behavior pertaining to motor function,Reference Evensen, Vik and Helbostad 3 , Reference Fattal-Valevski, Leitner and Kutai 4 learning and memory,Reference O'Keeffe, O'Callaghan, Williams, Najman and Bor 5 and increased susceptibility to anxiety, depressionReference Gale and Martyn 6 and schizophrenia.Reference Susser and Lin 7 Further investigations have examined the association between behavioral disorders and cardiovascular disease, specifically the influence of anxiety and depression.Reference Frasure-Smith and Lesperance 8 However, there are limited animal studies examining the effects of adverse early development on motor function, learning and memory and neurological disorders, such as depression and schizophrenia.
Our study employs a well-established uteroplacental insufficiency model, which is the common cause of growth restriction in Western society, restricting blood supply to the uterus and therefore oxygen and nutrients to the fetuses following bilateral uterine vessel ligation in rats, leading to prenatal and postnatal growth restriction.Reference O'Dowd, Kent, Moseley and Wlodek 9 – Reference Wlodek, Westcott and O'Dowd 11 We have shown that prenatal and postnatal growth restriction of offspring can program hypertension, nephronReference Wlodek, Westcott, Siebel, Owens and Moritz 12 and pancreaticReference Siebel, Gallo, Guan, Owens and Wlodek 13 deficits and impaired glucose toleranceReference Siebel, Mibus and De Blasio 14 later in life, most prominently in males.Reference Wadley, Siebel and Cooney 15 Uteroplacental insufficiency also results in a reduction in litter size, which is controlled for in the present study by inclusion of an additional experimental group.Reference Wlodek, Westcott, Siebel, Owens and Moritz 12 , Reference Wadley, Siebel and Cooney 15 We have previously demonstrated that a reduction in litter size impairs mammary development with altered milk composition and reduced quantity, resulting in postnatal growth restrictionReference Wadley, Siebel and Cooney 15 programming, increased adult blood pressure and reduced nephron number.Reference Wlodek, Westcott, Siebel, Owens and Moritz 12
Experimental evidence has shown that growth restriction leads to a reduction in number and altered morphology of neurons in specific brain regions, leading to subsequent behavioral changes.Reference Mallard, Loeliger, Copolov and Rees 16 , Reference Rees, Bocking and Harding 17 Human studies in childrenReference Fattal-Valevski, Leitner and Kutai 4 and adolescentsReference Evensen, Vik and Helbostad 3 born small for gestational age have revealed motor function impairment. An experimental maternal malnourishment study assessed coordination and balance in prenatally and/or postnatally growth-restricted rat offspring using the rotarod test. While prenatal growth restriction had no influence on motor function, impaired lactation resulted in poor motor skills in the offspring.Reference Smart, Dobbing, Adlard, Lynch and Sands 18
There is evidence that supports the in utero programming of learning deficits.Reference Fattal-Valevski, Leitner and Kutai 4 , Reference O'Keeffe, O'Callaghan, Williams, Najman and Bor 5 However, further studies are required to verify the memory and attention deficit in low birth weight infants.Reference Sommerfelt, Andersson and Sonnander 19 In animals, the Morris water maze (MWM) is a well-validated model for assessing spatial memory and learning.Reference Morris, Garrud, Rawlins and O'Keefe 20 Another memory test, the Y-maze is based on the rat's innate tendency to explore a novel environment (spontaneous exploratory behavior) in the absence of anxiogenic or noxious stimuli.Reference Dellu, Mayo, Cherkaoui, Le Moal and Simon 21
Human epidemiological studies support the hypothesis that anxiety and depression in adulthood are related to birth weight.Reference Costello, Worthman, Erkanli and Angold 22 In animal studies, anxiogenic behavior is investigated using the elevated plus maze (EPM) examining the innate conflict to explore a novel environment and avoid open areas and has been validated using anxiolytic drugs.Reference Pellow, Chopin, File and Briley 23 Depressive-like behavior has been widely assessed in rodents using the Porsolt forced swim test.Reference Porsolt, Le Pichon and Jalfre 24 A study in growth-restricted Wistar rats used the EPM and Porsolt forced swim test finding no difference in anxiogenic or depressive-like behavior.Reference Adori, Zelena and Timar 25 However, growth restriction was induced by 3,4-methylenedioxymethamphetamine, which is not a common model. In human studies, there is increasing evidence that the in utero environment is associated with the development of schizophrenia, with patients more likely to have lower birth weights than normal birth weight individuals.Reference Wahlbeck, Forsen, Osmond, Barker and Eriksson 26 Specifically, children exposed in early gestation to an undernourished environment have an increased risk of developing schizophrenia.Reference Susser and Lin 7 Although schizophrenia per se cannot be identified in animals, deficits in sensory gating are commonly reported among schizophrenic patients and animal models by measuring prepulse inhibition (PPI).Reference Grillon, Ameli, Charney, Krystal and Braff 27 No animal studies to date have investigated the association between low birth weight and sensorimotor gating function.
Due to many conflicting reports in the literature and the overall lack of evidence for a relationship between low birth weight and behavioral deficits, the present study employed eight well-characterized behavioral tests to investigate the effect of low birth weight on behavior in male and female rats. The aim of this study was to determine if prenatal and postnatal growth restriction (Restricted), and/or altered postnatal lactation environment alone (Reduced Litter), results in adverse behavioral outcomes in adult male and female rats. It was hypothesized that offspring from both the Restricted and Reduced Litter groups would have adverse behavioral outcomes compared with Controls.
