Clinoptilolite is a very common natural zeolite with the formula (Na,K,Ca)4Al6Si30O72·24H2O. It belongs to the heulandite group (Anielak, Reference Anielak2006). The unit cell of clinoptilolite is monoclinic with space group C2/m. Clinoptilolite has a higher Si/Al ratio (>4) and greater thermal stability than other zeolites of the heulandite group (Kitsopoulos, Reference Kitsopoulos2001; Sprynsky et al., Reference Sprynsky, Buszewski, Terzyk and Namiesnik2006; Pleśniak & Trzop, Reference Pleśniak and Trzop2016; Ambrozova et al., Reference Ambrozova, Kynicky, Urubek and Nguyen2017). The clinoptilolite structure consists of three types of channels. The A channels are made of ten-membered rings with a diameter of 0.31–0.76 nm. The C channels are limited by eight-membered rings with a diameter of 0.26–4.70 nm and are parallel to the A channels. The B channels, similarly to the C channels, are limited by eight-membered rings and intersect the A and B channels (Passaglia & Sheppard, Reference Passaglia, Sheppard, Bish and Ming2001; Ambrozova et al., Reference Ambrozova, Kynicky, Urubek and Nguyen2017).
Zeolites have acid–base and oxidation-reducing active centres that give them unique adsorptive and catalytic properties (Jamrozik et al., Reference Jamrozik, Gonet, Stryczek, Wojaczek and Maciołek2011; Kasperkowski, Reference Kasperkowski2014). The catalytic properties of clinoptilolite have been tested in the catalytic degradation of polyethylene, polypropylene and polystyrene polymers (Lee et al., Reference Lee, Woo, Chung, Kim, Lee and Lee2002b), isomerization of 1-butene to isobutene (Lee et al., Reference Lee, Yoon, Kim and Park2002a), isomerization of α-pinene (Allahverdiev et al., Reference Allahverdiev, Irandoust and Murzin1999), toluene oxidation (Pozan et al., Reference Pozan, Ozc and Boz2010) and photodegradation of pollutants (Zabihi-Mobarakeh & Nezamzadeh-Ejhieh, Reference Zabihi-Mobarakeh and Nezamzadeh-Ejhieh2015).
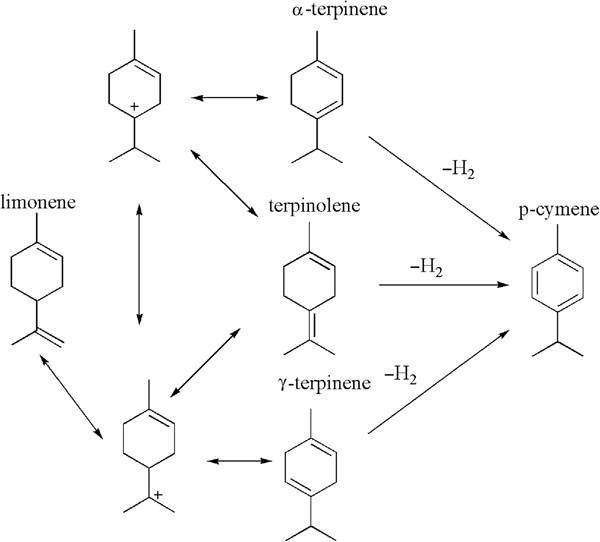
Limonene is the main component of orange oil, which is obtained from waste orange peel. It is an inexpensive terpene that may be transformed into valuable compounds via epoxidation, oxidation or isomerization processes (Wróblewska, Reference Wróblewska2014). Limonene isomerization yields γ-terpinene, α-terpinene and terpinolene as the main products. Moreover, dehydrogenation of the products of limonene isomerization and the formation of p-cymene are also observed (Fig. 1) (Retajczyk, Reference Retajczyk and Wróblewska2017).
