Introduction
The genus Monochamus Dejean (Coleoptera: Cerambycidae) is widely distributed, mainly in the Nearctic, Palaearctic, and Ethiopean biogeographic regions (Löbl and Smetana Reference Löbl and Smetana2010; Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013; Anonymous 2014). This group of species predominantly attacks coniferous host trees, typically attacking recently dead, dying, or stressed trees (Hanks Reference Hanks1999; Akbulut and Stamps Reference Akbulut and Stamps2012). Feeding by their larvae can play an important ecological role in terms of the breakdown of woody material and recycling of nutrients (Linsley Reference Linsley1959). However, a number of species can be considered serious forest insect pests, with larval tunnelling significantly degrading the value of logs for lumber (Safranyik and Raske Reference Safranyik and Raske1970). Moreover, some species can attack and kill trees following forest disturbances (Gandhi et al. Reference Gandhi, Gilmore, Katovich, Mattson, Spence and Seybold2007). Finally, adults of numerous Monochamus species can transmit pinewood nematode, Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle, the causal agent of pine wilt disease (Akbulut and Stamps Reference Akbulut and Stamps2012). Introduction of non-native Monochamus species to new geographic locations through international trade is an increasing concern. Haack (Reference Haack2006) reported 432 known interceptions of Monochamus at United States of America ports from 1985 to 2000, arriving in shipments from 29 different countries worldwide. Thus, early detection of Monochamus species that may be accidentally introduced to new habitats is critical.
Host orientation in the Cerambycidae, and specifically for some species in the genus Monochamus, is known to be facilitated by numerous volatile host plant chemicals, such as ethanol and α-pinene (Chénier and Philogène Reference Chénier and Philogene1989; Allison et al. Reference Allison, Borden, McIntosh, de Groot and Gries2001, Reference Allison, Morewood, Borden, Hein and Wilson2003, Reference Allison, Borden and Seybold2004; Pajares et al. Reference Pajares, Ibeas, Diez and Gallego2004; Sweeney et al. Reference Sweeney, de Groot, MacDonald, Smith, Cocquempot and Kenis2004, Reference Sweeney, Gutowski, Price and de Groot2006; Ginzel and Hanks Reference Ginzel and Hanks2005; Miller Reference Miller2006; Fan et al. Reference Fan, Sun and Shi2007; Costello et al. Reference Costello, Negron and Jacobi2008; Francardi et al. Reference Francardi, De Silva, Pennacchio and Roversi2009; Miller et al. Reference Miller, Asaro, Crowe and Duerr2011; Hanks et al. Reference Hanks, Millar, Mongold-Diers, Wong, Meier and Reagel2012), and these are commonly used in trapping surveys for wood-boring insects (Brockerhoff et al. Reference Brockerhoff, Jones, Kimberley, Suckling and Donaldson2006; Witzgall et al. Reference Witzgall, Kirsch and Cork2010). Moreover, some Monochamus species also use bark beetle pheromones as kairomones to orient towards susceptible host trees (Billings and Cameron Reference Billings and Cameron1984; Allison et al. Reference Allison, Morewood, Borden, Hein and Wilson2003, Reference Allison, McKenney, Miller and Gimmel2013; Miller and Asaro Reference Miller and Asaro2005; Miller et al. Reference Miller, Asaro, Crowe and Duerr2011, Reference Miller, Dodds, Eglitis, Fettig, Hofstetter and Langor2013; Pajares et al. Reference Pajares, Alvarez, Hall, Douglas, Centeno and Ibarra2013). For example, ipsenol, an aggregation pheromone of Ips De Geer and Dendroctonus Erichson species (Coleoptera: Curculionidae: Scolytinae), has been shown to increase trap captures of Monochamus species (Allison et al. Reference Allison, Borden, McIntosh, de Groot and Gries2001; de Groot and Nott Reference de Groot and Nott2004; Pajares et al. Reference Pajares, Ibeas, Diez and Gallego2004; Ibeas et al. Reference Ibeas, Gallego, Diez and Pajares2007). It has also been demonstrated that many cerambycids produce sex pheromones or aggregation pheromones (Lacey et al. Reference Lacey, Ginzel, Millar and Hanks2004, Reference Lacey, Ginzel, Moreira and Hanks2009; Liendo et al. Reference Liendo, Morillo, Sanches, Munoz, Guerra and Cabrera2005; Silk et al. Reference Silk, Sweeney, Wu, Price, Gutowski and Kettela2007; Ibeas et al. Reference Ibeas, Diez and Pajares2008; Ray et al. Reference Ray, Millar, McElfresh, Swift, Barbour and Hanks2009, Reference Ray, Zunic, Alten, McElfresh, Hanks and Millar2011; Fonseca et al. Reference Fonseca, Vidal and Zarbin2010; Nehme et al. Reference Nehme, Keena, Zhang, Baker, Xu and Hoover2010; Rodstein et al. Reference Rodstein, Millar, Barbour, McElfresh, Wright and Barbour2011). Moreover, the structural motifs for these pheromones are highly conserved, that is, the same or similar compounds are shared by many species in the same subfamilies, especially the Cerambycinae, Spondylidinae, and Lamiinae (Hanks et al. Reference Hanks, Millar, Moreira, Barbour, Lacey and McElfresh2007; Hanks and Millar Reference Hanks and Millar2013). For example, Mitchell et al. (Reference Mitchell, Graham, Wong, Reagel, Striman and Hughes2011) showed that (E,Z)-6,10-dimethyl-5,9-undecadien-2-ol [(E,Z)-fuscumol] and (E,Z)-6,10-dimethyl-5,9-undecadien-2-yl acetate [(E,Z)-fuscumol acetate], which are pheromones used by Tetropium fuscum (Fabricius), T. cinnamopterum (Kirby) (Spondylidinae) (Silk et al. Reference Silk, Sweeney, Wu, Price, Gutowski and Kettela2007), and Hedypathes betulinus (Klug) (Lamiinae) (Fonseca et al. Reference Fonseca, Vidal and Zarbin2010), were attractive to several species of Lamiinae. Furthermore, Mitchell et al. (Reference Mitchell, Graham, Wong, Reagel, Striman and Hughes2011) showed that fuscumol acetate was attractive to two species in the subfamily Cerambycinae, including Xylotrechus colonus (Fabricius), for which the pheromone blend does not contain fuscumol acetate (Lacey et al. Reference Lacey, Ginzel, Moreira and Hanks2009). Xylotrechus colonus is highly polyphagous, feeding on virtually all eastern hardwood species (Yanega Reference Yanega1996), and may respond to fuscumol acetate as a kairomone that indicates suitable hosts for oviposition (Mitchell et al. Reference Mitchell, Graham, Wong, Reagel, Striman and Hughes2011).
Recently, it was determined that 2-undecyloxy-1-ethanol (monochamol) is a male-produced aggregation pheromone for Monochamus galloprovincialis (Olivier) (Pajares et al. Reference Pajares, Alvarez, Ibeas, Gallego, Hall and Fahman2010), increasing trap captures by 80–140% when combined with a kairomone blend. Later studies showed that monochamol is also produced by and attractive to M. alternatus Hope (Teale et al. Reference Teale, Wickham, Zhang, Chen, Hanks and Millar2011), M. scutellatus (Say) (Fierke et al. Reference Fierke, Skabeikis, Millar, Teale, McElfresh and Hanks2012), M. carolinensis (Olivier), M. titillator (Fabricius) (Allison et al. Reference Allison, McKenney, Millar, McElfresh, Mitchell and Hanks2012), and M. sutor (Linnaeus) (Pajares et al. Reference Pajares, Alvarez, Hall, Douglas, Centeno and Ibarra2013). Field trapping experiments have provided evidence that monochamol is also attractive to M. notatus (Drury) (Fierke et al. Reference Fierke, Skabeikis, Millar, Teale, McElfresh and Hanks2012), M. clamator (LeConte), and M. obtusus Casey (Macias-Samano et al. Reference Macias-Samano, Wakarchuk, Millar and Hanks2012), suggesting it may be a pheromone for these species as well. This cumulative evidence strongly suggests that monochamol, combined with appropriate kairomones, may be an effective lure for attraction of Monochamus species in general, and therefore, a useful tool for surveillance.