Method
Animals
This study was approved by The University of Melbourne Animal Ethics Subcommittee (Ethics approval number: 0707427). Wistar Kyoto (WKY) rats were housed under standard conditions: 21 ± 1°C, 12 h light/dark cycle, with light on at 7 am and ad libitum food and water.
Pregnant females (n = 30, 11–14 weeks of age) were randomly allocated into the Restricted or Control group and bilateral uterine vessel ligation or sham surgery was performed, respectively. On day 18 of gestationReference Wlodek, Mibus and Tan 10 , Reference Wlodek, Westcott and O'Dowd 11 they were anesthetized with an intraperitoneal injection of Ketamine (50 mg/kg, Parnell Laboratories, New South Wales, Australia) and Xylazine (10 mg/kg, Troy Laboratories, New South Wales, Australia). Uteroplacental insufficiency surgery was performed via ligation of the left and right uterine vessels (Restricted group). Sham surgery was performed in the same manner in the Control group, without uterine vessel ligation.Reference Wlodek, Mibus and Tan 10 , Reference Wlodek, Westcott and O'Dowd 11 The day after birth on day 22 of gestation (day 1), in an additional control group that underwent the sham surgery, litter size was reduced to match the number of pups in the Restricted group (n = 5, including at least two pups of each sex; Reduced Litter group).Reference O'Dowd, Kent, Moseley and Wlodek 9 , Reference Wlodek, Westcott, Siebel, Owens and Moritz 12 , Reference Wadley, Siebel and Cooney 15 A total of 10 females and 10 males from 10 different litters were included from the three experimental groups and pups were weaned at day 35 after birth.Reference Wlodek, Mibus and Tan 10 , Reference Wlodek, Westcott and O'Dowd 11 Body weights of offspring were recorded on days 1, 6, 14 and 35, then at 2, 3, 4 and 5 months of age. One week after completion of behavioral testing, rats were weighed and euthanized with an intraperitoneal overdose injection of Ketamine (300 mg/kg) and Xylazil (225 mg/kg) and whole brains weighed.
Behavioral tests
Behavioral tests were carried out between 5.5 and 6.5 months of age. It is possible that the behavioral response to a particular test may affect the performance in subsequent tests. In order to minimize stress-related influences from one test to another, tests were performed in the same order and time period for each rat from least stressful (locomotor activity) to most stressful (Porsolt forced swim test), with a minimum of 2 days rest between tests.
Motor function
To investigate general motor activity, rat movement was recorded every 500 ms over a 3-h time period in a locomotor cell apparatus (Truscan 2.0, Coulburn Instruments, Allentown, PA, USA).Reference Craft, Clark, Hart and Pinckney 28 Muscle strength was measured using the grip strength test meter (Bioseb, Paris, France), where rats gripped a metal grid and were pulled by the base of the tail until they released. The average force of the five trials for forelimb and all limb strength was calculated.
The rotarod (Ugo Basile, Varese, Italy) was used to assess motor performance and coordination.Reference Rogers, Campbell, Stretton and Mackay 29 The rotarod test consisted of a training day and a test day, each comprising three trials spaced 30 min apart. The training consisted of two trials at increased speed (4–16 rpm) and one constant speed trial at 20 rpm for 3 min each. During the test, the rotarod accelerated from 4 to 40 rpm for 5 min. When the rat fell off the rotarod, the time was recorded. The average time to fall off was expressed as a percentage of the total time.
Learning and memory
The MWM tests spatial memoryReference Morris, Garrud, Rawlins and O'Keefe 20 and consists of a 1.9 m circular pool filled with opaque water (25 ± 2°C) to increase the contrast with the white rat for recording purposes. The pool was arbitrarily divided into four equal quadrants designated north-east, north-west, south-east and south-west. A transparent platform (12.5 cm diameter) located in the center of the quadrant was submerged 1 cm below the water to ensure it was not visible to rats. During the acquisition phase (days 1–7), the platform was randomly placed in the center of one of the quadrants and the rat was placed in the pool facing the wall at one of the compass points (north, east, south or west). The rat was allowed to search for the platform for 2 min. Once located, the rat was left to remain on the platform for 30 s before being removed from the pool. If the rat failed to locate the platform within the 2 min period it was led to the platform and left to remain on the platform for 30 s. This was repeated for the remaining compass points, with a total of four trials per day for each rat with the platform location remaining the same for each rat for the 7 days of the acquisition phase. The parameters measured were latency to reach the platform, distance travelled and velocity. For the probe test (day 8) the platform was removed and the rat was placed in the pool facing the wall on the opposite quadrant to the platform location in the acquisition phase and allowed to swim for 1 min. The parameters measured for the probe test were latency to reach the previous location of the platform, distance travelled, velocity and time spent in each of the four quadrants. Swimming activity was tracked by video and analyzed using Ethovision software (Noldus Information Technology, Wageningen, the Netherlands).
The Y-maze also tests spatial memory in rats.Reference Dellu, Mayo, Cherkaoui, Le Moal and Simon 21 The apparatus comprises three identical arms: the home, familiar or novel arm (50 × 12 × 30 cm each and 120° apart). Extra-maze cues were positioned so as to be visible from most points inside the maze. The first component of this test was a familiarization trial with the novel arm closed. The rat was placed at the end of the home arm facing the wall and was allowed to explore the home and familiar arms for 10 min. Two hours later, the test trial began with the rat placed in the maze and allowed to explore all three arms for 5 min, with the novel arm also being accessible. Allocation of the arms (home, familiar and novel) was randomly assigned. The number of entries and time spent in each arm, the latency into the novel arm and velocity were recorded via video and analyzed using Ethovision software.