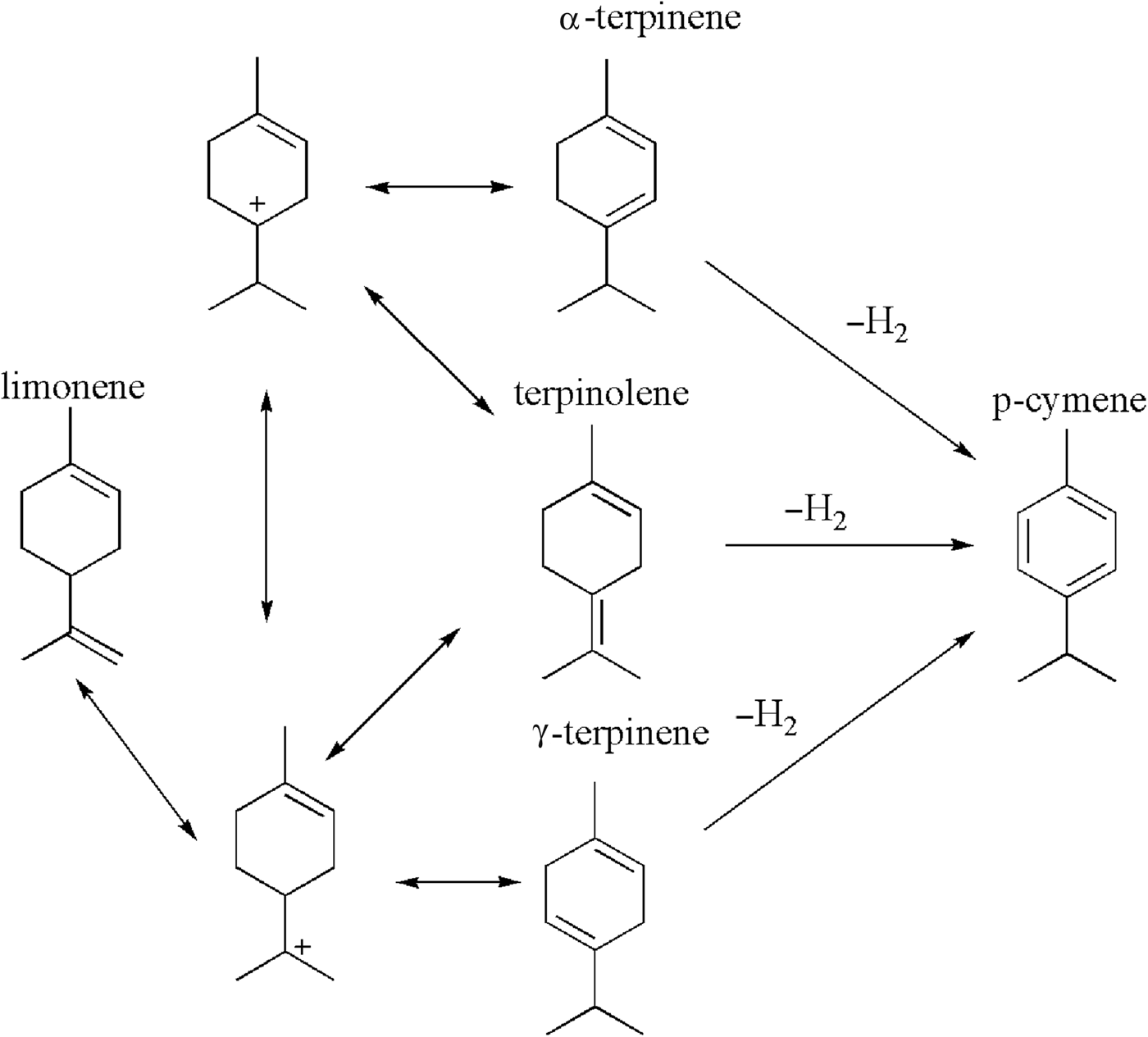
Fig. 1. The mechanism of the isomerization and dehydrogenation of limonene.
Among others approaches, the isomerization of limonene may be carried out using heterogeneous catalysts. This process was carried out using anatase as the catalyst (Johnson, Reference Johnson1985). Terpinolene was the main product obtained with a selectivity of 70%, and the conversion of limonene amounted to 55%. The isomerization was carried out at 100°C for 4 h in a nitrogen atmosphere and in the presence of sodium acetate buffer (Johnson, Reference Johnson1985).