Our objectives were to determine: (1) the response of additional Monochamus species to monochamol; (2) the influence of potential kairomones (host plant volatiles, bark beetle pheromones) and other cerambycid beetle pheromones (E,Z)-fuscumol and (E,Z)-fuscumol acetate) on those responses; (3) the effect of monochamol release rate on trap catches; and (4) the efficacy of specific lure combinations for detection of Monochamus species in traps. We conducted field trials in Canada (Ontario and New Brunswick), Poland (Białowieża), and China (Jilin Province) to include geographic areas with Monochamus species for which attraction to monochamol has not yet been determined. The sites in Canada contained M. marmorator Kirby and M. mutator LeConte in addition to M. carolinensis, M. notatus, and M. scutellatus. The site in Poland was known to have populations of the black fir sawyer, M. urussovii (Fischer), a species that can cause significant tree mortality in Siberia, Russia (Gavrikov and Vetrova Reference Gavrikov and Vetrova1991), as well as M. galloprovincialis, M. sutor, and rarely, M. saltuarius Gebler. The site in China was thought to have populations of M. saltuarius as well as M. guttatus Bless, which feeds on oaks (Quercus Linnaeus; Fagaceae), maples (Acer Linnaeus; Sapindaceae), and other broad-leaved species (Cherepanov Reference Cherepanov1990).
In addition to testing the influence of known kairomones, such as α-pinene, ethanol, and ipsenol, on response of Monochamus species to monochamol, we also tested Manuka oil and cubeb oil lures, derived from the flowering plants, Leptospermum scoparium Forster and Forster (Myrtaceae), and Piper cubeba Linnaeus (Piperaceae). The latter two lures were included because they have been shown to attract other beetle species (Crook et al. Reference Crook, Khrimian, Francese, Fraser, Poland and Sawyer2008; Hanula and Sullivan Reference Hanula and Sullivan2008; Hanula et al. Reference Hanula, Sullivan and Wakarchuk2013) and contain many sesquiterpenes and monoterpenes found in both conifers and hardwoods (Jactel and Kleinhentz Reference Jactel and Kleinhentz1997; Perry et al. Reference Perry, Brennan, Van Klink, Harris, Douglas and McGimpsey1997; Hong et al. Reference Hong, Na, Choi, Choi and Jeung2004; Huber et al. Reference Huber, Philippe, Madilao, Sturrock and Bohlmann2005; Chen et al. Reference Chen, Tang, Gao, Chen and Li2006; Singh et al. Reference Singh, Marimuthu, de Heluani and Catalan2007). We included cubeb oil in our trapping experiment near Jilin, China, as a potential attractant of the hardwood-feeding species, M. guttatus. Finally, we tested the combination of (E,Z)-fuscumol and (E,Z)-fuscumol acetate for its potential interference or synergism with monochamol on attraction of Monochamus species because they are known pheromones and/or attractants of several species of Cerambycidae (Silk et al. Reference Silk, Sweeney, Wu, Price, Gutowski and Kettela2007; Sweeney et al. Reference Sweeney, Silk, Gutowski, Wu, Lemay and Mayo2010; Mitchell et al. Reference Mitchell, Graham, Wong, Reagel, Striman and Hughes2011) and therefore, like monochamol, are potential components of multicomponent lures for survey and detection of exotic wood borers (Hanks et al. Reference Hanks, Millar, Mongold-Diers, Wong, Meier and Reagel2012; Wong et al. Reference Wong, Mitchell, Striman, Millar and Hanks2012; Wickham et al. Reference Wickham, Harrison, Lu, Guo, Millar and Hanks2014).
Materials and methods
Two different trapping experiments were conducted in 2012 to test for attraction of Monochamus species to monochamol with and without different kairomones. Each experiment was conducted twice, once at each of two different sites. A third experiment was conducted in 2013 at one site to test for the effects of monochamol, (E,Z)-fuscumol, (E,Z)-fuscumol acetate, and plant volatiles on trap captures of Monochamus species.
Sites
Experiment 1 was conducted in mixed conifer–hardwood stands in the Acadia Research Forest near Noonan, New Brunswick, Canada (45.9990°N, 66.2623°W) and Białowieża, Poland (52.6981°N, 23.7687°E). The site in New Brunswick consisted of a mixture of Picea rubens Sargent (Pinaceae); P. mariana (Miller) Britton, Sterns, and Poggenburg (Pinaceae); Abies balsamea (Linnaeus) Miller (Pinaceae); Pinus strobus Linnaeus (Pinaceae); Acer rubrum Linnaeus (Sapindaceae); Betula alleghaniensis Britton (Betulaceae); and B. papyrifera Marshall (Betulaceae). The site in Poland was natural forest dominated by Picea abies (Linnaeus) Karsten (Pinaceae), Pinus sylvestris Linnaeus (Pinaceae), Betula pendula Roth (Betulaceae), Quercus robur Linnaeus (Fagaceae), Carpinus betulus Linnaeus (Betulaceae), and Populus tremula Linnaeus (Salicaceae). Experiment 2 was conducted in Ontario, Canada (46.3418°N, 83.5636°W) in a 50-year-old to 60-year-old mixed-softwood plantation of Picea glauca (Moench) Voss (Pinaceae), P. mariana, Pinus resinosa Aiton (Pinaceae), P. banksiana Lambert (Pinaceae), and A. balsamea and in the Acadia Research Forest, New Brunswick, in a stand dominated by A. balsamea with scattered P. rubens, and A. rubrum. Experiment 3 was conducted in a mixed deciduous–conifer forest within the Jiaohe Administration Bureau of the Forest Experimental Zone, Jilin province, China (43.8500°N, 127.5830°E). This forest contained mature Quercus mongolica Fischer ex Ledebour (Fagaceae), Tilia amurensis Ruprecht (Malvaceae), T. mandshurica Ruprecht and Maximowicz (Malvaceae), Betula platyphylla Sukaczev (Betulaceae), Pinus koraiensis Siebold and Zuccarini (Pinaceae), Acer mono Maximowicz (Sapindaceae), A. triflorum Komarov (Sapindaceae), Populus davidiana Dode (Salicaceae), and Fraxinus rhynchophylla Hance (Oleaceae).
Lures
Monochamol lures were purchased from ConTech Inc. (Delta, British Columbia, Canada) and Synergy Semiochemicals (Burnaby, British Columbia, Canada) in 2012 and from the latter company in 2013 (Table 1). Manuka oil and cubeb oil lures were purchased from Synergy Semiochemicals. Ipsenol (50/50±) bubble caps and ultra-high release rate lures of ethanol and (95/5± enantiomers) α-pinene were obtained from ConTech Inc. Ipsenol was included as a synergist kairomone in experiment 2. Ethanol and α-pinene were included as kairomones in experiments 1 and 2. Lures of racemic (E/Z)-fuscumol and racemic (E/Z)-fuscumol acetate were purchased from Sylvar Technologies Inc. (Fredericton, New Brunswick, Canada). Lures were not replaced during the 8–10 week duration of the experiments.
Table 1 List of lures used in experiments.
Note: *Pouches and bubble caps are membrane-bound reservoir devices whereas rubber septa are monolithic devices.
Trapping protocols
Twelve-unit Lindgren funnel traps (Lindgren Reference Lindgren1983) (ConTech Inc) were used in Canada. Black panel intercept traps (AlphaScents Inc, West Linn, Oregon, United States of America) were used in Poland and China. Traps in New Brunswick were treated with Fluon® to increase catches (Graham et al. Reference Graham, Mitchell, Reagel, Barbour, Millar and Hanks2010; Allison et al. Reference Allison, Wood Johnson, Meeker, Strom and Butler2011); traps in Ontario were treated with Rain-X® for the same reason (de Groot and Nott Reference de Groot and Nott2003), but traps in Poland and China were not treated. Traps were suspended from rope tied between two trees, with the bottom of the collection cup ~1.5 metres above ground and at least one metre between the trap and adjacent trees. Wet traps were used at all sites. Collecting cups were filled half way with a 50% solution of propylene glycol in water in Ontario, a 50% solution of ethylene glycol in water in Poland, and a saturated solution of table salt in water in both New Brunswick and China. At all sites, a drop or two of liquid dish detergent was added to trapping solution to reduce surface tension. In each experiment, lure treatments were replicated eight times using a randomised complete block design, with about 30 metres spacing between traps and at least 30 metres spacing between blocks. Traps were checked every two or three weeks, and all specimens were preserved in 70% ethanol. All Monochamus specimens were identified to species, and voucher specimens were deposited in the collections of the Great Lakes Forestry Centre, Sault Ste. Marie, Ontario, Canada; Atlantic Forestry Centre, Fredericton, New Brunswick, Canada; Forest Research Institute, Białowieża, Poland; and Beihua University, Jilin, China.