Anxiogenic and depressive-like behavior
The EPM tests anxiogenic behaviorReference Pellow, Chopin, File and Briley 23 and comprises four arms (44 × 12.5 cm), forming a cross-like structure elevated 70 cm above the floor. Two opposing arms were open without side walls and the other two opposing arms were closed with side walls 10 cm high. The rat was placed in the center of the maze facing an open arm and allowed to explore the maze for 10 min. The number of entries into each arm and time spent in those arms were recorded.
The Porsolt forced swim test was used to test depressive-like behavior.Reference Porsolt, Le Pichon and Jalfre 24 This consists of a transparent rectangular glass tank (28 × 20 × 50 cm) containing water to a depth of 28 cm (23 ± 2°C). This ensures that animals were not able to rest their tail on the bottom of the tank. On day 1, the rat was placed in the tank and observed for 10 min. On day 2, the rat was once again placed in the tank for 10 min. The amount of time spent floating (equivalent to non-escaping behavior) was recorded and analyzed using Ethovision software.
Sensorimotor gating function
To assess sensorimotor gating function the PPI test was employed.Reference Carter, Lione and Humby 30 Rats were placed inside a sound-attenuating box with a 70 dB background noise (SR-LAB Startle Response chamber, San Diego Instruments Inc., San Diego, CA, USA). The first 5 min was an acclimatization period, with only background noise (70 dB) to establish baseline reading. A total of ‘80 prepulse–pulse stimuli’ were then delivered (ISI 7 and 21 s). The first and last 10-pulse stimuli (first and last block) consisted of a strong pulse-alone stimulus (115 dB pulse, 50 ms). The middle 60 prepulse–pulse stimuli (second and third blocks) consisted of the stimulus (115 dB) preceded by a 20 ms prepulse of 4, 8 or 16 dB over baseline. Startle habituation was measured by comparing the average startle responses obtained in the first and last block of pulse-alone stimuli trials and the middle block trials involving prepulse stimuli. Percentage of PPI was calculated to determine if a sensorimotor gating deficit was present.Reference van den Buuse, Martin and Brosda 31
Statistical analyses
For most measures and behavioral tests, two-way analysis of variance (ANOVA) was performed to determine significant differences between experimental groups and sexes (SPSS-X, SPSS Inc., Encinitas, CA, USA). One-way ANOVAs were performed between experimental groups within a sex when a significant interaction was detected, followed by Student–Newman–Keuls post hoc test. A multivariate analysis of variance (MANOVA) was used for PPI data, followed by Fisher's least significant difference post hoc test. Student's t-tests were performed to determine significant differences between sexes for a given experimental group. The one exception to this was the MWM, which used repeated-measures ANOVA to identify differences within each group over time. All data are expressed as the mean ± s.e.m., with a significance level of P < 0.05.
Results
Litter size, body and brain weights
At birth, litter size for the Restricted group was smaller (6.50 ± 0.64) than the Control (8.80 ± 0.44) and Reduced Litter (10.10 ± 0.69) groups (P < 0.05). At birth (day 1), Restricted male and female offspring were lighter than Control and Reduced Litter males and females (P < 0.05). This significant difference in weight was still evident on days 6, 14 and 35 for males and females (P < 0.05; Table 1). Restricted female, but not male, offspring were lighter than Controls during the behavioral testing period up until post-mortem (P < 0.05; Table 1). Reduced Litter females were heavier than Controls on day 35 and at 2 months of age, suggesting a period of accelerated growth (P < 0.05). There was no difference in absolute or relative brain weight between groups for either sex; however, due to the significant differences in body weight between sexes at post-mortem, the relative brain weight for females was heavier than males for all experimental groups (P < 0.05; Table 1).
Table 1 Body and brain weights

Data are expressed as mean ± s.e.m. (n = 7–10 per group per sex). Significant differences (P < 0.05) between groups are represented by letters, so that ‘a’ is different from ‘b’, but not different from ‘ab’. *P < 0.05 between sexes for an experimental group.
Motor function
There were no differences in motor activity measured by total time spent moving, total distance travelled and average velocity in the locomotor test between groups or sexes (Table 2). There was no significant difference between groups in muscle strength exerted in the grip strength test for either sex, when gripping with fore limbs only or all limbs, although males did grip with more strength than females (data not shown). Males from the Restricted group and those from Reduced Litter had a 30–34% longer latency to fall from the accelerating rotarod when compared with Control males (P < 0.05; Fig. 1a). Control females had a longer latency to fall from the rotarod when compared with Control males (P < 0.05; Fig. 1).
Table 2 The effects of growth restriction on Locomotor test parameters in male and female offspring

Data are expressed as mean ± s.e.m. (n = 10 per group per sex).