The isomerization of limonene was also carried out using bentonites from Serra de Dentro as the catalysts (Catrinescu et al., Reference Catrinescu, Fernandes, Castilho and Breen2006). The most active catalyst was bentonite modified by Ni. During the isomerization, the following products were obtained: 20% terpinolene, 12% α-terpinene, 4% γ-terpinene and 8% isoterpinolene. The experiments were carried out at 150°C for 25 min in the presence of n-dodecane as the solvent.
Spanish sepiolite has been used for catalytic isomerization and dehydroaromatization of limonene to p-cymene (Martin-Luengo et al., Reference Martin-Luengo, Yates, Rojoa, Arribas, Aguilar and Hitzkya2010). In the isomerization process, microwave heating was used to obtain products in a short period of time. After 5 min at 210°C, the reaction products were 28% α-terpinene, 20% γ-terpinene and 27% α-terpinolene. The same catalyst, modified with Ni, yielded p-cymene with 100% selectivity and 100% conversion of limonene after 20 min of reaction.
In a recent contribution, the isomerization of limonene over the mesoporous titanium silicate catalyst Ti-SBA-15 was reported (Retajczyk & Wróblewska, Reference Retajczyk and Wróblewska2017). The main product of isomerization was α-terpinene, while the main product of dehydroaromatization was p-cymene. The yield of α-terpinene was 31.33 mol.% after 180 min at 160°C for 15 wt.% catalyst content, while the yield of p-cymene reached 50.66 mol.% after 1380 min of reaction time.
The products of limonene isomerization are used widely as additives in food, cosmetics or pharmaceuticals (Retajczyk & Wróblewska, Reference Retajczyk and Wróblewska2017). Terpinolene has excellent antibacterial and antifungal properties (Eftekhara et al., Reference Eftekhara, Yousefzadia, Aziziana, Sonbolib and Salehi2005; Lee et al., Reference Lee, Chen, Chen, Chang, Huang, Huang and Wang2013). Moreover, it may be used to treat depression (Ito & Ito, 2013) and in anticancer therapy (Aydin et al., Reference Aydin, Turkez and Tasdemir2013). p-Cymene is used for cresol production and also as a solvent for paints and varnishes, as a heat-transfer agent and as an additive to scents and musky perfumes (Roberge et al., Reference Roberge, Buhl, Niede, Niederer and Hölderich2001).
The purpose of this contribution was to determine the most favourable conditions for obtaining terpinolene and p-cymene during the process of isomerization of limonene over clinoptilolite. In this study, the influences of temperature, catalyst content and reaction time were tested. Limonene was selected because it is a cheap, easily available and renewable raw material (waste orange peel is the source of limonene with a purity of ~98%). Moreover, clinoptilolite used as the catalyst in the isomerization process is a cheap and readily available zeolite. In addition, limonene isomerization does not require the use of solvents; hence, it is environmentally friendly and a good alternative to other methods used to obtain terpinolene and p-cymene. The establishment of optimum conditions to obtain terpinolene and p-cymene will allow for the use of biomass waste (citrus peel) and price reductions of terpinolene and p-cymene.
Materials and methods
Raw materials
For the isomerization of limonene, the following raw materials were used: R-(+)-limonene (97%, Sigma, Kawasaki, Japan) and clinoptilolite (zeolite) as the catalyst (95% clinoptilolite, Turkey). During gas chromatography (GC) analyses, the following templates were used: terpinolene (>85%, Sigma Aldrich, Japan), α-terpinene (>85%, Aldrich, China), γ-terpinene (>97%, Aldrich, USA) and p-cymene (>99%, Aldrich, USA).