Experiment 1: effect of monochamol, with and without plant volatiles, on trap capture of Monochamus species
Experiment 1 was designed to test for interactions between monochamol and kairomones (various host plant volatiles) on trap capture of Monochamus species. Treatments were as follows: (1) monochamol (M); (2) manuka oil (MK); (3) (95/5±) α-pinene+ethanol (APE); (4) M+MK; (5) M+APE; (6) MK+APE; (7) M+MK+APE; and (8) unbaited control. Traps were deployed from 14 June to 22 August 2012 in New Brunswick and from 4 June to 30 July 2012 in Poland.
Experiment 2: effect of monochamol release rate, with and without plant volatiles, on trap captures of Monochamus species
Experiment 2 evaluated capture of Monochamus species in response to different release rates of monochamol, with or without kairomones. Treatments included: (1) high release monochamol (MH); (2) low release monochamol (ML); (3) MH+(95/5±) α-pinene+ethanol+ipsenol (AEI); (4) ML+AEI; (5) AEI; and (6) unbaited control. Traps were deployed from 12 June to 7 August 2012 in Ontario and from 14 June to 10 September 2012 in New Brunswick.
Experiment 3: effect of monochamol, fuscumol and fuscumol acetate, and cubeb oil on trap captures of Monochamus species
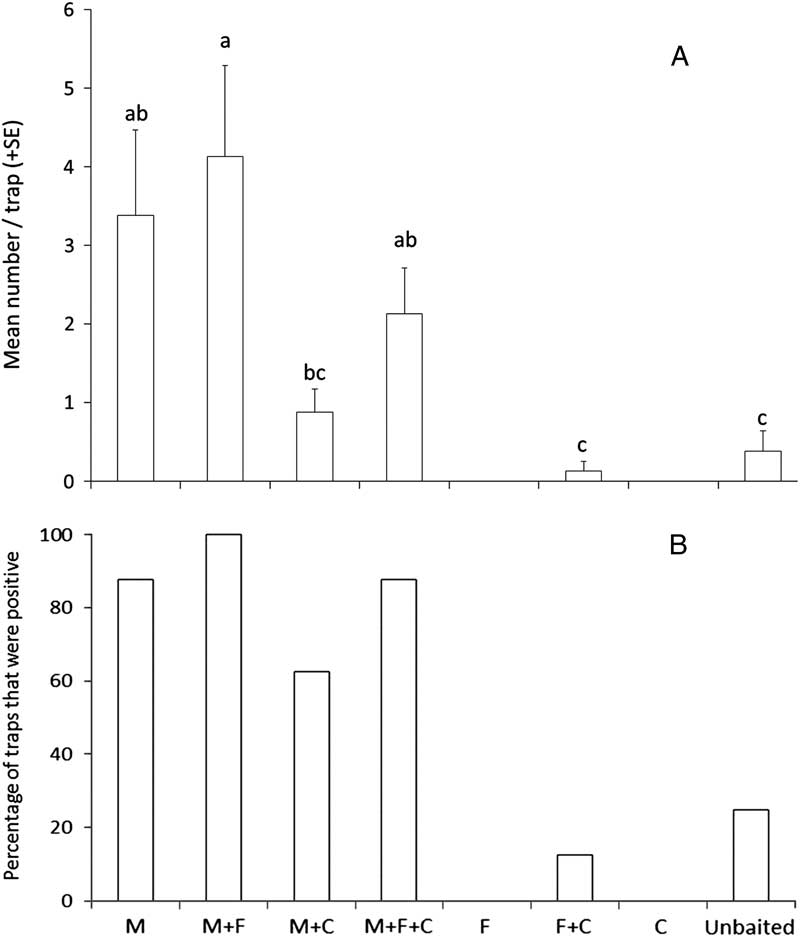
Experiment 3 tested the efficacy of monochamol, cubeb oil, (E/Z) fuscumol, and (E/Z)-fuscumol acetate on capture of Monochamus spp. in intercept traps. Treatments were: (1) monochamol (M); (2) fuscumol+fuscumol acetate (F); (3) cubeb oil (C); (4) M+F; (5) M+C; (6) F+C; (7) M+F+C; and (8) unbaited control. Traps were deployed from 23 May to 1 August 2013 in Jilin, China.
Statistical analysis
Data were analysed separately by experiment, site (e.g., Ontario, New Brunswick, Poland, China), and Monochamus species, using summed total captures per trap over the duration of the trapping period. Data were analysed separately by sex for M. scutellatus, for which catches were very abundant, and with sexes pooled for all other species. For less commonly intercepted Monochamus species, or where there was a high frequency of zeroes in the data set (>30% of observations with zero captures), data were analysed using a Kruskal–Wallis (K-W) rank sum test to test for differences among treatments. Alternatively, data for species captured in high enough numbers and where >70% of traps caught at least one beetle were analysed using the analysis of variance (ANOVA) function in R version 3.0.2 (R Development Core Team 2013), with treatment and block as factors. For the ANOVA analyses, transformation of the data before analysis was not necessary based on evaluation of residuals for normality (plot function in R). Before either analysis, any treatments with zero trap captures were removed from the data set; treatments with zero captures were excluded to avoid having treatments with zero mean and variance (Reeve and Strom Reference Reeve and Strom2004). Results for all treatments are included in figures for illustrative purposes. To compare mean captures among treatments, either a Wilcoxon rank sum test or a Tukey’s multiple comparison of means test was conducted for data sets analysed by K-W rank sum test or ANOVA, respectively. For the Wilcoxon rank sum test, the P value was adjusted using the Bonferroni method (Zar Reference Zar1984). Finally, to test the effect of lure treatment on the rate of detecting a given species, we used Cochran’s Q test for dichotomous nominal-scale data in randomised blocks (Zar Reference Zar1984) to test the hypothesis that the proportion of traps that captured at least one specimen of a given species was the same for all lure treatments. Cochran’s Q is distributed approximately as χ2 as long as the number of data is large, so we calculated it only for those species where the number of lure treatments (a) multiplied by the number of trap blocks in which at least one (but not all) treatments captured that species (b) was ⩾24 (Zar Reference Zar1984).
Results
Experiment 1: effect of monochamol, with and without plant volatiles, on trap capture of Monochamus species
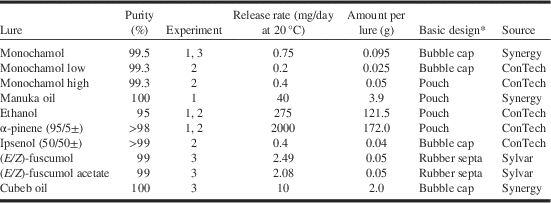
In experiment 1, 310 M. scutellatus and 205 M. urussovii were captured in New Brunswick and Poland, respectively. There were significant differences in mean total catch per trap among treatments of both M. scutellatus (K-W χ2=45.9, df=7, P<0.0001) and M. urussovii (K-W χ2=30.0, df=6, P<0.0001). No M. urussovii were captured in unbaited traps. The highest mean catches for both species were in traps baited with combinations of monochamol and host volatiles, particularly α-pinene+ethanol (Figs. 1A, 1B). Addition of manuka oil to monochamol+α-pinene+ethanol did not significantly increase mean trap captures for either species (Figs. 1A, 1B). However, there was evidence that trap captures of both species were maximised by the combination of monochamol and host volatiles; mean catch was significantly greater in traps baited with monochamol+manuka oil, α-pinene, and ethanol than in traps baited with either monochamol alone or the host volatiles alone (Figs. 1A, 1B).