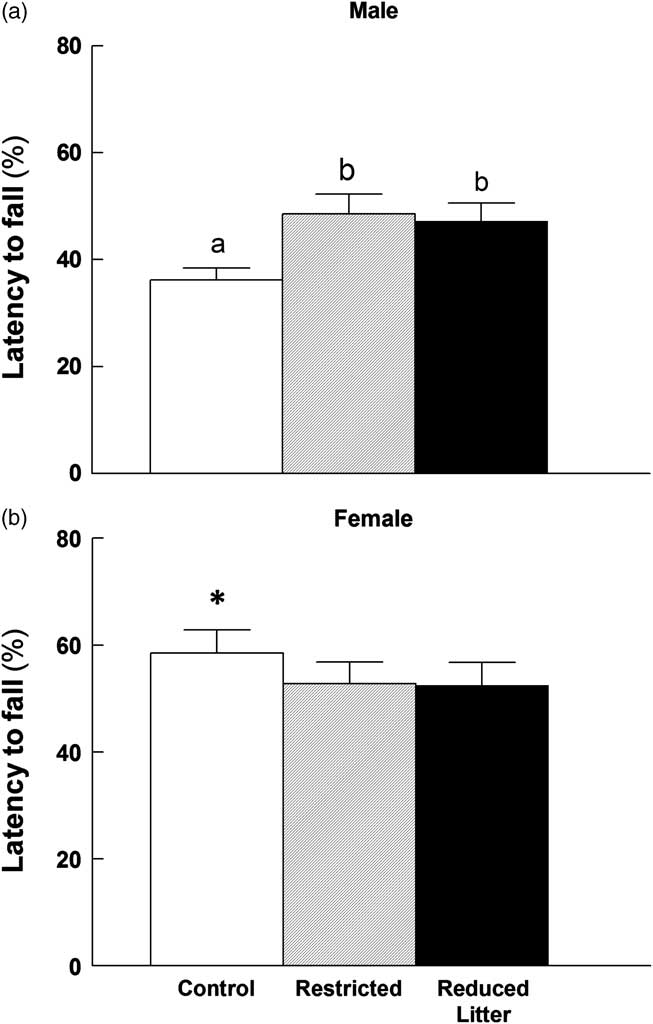
Fig. 1 Latency to fall in the rotarod test for male (a) and female (b) offspring. Control, Restricted and Reduced Litter groups are represented by open, hatched and closed bars, respectively. Data are expressed as mean ± s.e.m. (n = 9–10 per group per sex). Significant differences (P < 0.05) between groups within sex are represented by letters, so that ‘a’ is different from ‘b’. Significant differences (P < 0.05) between sexes within groups are represented by ‘*’.
Learning and memory
In the acquisition phase of the MWM overall, there were no differences in latency to reach the platform between male groups (Fig. 2a). In contrast, Restricted females took longer to find the platform on days 1 and 2 (P < 0.05) when compared with Control females (Fig. 2b). Reduced Litter females took longer to find the platform only on day 1 when compared with Controls (Fig. 2b). Male and female groups had the same latency to reach the platform for the remainder of the acquisition phase.
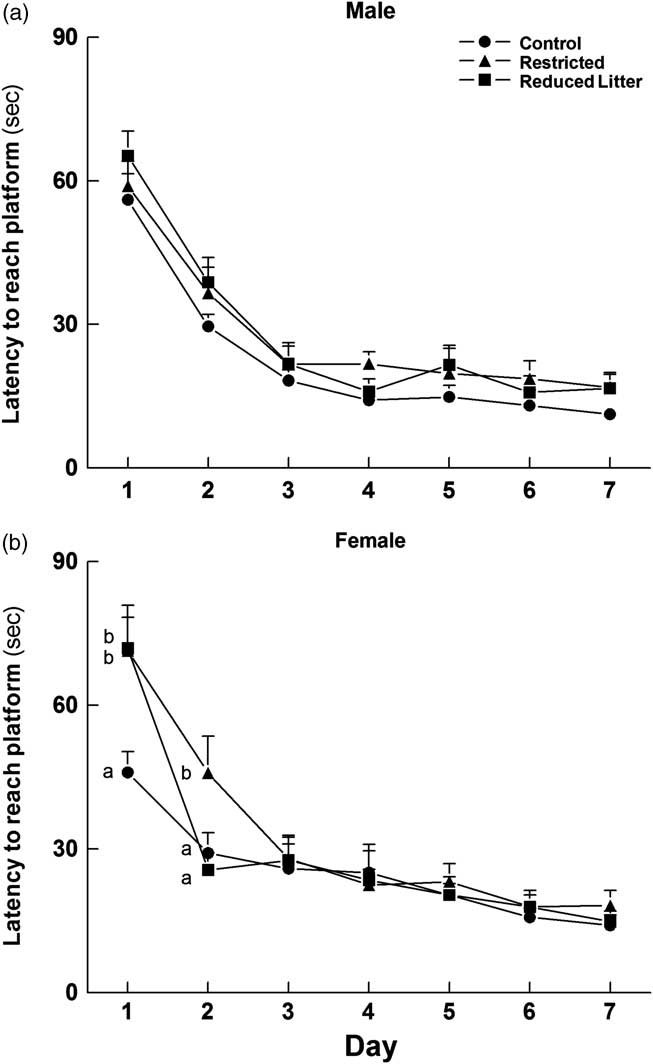
Fig. 2 Latency to reach the platform during the acquisition phase of the MWM test for male (a) and female (b) offspring. Circles, triangles and squares represent Control, Restricted and Reduced Litter groups respectively. Data are expressed as mean ± s.e.m. (n = 9–10 per group per sex). Significant differences (P < 0.05) between groups within sex are represented by letters on the graph, so that ‘a’ is different from ‘b’.