Characterization of clinoptilolite
Ultra-high-resolution field emission scanning electron microscope (UHR FE-SEM; Hitachi SU8020) coupled with energy-dispersive X-ray spectroscopy (EDX) analysis was used to determine the microstructure of clinoptilolite and the major chemical elements present in the zeolite. The chemical composition was determined with energy-dispersive X-ray fluorescence (ED-XRF) spectrometry (Epsilon 3 XLE XRF spectrometer, Malvern PANalytical). X-ray diffraction (XRD) analysis was carried out with an X'Pert PRO diffractometer (PANalytical) over the range 5–50°2θ with a 1°/min 2θ scanning step using Cu-Kα radiation. Joint Committee on Powder Diffraction Standards (JCPDS) cards were used to identify the phases present in the samples. The characterization of the porous texture was performed by N2 adsorption–desorption at 77 K using a Quadrasorb automatic adsorption system (Quantachrome Instruments). The specific surface areas were calculated by the multipoint Brunauer–Emmett–Teller (BET) method in the relative pressure p/p 0 range of 0.05–0.24 of the isotherms. Within this partial pressure range, the linearity of 1/(V[(p 0 / p) − 1]) versus the p/p 0 plot was satisfactory, where p 0 = 7622 Torr and V is the volume of gas adsorbed at p/p 0. The total pore volume was estimated at p/p 0 = 0.99. The Barrett, Joyner and Halenda (BJH) method was applied for calculating pore-size distributions using the desorption branch of the isotherms. The BJH method calculates the pore size distributions based on the modified Kelvin's equation:
where p/p 0 is the relative pressure at which capillary condensation occurs, γ is the vapour/liquid surface tension, V m is the molar volume of liquid nitrogen, r is the pore radius, r t is the thickness of the adsorbed layer, R is the gas constant and T is temperature. Micropore volume was estimated using the t-plot method. The thermogravimetric (TG) analysis was performed on an IMI instrument (Hiden Isochema) under nitrogen flow with a heating rate of 2°C/min.
Isomerization of limonene over the clinoptilolite catalyst
Studies of the influence of the reaction time, temperature and clinoptilolite content on limonene isomerization were performed in a 25 cm3 glass reactor equipped with a reflux condenser and a magnetic stirrer with a heating function. In the experiments, 5.00 g of limonene and an appropriate amount of clinoptilolite (0.25, 0.50 or 0.75 g, respectively) were inserted into the reactor, which was placed in the paw and then immersed in an oil bath. Limonene isomerization was tested at 175°C, 165°C and 155°C under stirring (500 rpm). Samples of the reaction mixture were taken after 15, 30, 60, 120, 180, 240, 300 and 1440 min of reaction.
Analysis by gas chromatography
The qualitative and quantitative analyses of the reaction mixtures were performed by GC using a FOCUS apparatus (Thermo Fisher Scientific, Waltham, MA, USA), equipped with a flame-ionization detector, a capillary column ZEBRON ZB-WAXplus type (0.32 mm × 30 m × 0.5 µm, filled with polyethylene glycol) and an autosampler. The parameters of the analyses were as follows: helium pressure = 60 kPa; temperature of the sample chamber = 240°C; detector temperature = 250°C; and thermostat temperature raised as follows: isothermally to 60°C for 2 min, increase of temperature at a rate of 10°C/min to 240°C, isothermally to 240°C for 4 min and finally cooling to 60°C. The samples for GC analysis were prepared as follows: the catalyst was separated from the reaction mixture by centrifugation and the solution was diluted with acetone to a 1:3 sample:acetone ratio. The quantitative analyses were performed with the external standard method. Subsequently, the mass balances for appropriate syntheses were counted and the main functions describing the process of isomerization of limonene were calculated.
Results and discussion
Characterization of clinoptilolite
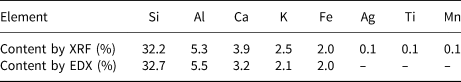
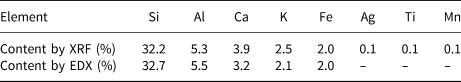
The chemical composition of clinoptilolite showing the abundances of the individual elements in clinoptilolite determined by XRF and SEM-EDX is listed in Table 1. The standard errors of the individual elements for EDX ranged from 0.03% to 0.25%.
Table 1. Chemical composition of clinoptilolite determined by XRF and SEM-EDX.
The elemental map shows the spatial distribution of elements in a clinoptilolite sample (Fig. 2). All elements are incorporated uniformly in the structure of clinoptilolite.