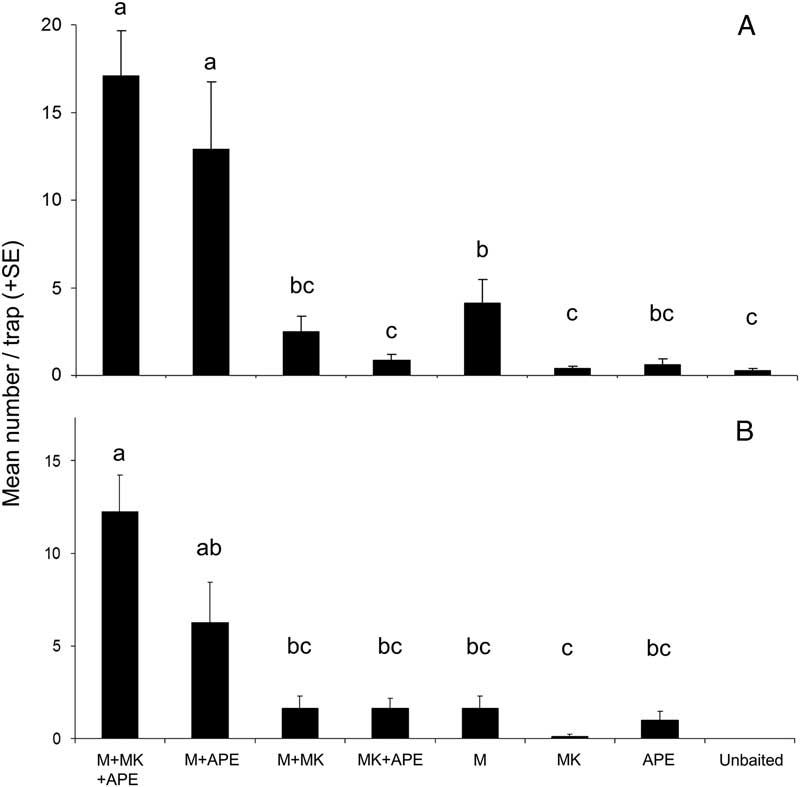
Fig. 1 Mean number per trap (+SE) of: (A) Monochamus scutellatus in New Brunswick (14 June–22 August 2012); and (B) M. urussovii in Poland (4 June–30 July 2012) in traps baited with monochamol (M), with or without the host volatile lures, Manuka oil (MK), and the combination of α-pinene and ethanol (APE). Treatments with zero captures were excluded from analysis. Bars with different letters are significantly different (P⩽0.05).
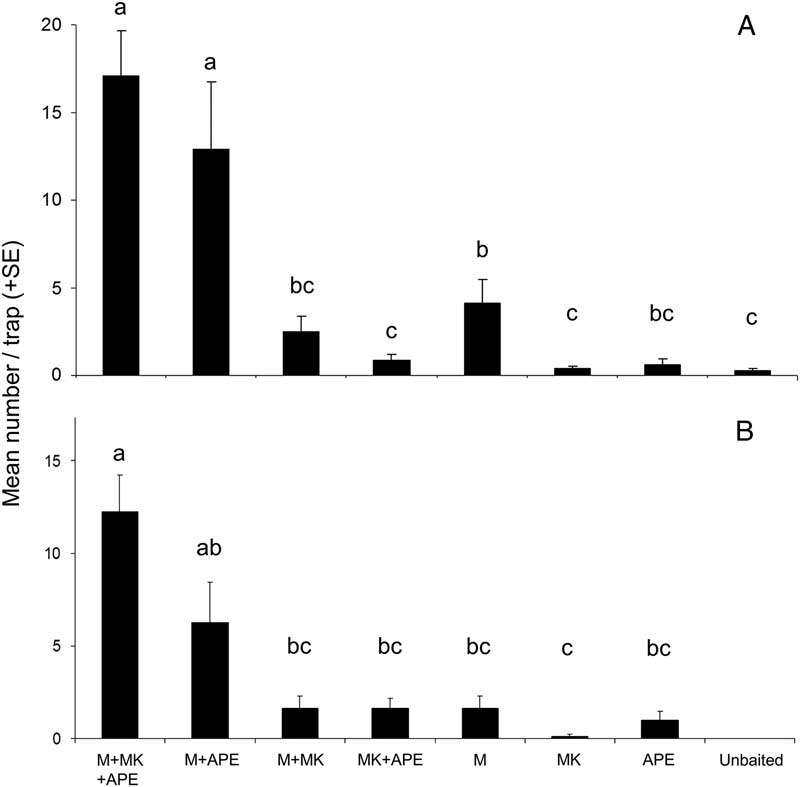
Lure treatments also differed significantly in the proportion of traps that detected M. scutellatus (Q=22.8, P<0.01) and M. urussovii (Q=29.9, P<0.001) (Figs. 2A, 2B). Presence of host volatiles did not affect the proportion of traps that captured M. scutellatus. Traps baited with monochamol alone or monochamol+α-pinene and ethanol detected M. scutellatus in all eight blocks. However, monochamol+manuka oil, α-pinene, and ethanol was the only lure treatment to detect M. urussovii in all eight trap blocks, providing further evidence of synergism between monochamol and plant volatiles.
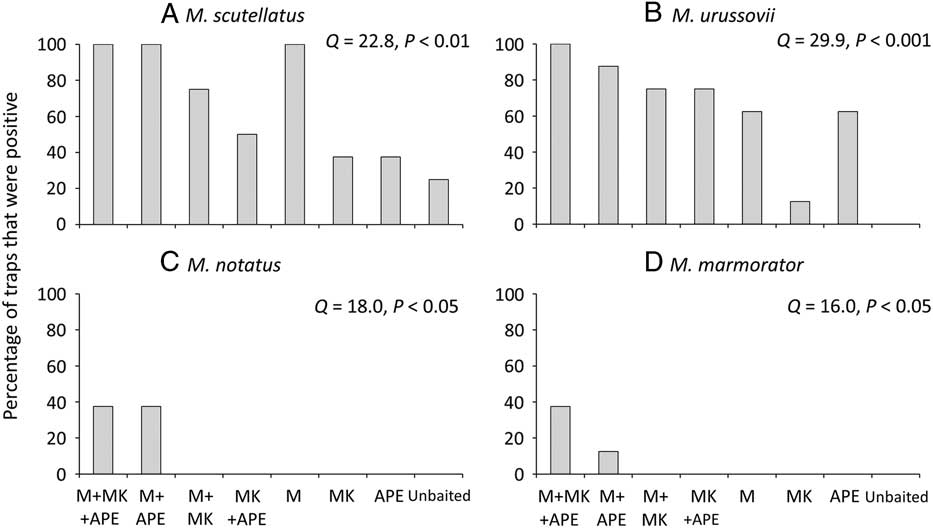
Fig. 2 Percentage of traps baited with monochamol (M), manuka oil (MK), α-pinene+ethanol (APE), or combinations thereof, that captured at least one specimen of (A) M. scutellatus, (B) M. urossovii, (C) M. notatus, and (D) M. marmorator in field trapping experiments in New Brunswick, Canada (A, C, D) and Poland (B) in 2012.
Three additional species of Monochamus were captured in experiment 1. In New Brunswick, low numbers of M. notatus (three females and four males) and M. marmorator (five females) were captured. Lure treatments differed significantly in the proportion of traps that detected M. notatus (Q=18.0, P<0.05) and M. marmorator (Q=16.0, P<0.05) (Figs. 2C, 2D). Traps baited with monochamol+manuka oil, α-pinene, and ethanol captured M. marmorator and M. notatus in three of eight blocks; traps baited with monochamol+α-pinene and ethanol captured M. marmorator in one of eight blocks and M. notatus in three of eight blocks. None of the other lure treatments captured either species. Finally, a single specimen of M. saltuarius was captured in a trap baited with monochamol at the site in Poland, where it is considered very rare.
Experiment 2: effect of monochamol release rate, with and without kairomones, on trap captures of Monochamus species
A total of 436 and 1332 Monochamus beetles were captured in softwood stands in Experiment 2 in New Brunswick and Ontario, respectively. Four species were detected in both provinces (M. scutellatus, M. notatus, M. carolinensis, and M. marmorator), with a fifth species also detected in Ontario (M. mutator).