In the probe test (day 8), within each experimental group, males spent more time in the target quadrant than the other quadrants (P < 0.05; Fig. 3a). The Control and Reduced Litter females spent the same time in each of the four quadrants. However, the Restricted females spent more time in the target quadrant compared with the adjacent left and opposite quadrants (P < 0.05), but not the adjacent right (Fig. 3b). There was no difference between time spent in the target quadrant between sexes for each experimental group, or when compared across groups within each sex. Additionally, there was no difference between groups in average distance travelled or velocity for the acquisition phase and probe test, and the only sex difference was observed in Restricted offspring, where males travelled further and with greater velocity than females (P < 0.01, data not shown).
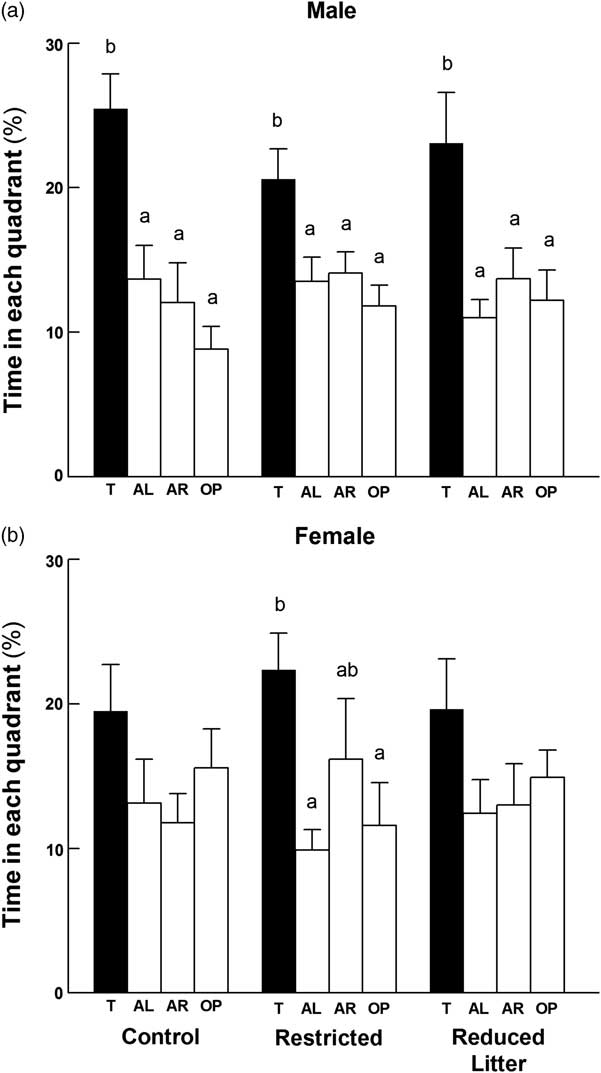
Fig. 3 Time spent in each quadrant during the MWM probe test for male (a) and female (b) offspring. The four quadrants are denoted by T = target quadrant, AL = adjacent left quadrant, AR = adjacent right quadrant and OP = opposite quadrant. Data are expressed as mean ± s.e.m. (n = 8–10 per group per sex). Significant differences (P < 0.05) within groups within sex are represented by letters, so that ‘a’ is different from ‘b’, but not different from ‘ab’.
In the Y-maze test, females from the Restricted group spent more time in the novel arm compared with Control and Reduced Litter females and Restricted males (P < 0.05; Fig. 4). There was no difference between male groups in time spent in the novel arm of the Y-maze. There were no differences between groups or sex in latency to first enter, number of entries into the novel arm and time spent in the home and familiar arms. Velocity and distance travelled was the same across groups; however, females had an increased velocity and distance travelled when compared with males (P < 0.05, data not shown).
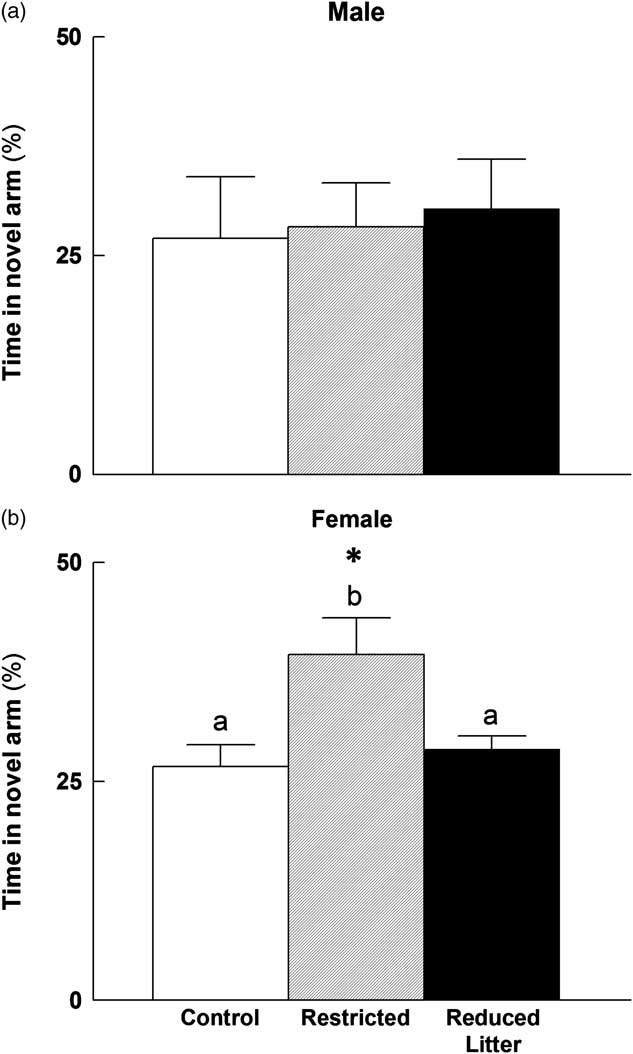
Fig. 4 Time spent in the novel arm of the Y-maze test for male (a) and female (b) offspring. Control, Restricted and Reduced Litter groups are represented by open, hatched and closed bars, respectively. Data are expressed as mean ± s.e.m. (n = 9–10 per group per sex). Significant differences (P < 0.05) between groups within sex are represented by letters, so that ‘a’ is different from ‘b’. Significant differences (P < 0.05) between sexes within groups are represented by ‘*’.