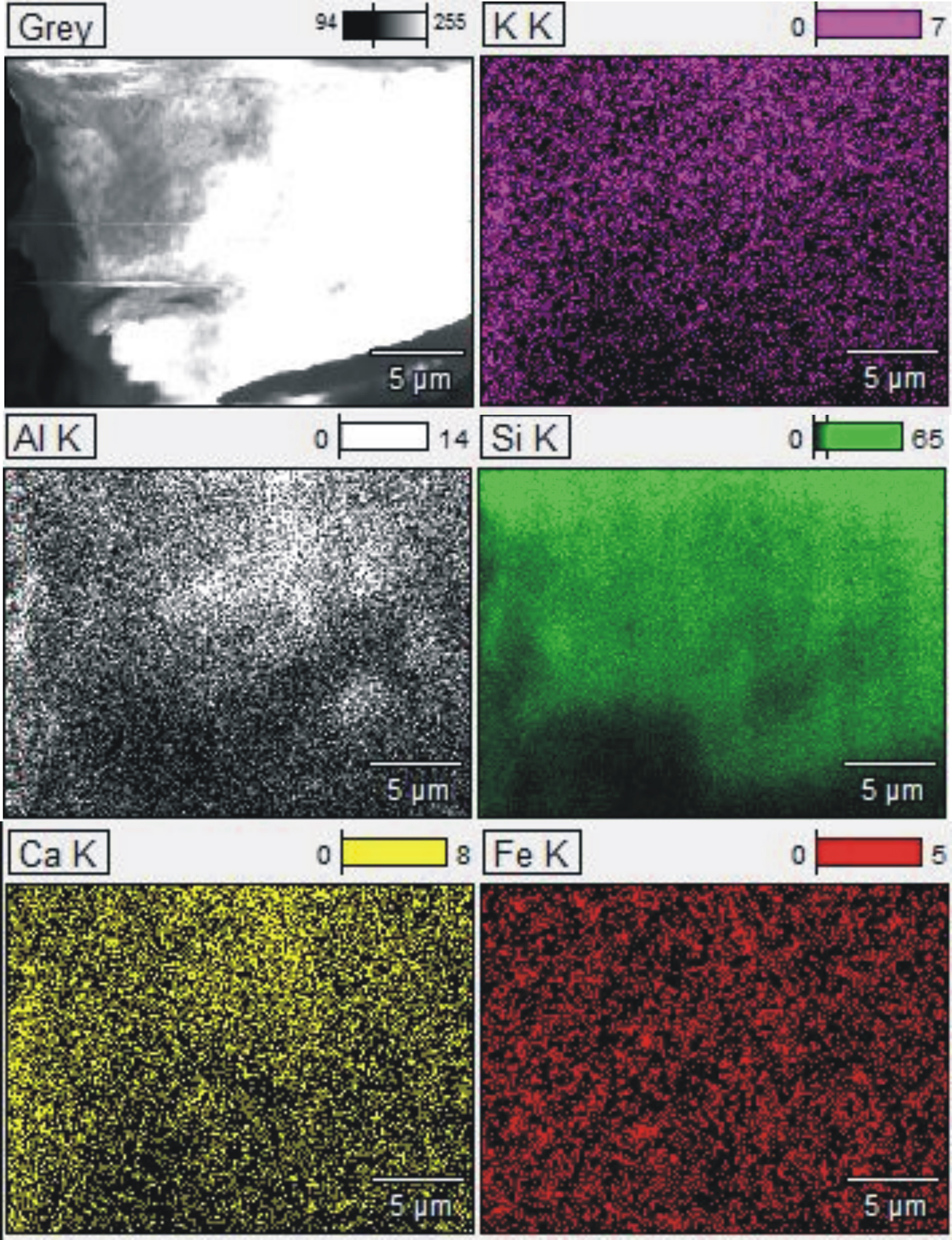
Fig. 2. EDX mapping images for K, Al, Si, Ca and Fe in clinoptilolite.
Using SEM, the original clinoptilolite is characterized by platy crystals of various sizes (Fig. 3).

Fig. 3. SEM image of the original clinoptilolite.