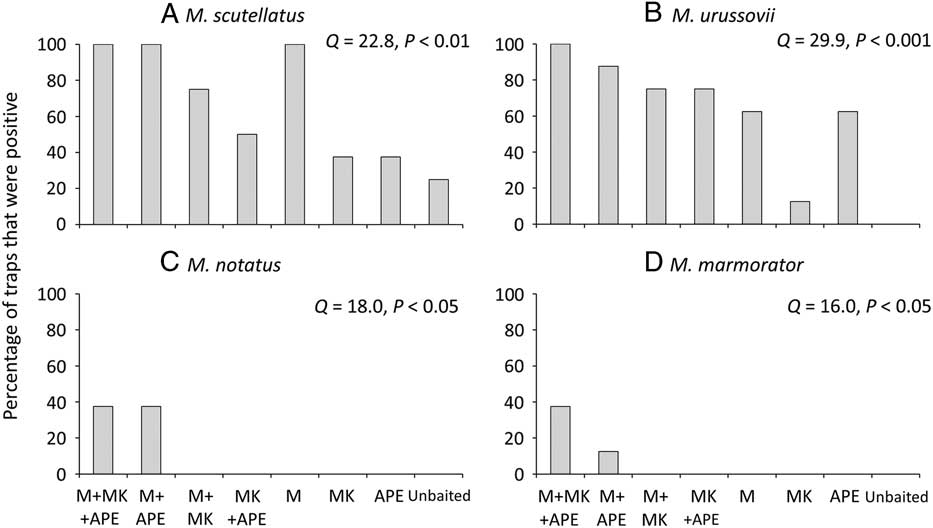
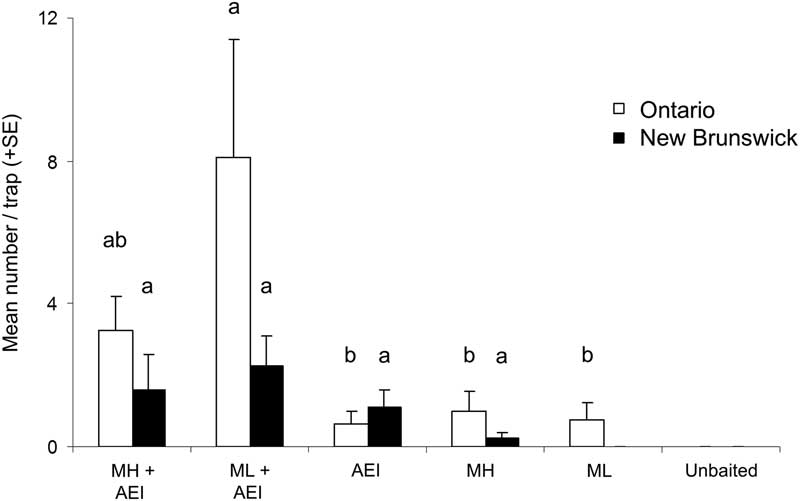
For M. scutellatus in Ontario and New Brunswick, the combination of monochamol and kairomones (α-pinene+ethanol+ipsenol) had the highest mean trap captures for both males and females (Table 2; Figs. 3A, 3B). Significantly higher numbers of female M. scutellatus were captured in Ontario in traps baited with either dose of monochamol plus kairomones than with any other treatment. There was no significant effect of monochamol release rate on mean trap captures of either sex in either trapping location (Fig. 3). Unbaited traps caught few or no M. scutellatus.
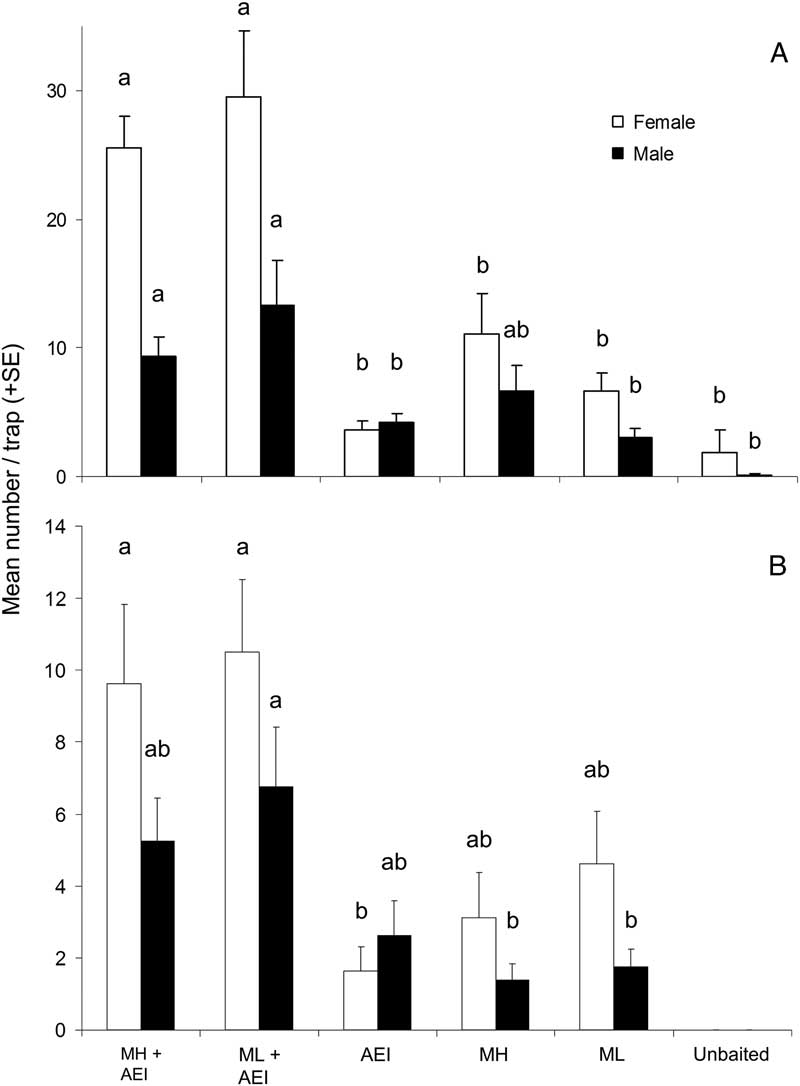
Fig. 3 Mean (+SE) number of female and male Monochamus scutellatus per trap in (A) Ontario, Canada, and (B) New Brunswick, Canada in traps baited with two release rates of monochamol (low: ML; high: MH) with versus without kairomones (α-pinene, ethanol, and ipsenol, AEI). Treatments with zero captures were excluded from analysis. Means with different letters are significantly different among treatments, analysed separately by location and sex (P⩽.05).
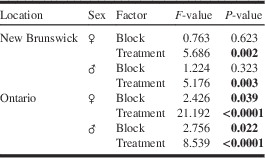
Table 2 Results of experiment 2 testing the effect of monochamol at two release rates, alone and combined with a kairomone lure blend (α-pinene, ethanol, ipsenol) for trap capture of Monochamus scutellatus in New Brunswick and Ontario.
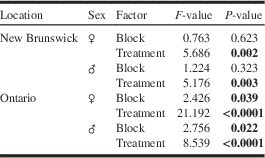
Note: Factors and P-values in bold were significant (P⩽0.05).
Captures of the other species of Monochamus were lower, but each demonstrated similar trends in trap captures among treatments. For M. notatus, there was a significant influence of treatment on mean trap captures in Ontario (K-W χ2=18.4, df=4, P=0.0011; Fig. 4); traps baited with low release monochamol plus kairomones captured more M. notatus than traps baited with either the pheromone or kairomones alone (Fig. 4). There was no significant difference in mean trap captures for M. notatus in New Brunswick (K-W χ2=3.5, df=3, P=0.32), but mean catches followed a trend similar to that in Ontario. Most M. marmorator were captured in traps baited with either release rate of monochamol plus kairomones, but differences were not significant (K-W χ2=6.4, df=3, P=0.08). Only one M. marmorator was captured in Ontario in a trap baited with low release monochamol+α–pinene, ethanol, and ipsenol. Trap captures of M. mutator followed a similar trend, with highest numbers in traps baited with monochamol (either release rate) plus kairomones, but differences were not significant (K-W χ2=5.4, df=5, P=0.37).