Anxiogenic and depressive-like behavior
In the EPM, as expected all animals spent the majority of the time in the closed arms (98.2–99.5%) compared with the open arms (0.5–2.1%). However, there were no differences between groups in time spent in the open or closed arms as a percentage of total time, for either sex. The number of entries into the open arms was not different between groups or sex; however, Reduced females had an increased number of entries into the closed arms when compared with the Control and Restricted females and Reduced males (P < 0.05). All groups for each sex travelled the same distance. There was no difference in velocity between groups, although Reduced females had an increased velocity when compared with Reduced males (P < 0.05, data not shown).
There was no difference between groups in time spent immobile in the Porsolt forced swim test, for both sexes. When comparing sexes, Control and Restricted males had a longer duration of immobility (12.6 and 19%, respectively) when compared with Control (3.4%) and Restricted (3.5%) females (P < 0.05, data not shown).
Sensorimotor gating function
The MANOVA revealed significant main effects of PPI (F 2, 96 = 460.54, P < 0.01), sex (F 1, 48 = 13.60, P = 0.001) and group (F 2, 48 = 4.68, P < 0.05). Most importantly, the sex by group by PPI interaction (F 4, 96 = 3.067, P < 0.05) was significant. Reduced Litter males had a decreased PPI at a prepulse of 4 dB compared with Control males (P < 0.05; Fig. 5a). Restricted males had a decreased PPI at 16 dB compared with Control and Reduced Litter males (P < 0.05; Fig. 5a). Reduced Litter females had a decreased PPI at a prepulse of 16 dB compared with Control females (P < 0.05; Fig. 5b) and Reduced Litter males. While there were no differences in PPI between groups at 8 dB for either sex, females in all experimental groups had decreased PPI at this prepulse compared with males.
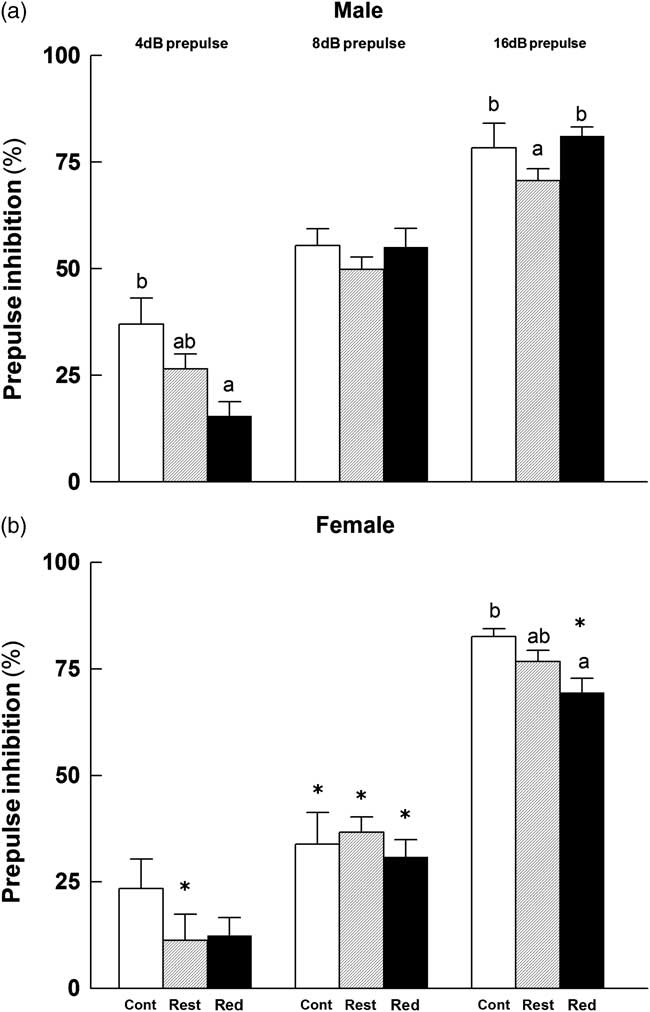
Fig. 5 Percentage of PPI (startle response) to prepulses of 4 dB, 8 dB or 16 dB for male (a) and female (b) offspring. Control (Cont), Restricted (Rest) and Reduced Litter (Red) groups are represented by open, hatched and closed bars, respectively. Data are expressed as mean ± s.e.m. (n = 9–10 per group per sex). Significant differences (P < 0.05) between groups within sex are represented by letters, so that ‘a’ is different from ‘b’, but not different from ‘ab’. Significant differences (P < 0.05) between sexes within groups are represented by ‘*’.
Discussion
This study supports previous evidence that uteroplacental insufficiency produces offspring of low birth weight with a concomitant reduction in litter size.Reference O'Dowd, Kent, Moseley and Wlodek 9 – Reference Wlodek, Westcott and O'Dowd 11 In the current cohort, Restricted offspring of both sexes weighed significantly less than the Control and Reduced Litter groups during lactation confirming sustained postnatal growth restriction. By 6–7 months of age, there was no difference in body or relative brain weight between the Restricted and Control males. This suggests a degree of accelerated growth but no evidence of brain sparing,Reference Westcott, Hirst, Ciurej, Walker and Wlodek 32 neither of which were consistently observed in previous cohorts at 6 months.Reference Wlodek, Westcott, Siebel, Owens and Moritz 12 , Reference Wadley, Siebel and Cooney 15 We have previously demonstrated that a poor postnatal lactation environment can be induced by a minor reduction in litter size producing offspring with body weights lower than Controls in early postnatal life. Even in the absence of postnatal growth restriction in the Reduced Litter cohort, there was evidence of altered lactation having an effect on behavioral responses and outcomes.
We found no differences in locomotor activity or grip strength between experimental groups, suggesting that these behavioral outcomes are not directly influenced by the prenatal or postnatal environment. It was anticipated that Restricted and Reduced Litter offspring would perform poorly on the rotarod, which assesses motor function, as they both experience a compromised nutritional environment around birth when the cerebellum is rapidly developing.Reference Dobbing and Sands 33 Unexpectedly, Restricted and Reduced Litter males showed improved balance compared with Controls. These results are inconsistent with a previous study where postnatal growth restriction induced by malnutrition in black and white hooded Lister rats resulted in poor performance or a decreased latency to fall from the accelerating rotarod.Reference Smart, Dobbing, Adlard, Lynch and Sands 18 Importantly, although the degree of growth restriction (12%) in male offspring was less than in our cohort (20%), malnutrition in this previous study was associated with a decrease in male body weight at the time of testing that may have had an impact on the interpretation of the results. At the time of testing in our study, correlation statistics (r = 0.15, P = 0.43) showed male body weight was not a confounder, and we followed a similar protocol to other rotarod studies.Reference Chapillon, Lalonde, Jones and Caston 34 However, both studies have now shown that rotarod performance can be influenced by the postnatal environment. It is suggested that adult rats, which have greater muscle strength than young rats, can passively cling on to the rotarod as opposed to walking in synchrony and therefore balance and coordination is not the only parameter measured.Reference Chapillon, Lalonde, Jones and Caston 34 In our study, tests were performed at the same age with no difference between groups in relative grip strength, potentially eliminating these variables.
When testing learning and memory using the MWM, overall we found no learning deficit following growth restriction in either females or males. Despite similar learning outcomes in the acquisition phase, Restricted females spent more time in the target quadrant than the adjacent left and opposite quadrants in the probe test and they also spent more time in the novel arm in the Y-maze test than Control and Reduced Litter groups. This suggests that they may have had increased spatial memory retention, which is contrary to our original hypothesis. The differences between groups cannot be attributed to impaired motor function, as velocity remained unchanged. One limitation of the present study is that the female rats were not assessed for stage of estrus before testing given the many behavioral tests scheduled over a predetermined time period. Perhaps future studies could eliminate any influence of estrous cycle by examining females when in diestrus, when they have been shown to have the same latency to reach the platform in the MWM as males.Reference Frye 35 However, this is unlikely to be a causal factor in the observed differences in female memory retention, as the results from the probe test correspond to those of the Y-maze and the interpretation was similar for these tests performed more than 2 weeks apart. Importantly, it has been shown there is no difference in time spent in the target quadrant of the probe test in estrus compared with proestrus.Reference Warren and Juraska 36
A rat is suggested to exhibit anxiogenic behavior in the EPM when there is a reduction in time spent in the open arms and shorter distance travelled compared with Control rats.Reference Pellow, Chopin, File and Briley 23 Rats have a preference for exploratory behavior in enclosed areas and have an aversion to open spaces, with this aversion being decreased by anxiolytic drugs.Reference Pellow, Chopin, File and Briley 23 It was anticipated that the most anxiogenic behavior would be exhibited in Restricted offspring. However, there were no differences in time spent in the arms, number of entries into the open arms and distance travelled between groups for either sex in the EPM test. This does not support a previous study, which used prenatally malnourished female rats that were growth restricted at birth and showed less anxious and more ‘impulsive’ behavior in the EPM when compared with Controls.Reference Almeida, Tonkiss and Galler 37 In contrast to maternal malnourishment, uteroplacental insufficiency is generally seen as a less severe model of growth restriction. There is the possibility that a ‘floor effect’ of the data occurred, in that the EPM may not have been sensitive enough to detect subtle effects of uteroplacental insufficiency or poor lactation on anxiogenic behavior, and therefore resulted in all rats scoring low in the test.
A rat is suggested to exhibit depressive-like behavior in the Porsolt forced swim test when there is an increased duration of immobility compared with Control rats.Reference Porsolt, Le Pichon and Jalfre 24 There were no differences between experimental groups, which is consistent with the locomotor activity test results. It has been reported that WKY rats display more anxiogenicReference Pare 38 and depressive-likeReference Pare 39 behavior compared with other strains, and therefore Control animals in the present study may be prone to these adverse behaviors, rendering any effect of a relatively subtle growth restriction difficult to detect. Perhaps a comparison with another rat strain in future studies may resolve this issue.