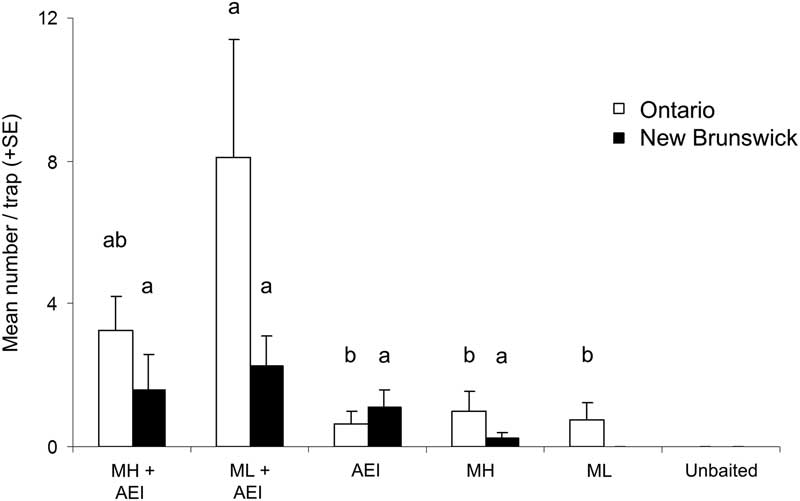
Fig. 4 Mean (+SE) number of Monochamus notatus per trap in Ontario, Canada (white bars) and New Brunswick, Canada (black bars) in traps baited with two release rates of monochamol (low: ML; high: MH) with versus without kairomones (α-pinene, ethanol, and ipsenol=AEI). Treatments with zero captures were excluded from analysis. Means with different letters are significantly different among treatments, separately by species and location (P⩽0.05).
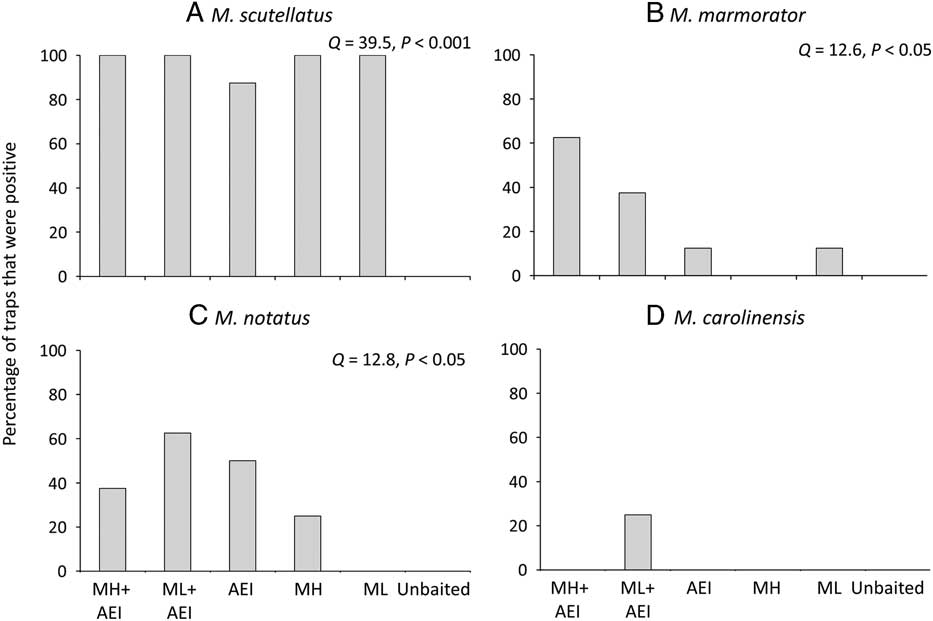
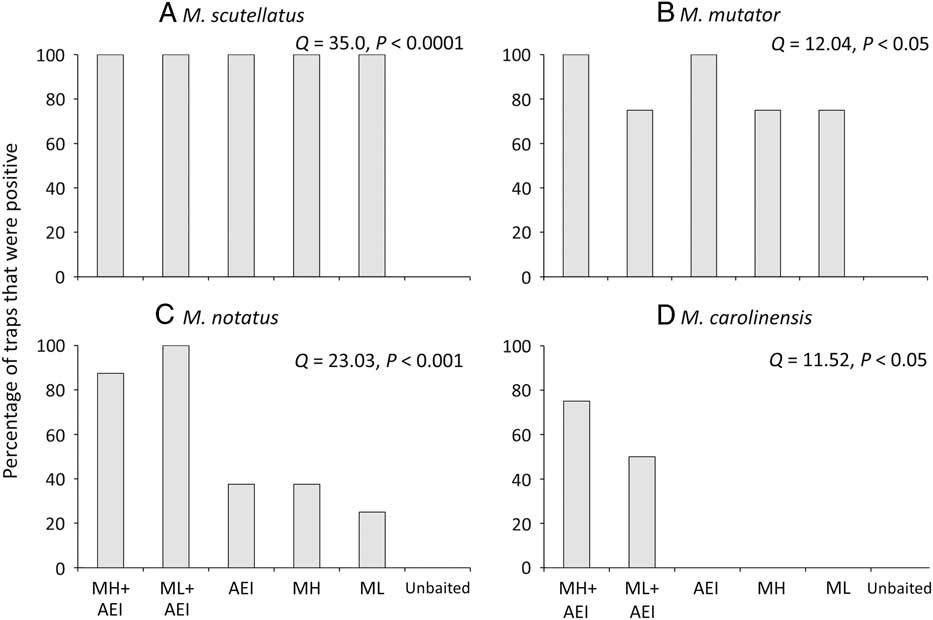
The proportion of traps that detected a given species differed significantly among lure treatments in both New Brunswick and Ontario. No Monochamus specimens were captured in unbaited traps. Monochamol or the kairomone blend of α–pinene, ethanol, and ipsenol by themselves were sufficient to detect the very common M. scutellatus in most or all trap blocks (New Brunswick: Q=39.5, P<0.001, Fig. 5A; Ontario: Q=35.0, P<0.0001, Fig. 6A). Similarly, all treatments, except the unbaited control, detected the common M. mutator in Ontario (Q=12.04, P<0.05, Fig. 6B). The proportion of traps that detected M. marmorator differed significantly among lure treatments in New Brunswick (Q=12.6, P<0.05); however, too few M. marmorator were captured in Ontario for analysis. The proportion of traps that captured M. notatus varied significantly among treatments in both New Brunswick (Q=12.8, P<0.05, Fig. 5C) and ON (Q=23.0, P<0.001, Fig. 6C) and at both locations, the greatest detection rate was in traps baited with low release monochamol plus kairomones (Figs. 5C, 6C). Finally, the proportion of traps that detected M. carolinensis in Ontario differed significantly among lure treatments (Q=11.5, P<0.05, Fig. 6D). All seven M. carolinensis specimens collected in Ontario were captured in traps baited with monochamol plus kairomones. Too few traps detected M. carolinensis in New Brunswick to analyse statistically. For the less common species, presence of the kairomones may be more critical than monochamol. For example, in New Brunswick, traps baited with monochamol alone at the low release rate failed to detect M. notatus, and traps baited with monochamol alone at the high release rate failed to detect M. marmorator, whereas both species were detected in at least some traps baited with kairomones alone (Figs. 5B, 5C). Traps baited with a combination of monochamol and kairomones had the highest detection rate of M. notatus, M. marmorator, and M. carolinensis (Figs. 5B–5D, 6C, 6D).
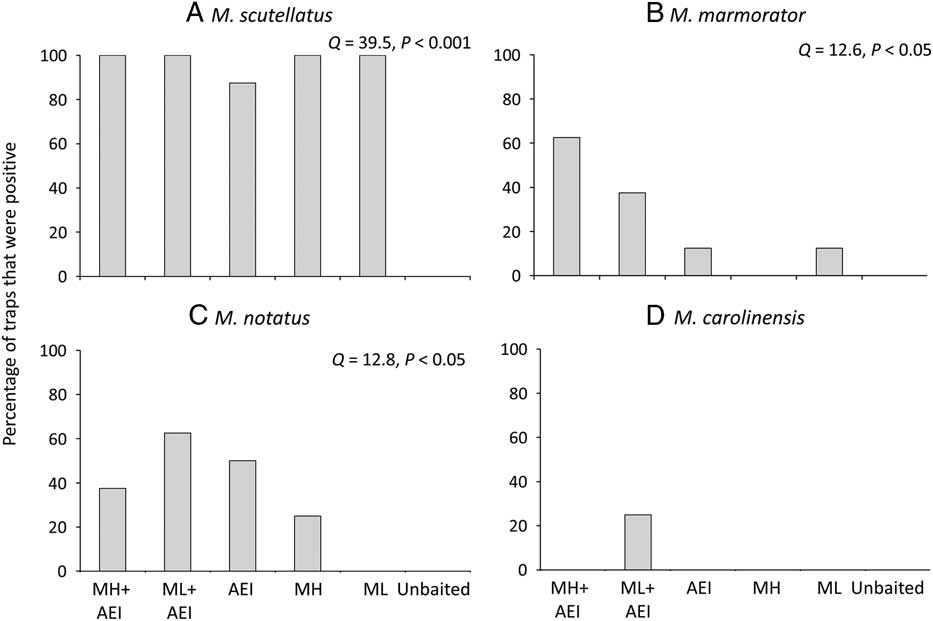
Fig. 5 Percentage of traps baited with monochamol emitted at either high (MH) or low release rate (ML), kairomones (α-pinene, ethanol, and ipsenol=AEI), or combinations thereof, that captured at least one specimen of (A) M. scutellatus, (B) M. marmorator, (C) M. notatus, and (D) M. carolinensis in field trapping experiments in New Brunswick, Canada in 2012.