The PPI test assesses ‘sensory gating’ where in the normal condition an animal would exhibit less of a startle response to a high-intensity stimulus if preceded by a non-startling weaker stimulus. PPI is reduced in patients with psychiatric disordersReference Grillon, Ameli, Charney, Krystal and Braff 27 and in rats with adverse development.Reference Varty and Higgins 40 , Reference Lipska, Swerdlow and Geyer 41 The present study reported that Reduced Litter and Restricted males and Reduced Litter females had decreased PPI compared with Controls that translates to ‘less gating’ to a loud noise when preceded by a softer noise. It is noteworthy that this reduction in PPI was at prepulses of 4 and 16 dB for males and only at 16 dB for females. While the identification of PPI is well documented,Reference Grillon, Ameli, Charney, Krystal and Braff 27 , Reference Swerdlow and Geyer 42 analysis and interpretation of results is limited to the presence of a decrease or deficit. The decreased PPI found in the Restricted and Reduced Litter offspring implies that an adverse prenatal and/or postnatal environment alters this sensorimotor gating process, which is in concordance with human studies showing that nutrient availability in utero is associated with the development of schizophrenia, with patients more likely to have lower birth weights than unaffected siblingsReference Lane and Albee 43 and normal birth weight individuals.Reference Wahlbeck, Forsen, Osmond, Barker and Eriksson 26
There is only limited evidence of sex-specific differences in behavioral responses in the context of growth restriction. Gender differences were evident in a model of growth restriction via bilateral uterine artery ligation performed a day earlier than in our cohort using the open field test, with a disturbance in locomotor activity observed in males only.Reference Tashima, Nakata, Anno, Sugino and Kato 44 Spontaneous motor activity is reduced in ovariectomized females with estradiol administration resulting in increased activity.Reference Colvin and Sawyer 45 Furthermore, a previous study reported that WKY females have longer path length than males and thus are more active.Reference Li and Huang 46 Ultimately, this suggests an influence of sex steroids. While it was anticipated that in our cohort there would be sex differences in the locomotor test, this was not the case. This may be accounted for by the less severe growth restriction model employed without a sufficient reduction in sex steroids to elicit a motor impairment. Testosterone is widely known for its role in muscle development, strength and maintenanceReference Morley, Kaiser, Sih, Hajjar and Perry 47 and is reflected by the increased grip strength demonstrated in our cohort of males compared with females. Female Controls performed better than males in the rotarod test, so future studies should determine hormone profiles before and immediately following a regimen of behavioral tests.
Estradiol plays a critical role in hippocampal synapse formation and maturation. Therefore, it may have been anticipated that estradiol would influence learning and memory performance. For example, administration of estradiol to ovariectomized female rats increases spatial working memory.Reference Packard and Teather 48 In contrast, gonadally intact females performed poorly in the MWM acquisition phase compared with untreated ovariectomized females.Reference Frye 35 However, we found no sex differences between our Control groups in the MWM or Y-maze.
There were no sex differences in anxiogenic behavior between our Controls, in contrast to a study where male WKY rats were generally more anxious than females.Reference Fernandes, Gonzalez, Wilson and File 49 Given the strict regimen employed in the present study an additional anxiety test, such as the open field test could not be included, but may be considered for future studies. In general, males exhibited more depressive-like behavior when compared with females in the Porsolt forced swim test. The sex differences demonstrated in the PPI test cannot be easily explained due to the complex nature of sensorimotor gating, which involves different structural and mechanistic processes that are not yet fully understood.Reference Swerdlow and Geyer 42 Previous studies using similar behavioral tests have generally used males only, thus our gender dimorphic responses reflect a novel finding.
Due to the variation in behavioral responses we observed in the different experimental groups, it is important that future studies include determination of a physiological stress response (e.g. corticosterone release) to each test. It is possible that uteroplacental insufficiency or reducing litter size may influence the response to stress, which may represent a predictive adaptive response and therefore alter particular behaviors. Additionally, due the sex-specific differences that were revealed in some tests, a full characterization of the endocrine profile for each individual animal would be informative. Further analyses could include hormones related to a variety of behaviors, such as leptin, insulin, thyroid hormones and sex steroids. Results from the suite of behavioral tests performed in this study may direct mechanistic studies. Future studies could incorporate assessment of estrogen receptors in and ultrastructure of hippocampal and cerebellar brain regions, due to their roles in motor functionReference Molinari, Leggio and Thaut 50 and learning and memory,Reference Morris, Garrud, Rawlins and O'Keefe 20 respectively. The direct effects of estrogen on these brain structures and related behavioral responses in our model may also help inform potential mechanisms of gender differences. Our results suggest that growth restriction in males may result in altered motor function later in life, whereas growth-restricted females appear to have enhanced memory, when compared with Controls. Furthermore, a compromised prenatal and/or postnatal environment was not directly associated with depressive-like behavior in this cohort of animals; however, it can influence sensorimotor gating. This study has revealed that critical growth periods before and after birth can program alterations in behavioral traits in a sex-specific manner.
Acknowledgements
The authors would like to thank Brett Purcell and Tim Brown for assistance with behavioral studies, as well as Kerryn Westcott for assistance with animal surgery. We acknowledge grant support from the National Health & Medical Research Council (NHMRC) of Australia (to M. E. W.). A. L. S. was supported by an NHMRC Peter Doherty Biomedical Research Fellowship.