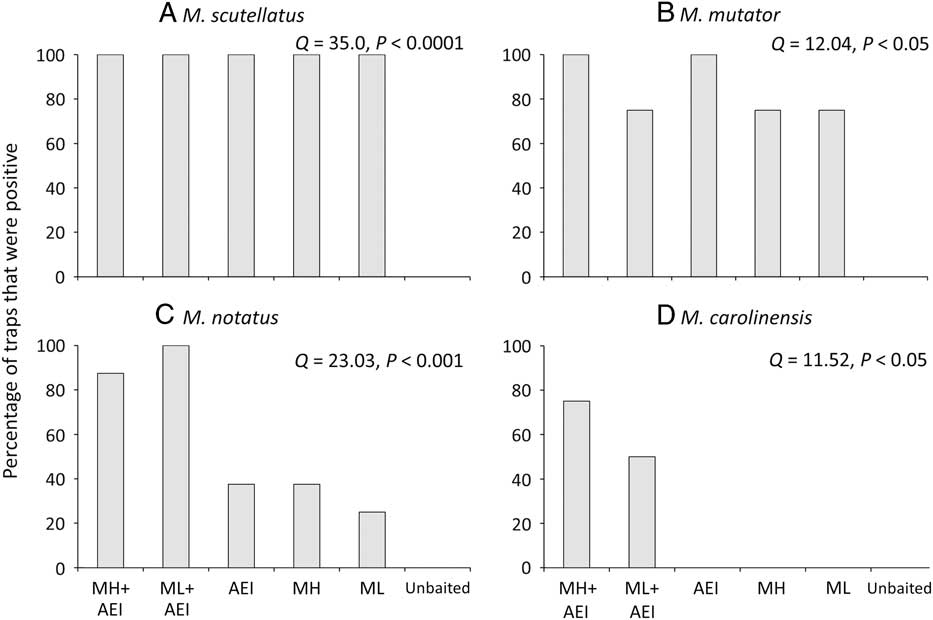
Fig. 6 Percentage of traps baited with monochamol emitted at either high (MH) or low release rate (ML), kairomones (α-pinene, ethanol, and ipsenol=AEI), or combinations thereof, that captured at least one specimen of (A) M. scutellatus, (B) M. mutator, (C) M. notatus, and (D) M. carolinensis in field trapping experiments in Ontario, Canada in 2012.
Experiment 3: effect of monochamol, fuscumol and fuscumol acetate, and cubeb oil on trap captures of Monochamus species
We captured 83 specimens of M. saltuarius, eight specimens of M. urussovii, and four specimens of M. sutor at the site in Jilin province, China. Lure treatment significantly affected mean total catch per trap of M. saltuarius (K-W χ2=24.17, df=6, P<0.0002) (Fig. 7A) as well as the proportion of traps that captured that species (Q=35.9, P<0.001) (Fig. 7B). Monochamol was significantly attractive to M. saltuarius, but the combination of (E/Z)-fuscumol and (E/Z)-fuscumol acetate was not attractive on its own and did not increase catches when added to traps baited with monochamol. Although not statistically significant, the addition of cubeb oil to traps baited with monochamol tended to reduce trap captures of M. saltuarius compared with traps baited with monochamol alone (Fig. 7A).
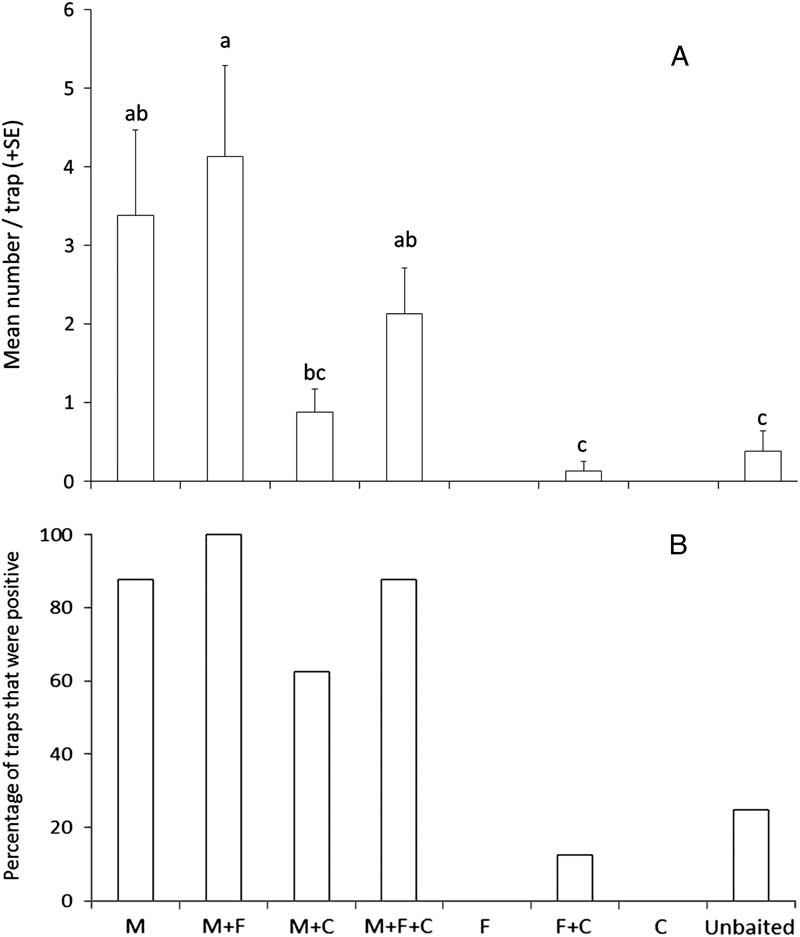
Fig. 7 Capture of M. saltuarius in intercept traps baited with monochamol (M), (E/Z)-fuscumol+(E/Z)-fuscumol acetate (F), cubeb oil (C), or left unbaited (blank) in the Jiaohe Administration Bureau of the Forest Experimental Zone, Jilin province, China, 2013. Each treatment was replicated eight times. (A) Mean total catch per trap. Treatments with zero captures were excluded from analysis. Means with different letters are significantly different among treatments (P⩽0.05); (B) Percentage of traps that captured at least one M. saltuarius.
All specimens of M. urussovii and M. sutor were captured in traps baited with monochamol or monochamol plus either cubeb oil or (E/Z)-fuscumol plus (E/Z)-fuscumol acetate, but the proportion of traps that detected either species did not differ significantly among lure treatments (Q=8.6, P>0.05). No specimens of M. guttatus were captured.
Discussion
There is an increasing body of evidence for the use of pheromones in the Cerambycidae (Lacey et al. Reference Lacey, Ginzel, Millar and Hanks2004, Reference Lacey, Ginzel, Moreira and Hanks2009; Liendo et al. Reference Liendo, Morillo, Sanches, Munoz, Guerra and Cabrera2005; Silk et al. Reference Silk, Sweeney, Wu, Price, Gutowski and Kettela2007; Ray et al. Reference Ray, Millar, McElfresh, Swift, Barbour and Hanks2009, Reference Ray, Zunic, Alten, McElfresh, Hanks and Millar2011; Fonseca et al. Reference Fonseca, Vidal and Zarbin2010; Nehme et al. Reference Nehme, Keena, Zhang, Baker, Xu and Hoover2010; Rodstein et al. Reference Rodstein, Millar, Barbour, McElfresh, Wright and Barbour2011). Moreover, there is evidence for cross-attraction of multiple species of cerambycid species to the same pheromone compounds (e.g., Hanks et al. Reference Hanks, Millar, Mongold-Diers, Wong, Meier and Reagel2012). We provide further evidence here that monochamol significantly influences mean trap captures of several Monochamus species and that attraction is increased and sometimes synergised when monochamol is combined with kairomones such as α-pinene, ethanol, and ipsenol. Our results support recent reports demonstrating the attraction to monochamol for three of the species: M. scutellatus (Fierke et al. Reference Fierke, Skabeikis, Millar, Teale, McElfresh and Hanks2012; Hanks et al. Reference Hanks, Millar, Mongold-Diers, Wong, Meier and Reagel2012; Macias-Samano et al. Reference Macias-Samano, Wakarchuk, Millar and Hanks2012); M. notatus (Fierke et al. Reference Fierke, Skabeikis, Millar, Teale, McElfresh and Hanks2012); and M. carolinensis (Allison et al. Reference Allison, McKenney, Millar, McElfresh, Mitchell and Hanks2012; Hanks et al. Reference Hanks, Millar, Mongold-Diers, Wong, Meier and Reagel2012). This is the first study to demonstrate attraction of M. saltuarius and M. urussovii to monochamol, and the first to provide potential evidence of attraction to monochamol by M. mutator and M. marmorator. Our data suggest that these species may also use monochamol as a pheromone component, but this must be confirmed by identification of monochamol in the effluvia of males. When combined with previous studies on M. galloprovincialis (Pajares et al. Reference Pajares, Alvarez, Ibeas, Gallego, Hall and Fahman2010), M. alternatus (Teale et al. Reference Teale, Wickham, Zhang, Chen, Hanks and Millar2011), M. titillator (Allison et al. Reference Allison, McKenney, Millar, McElfresh, Mitchell and Hanks2012), M. clamator and M. obtusus (Macias-Samano et al. Reference Macias-Samano, Wakarchuk, Millar and Hanks2012), and M. sutor (Pajares et al. Reference Pajares, Alvarez, Hall, Douglas, Centeno and Ibarra2013), there is now evidence that monochamol is attractive to 12 species within the genus and has excellent potential as a surveillance tool for survey and detection of Monochamus species.
Our results further demonstrated a synergism between monochamol and host volatiles for trap captures of several species of Monochamus, supporting results of previous studies. For all species except M. saltuarius, highest mean trap captures were observed in treatments that combined monochamol with host volatiles, in particular α-pinene and ethanol. Allison et al. (Reference Allison, McKenney, Millar, McElfresh, Mitchell and Hanks2012) also found that traps baited with the combination of monochamol plus α-pinene caught significantly more M. carolinensis and M. titillator than traps baited with either α-pinene or monochamol alone, although monochamol alone was also significantly attractive to M. carolinensis. Similar synergism of attraction to monochamol and kairomones has been reported for M. alternatus (Teale et al. Reference Teale, Wickham, Zhang, Chen, Hanks and Millar2011), M. galloprovincialis (Pajares et al. Reference Pajares, Alvarez, Ibeas, Gallego, Hall and Fahman2010), and M. sutor (Pajares et al. Reference Pajares, Alvarez, Hall, Douglas, Centeno and Ibarra2013) as well as other cerambycids, for example, Tetropium fuscum (Silk et al. Reference Silk, Sweeney, Wu, Price, Gutowski and Kettela2007; Sweeney et al. Reference Sweeney, Silk, Gutowski, Wu, Lemay and Mayo2010). Macias-Samano et al. (Reference Macias-Samano, Wakarchuk, Millar and Hanks2012) reported that traps baited with monochamol, α-pinene, and ethanol caught significantly more M. scutellatus, M. clamator, and M. obtusus than those baited with α-pinene and ethanol, but they did not test response to traps baited with monochamol alone. The addition of manuka oil to traps baited with monochamol did not significantly enhance captures of M. scutellatus in New Brunswick or M. urussovii in Poland, nor did the addition of cubeb oil increase captures of M. saltuarius in China. These results suggest that these complex blends of sesquiterpenes and monoterpenes are not suitable kairomones for the Monochamus species present at these sites and may be perceived as non-host volatiles.
We did not test ipsenol on its own versus combinations with monochamol or α-pinene+ethanol, and thus cannot determine its relative influence on trap capture of the species in our study. In experiment 1, which did not include ipsenol, we found that addition of α-pinene and ethanol to traps baited with monochamol was sufficient to increase captures of M. scutellatus; Macias-Samano et al. (Reference Macias-Samano, Wakarchuk, Millar and Hanks2012) demonstrated that the addition of ipsenol and ipsdienol to traps baited with monochamol, α-pinene, and ethanol did not increase catches of M. scutellatus or M. obtusus, but did increase captures of M. clamator. Thus, future experiments are necessary to elucidate the relative roles of host volatiles and bark beetle pheromones in attraction and trapping of Monochamus species.
The combination of fuscumol and fuscumol acetate was not attractive to Monochamus species in Jilin and did not significantly affect captures when added to traps baited with monochamol, cubeb oil, or both. Although these pheromones do not appear to increase the efficacy of detecting Monochamus species, neither did they reduce attraction. Fuscumol and fuscumol acetate have been shown to attract other species in the Lamiinae (Mitchell et al. Reference Mitchell, Graham, Wong, Reagel, Striman and Hughes2011; Wong et al. Reference Wong, Mitchell, Striman, Millar and Hanks2012) and Spondylidinae subfamilies (Silk et al. Reference Silk, Sweeney, Wu, Price, Gutowski and Kettela2007; Sweeney et al. Reference Sweeney, Silk, Gutowski, Wu, Lemay and Mayo2010) and thus may be useful in multi-component lures with monochamol for general trapping surveys of cerambycid beetles.
We found little or no effect of monochamol release rate on trap captures when presented alone or when combined with α-pinene, ethanol, and ipsenol. Response to pheromone release rate may be influenced by the kairomone lures deployed on the same traps: Pajares et al. (Reference Pajares, Alvarez, Ibeas, Gallego, Hall and Fahman2010) found that higher release rates of monochamol (0.16 versus 0.74 mg/day) increased captures of M. galloprovincialis in traps that were also baited with ipsdienol and 2-methyl-3-buten-2-ol but not in traps that were baited with ipsenol, 2-methyl-3-buten-2-ol, and α-pinene. Other studies used a single release rate of 0.7 mg/day with 95 mg/lure (Macias-Samano et al. Reference Macias-Samano, Wakarchuk, Millar and Hanks2012), 25 mg/lure (Teale et al. Reference Teale, Wickham, Zhang, Chen, Hanks and Millar2011; Fierke et al. Reference Fierke, Skabeikis, Millar, Teale, McElfresh and Hanks2012; Hanks et al. Reference Hanks, Millar, Mongold-Diers, Wong, Meier and Reagel2012; Hanks and Millar 2013) or 100 μg/hour with 50 mg/lure (Allison et al. Reference Allison, McKenney, Millar, McElfresh, Mitchell and Hanks2012) and found it was sufficient to elicit attraction. Overall, these results suggest that a lower, less expensive dose of the pheromone may suffice in monitoring programmes if combined with appropriate host volatile, thereby reducing the costs of survey and monitoring programmes for these species; however, additional testing is required.
Combined with previous studies, there is now evidence that monochamol is attractive to a dozen species of Monochamus. Increases in trap captures were most evident when combined with various host volatiles and kairomones. Further research is required to elucidate the optimum combination of pheromone, host volatiles, and bark beetle kairomones to maximise attraction to baited traps, to increase our understanding of the chemical ecology of these species and to develop lures for future use in detection and monitoring programmes.
Acknowledgements
This work was supported in part by Natural Resources Canada, Canadian Forest Service, United States Department of Agriculture Animal and Plant Health Inspection Service, Ontario Ministry of Natural Resources, Canadian Food Inspection Agency, Forest Protection Limited, and SERG International. The authors thank Katelyn Strong and Lin Tian Xi for permission to conduct experiments in the Acadia Research Forest, New Brunswick, and the Jiaohe Administration Bureau, Jilin, China, respectively. For technical assistance in the field and laboratory, the authors thank Chantelle Alderson, Chen Yibao, David Dutkievicz, Hugh Evans, Cory Hughes, Feng Li Chao, Isabelle Ochoa, Kathryn Nystrom, Lena van Seggelen, Krzysztof Sućko, Kalle Wainio, Vincent Webster, Wong Chong Shu, Li Lu Yao, Liu Yin, and Zhao Hong Rui. The authors thank Jeremy Allison and David Langor for constructive comments on an earlier draft of the manuscript. Finally, we thank Dave Davies for funding and support.