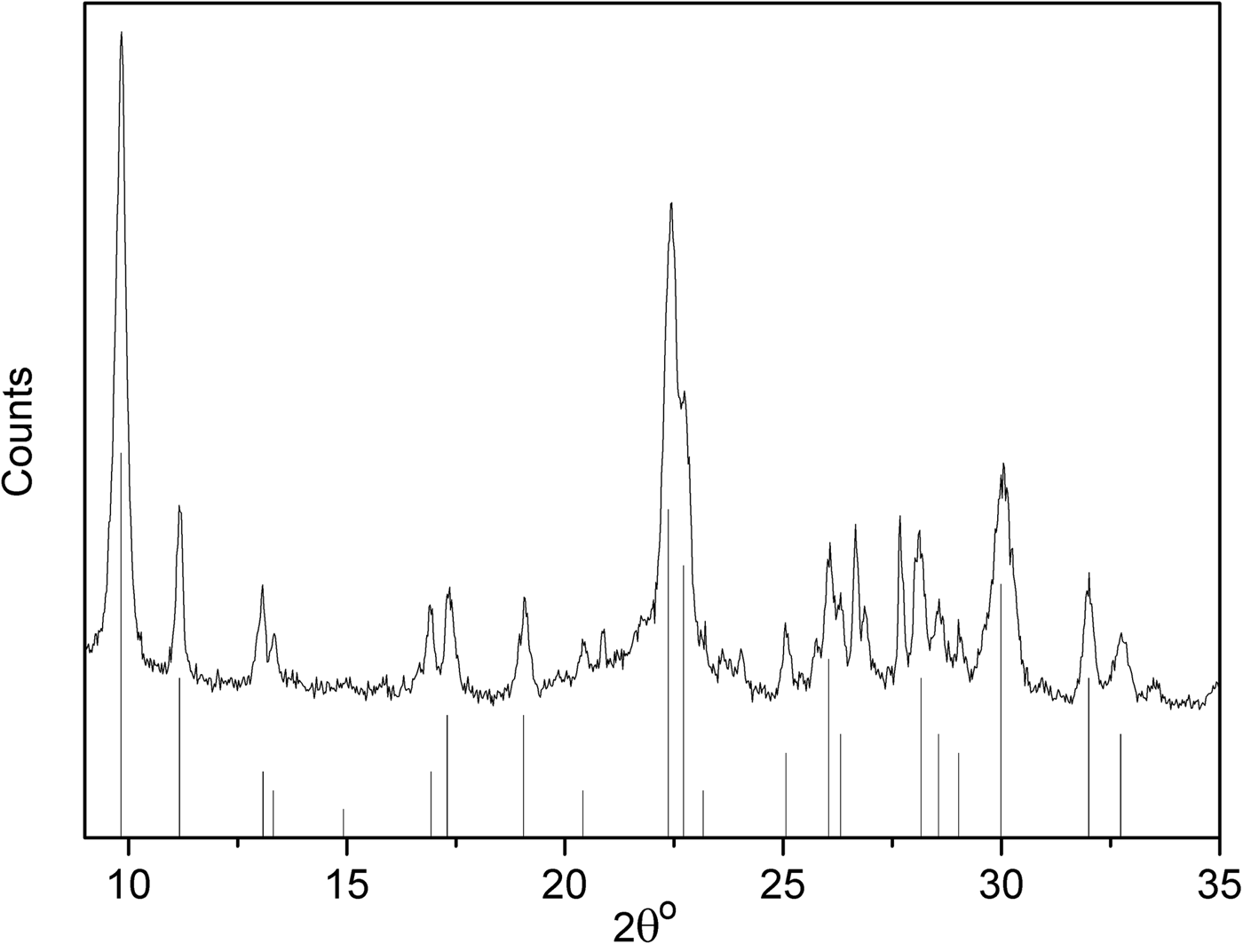
Figure 4 shows the XRD trace of the original clinoptilolite. The clinoptilolite is well crystallized and was fitted with the JCPDS card 25-1349 with the overall chemical formula: (Na,K,Ca)6(Si,Al)36O72·20H2O (Boles, Reference Boles1972). It is worth highlighting that Boles (1979) and others (Burlet, Reference Burlet1997; Leggo et al., Reference Leggo, Ledesert and Christie2006) argue that minor Fe might also be present, yielding the following structural formulae for clinoptilolite: Na3.78K1.31Ca0.61Mg0.23Fe0.15Al6.61Si29.19O72·20H2O (Boles, 1979) and K1.32Ca2.03Na0.4Mg0.32Fe0.05Al6.6Si29.4O72·22H2O (Burlet, Reference Burlet1997; Leggo et al., Reference Leggo, Ledesert and Christie2006).
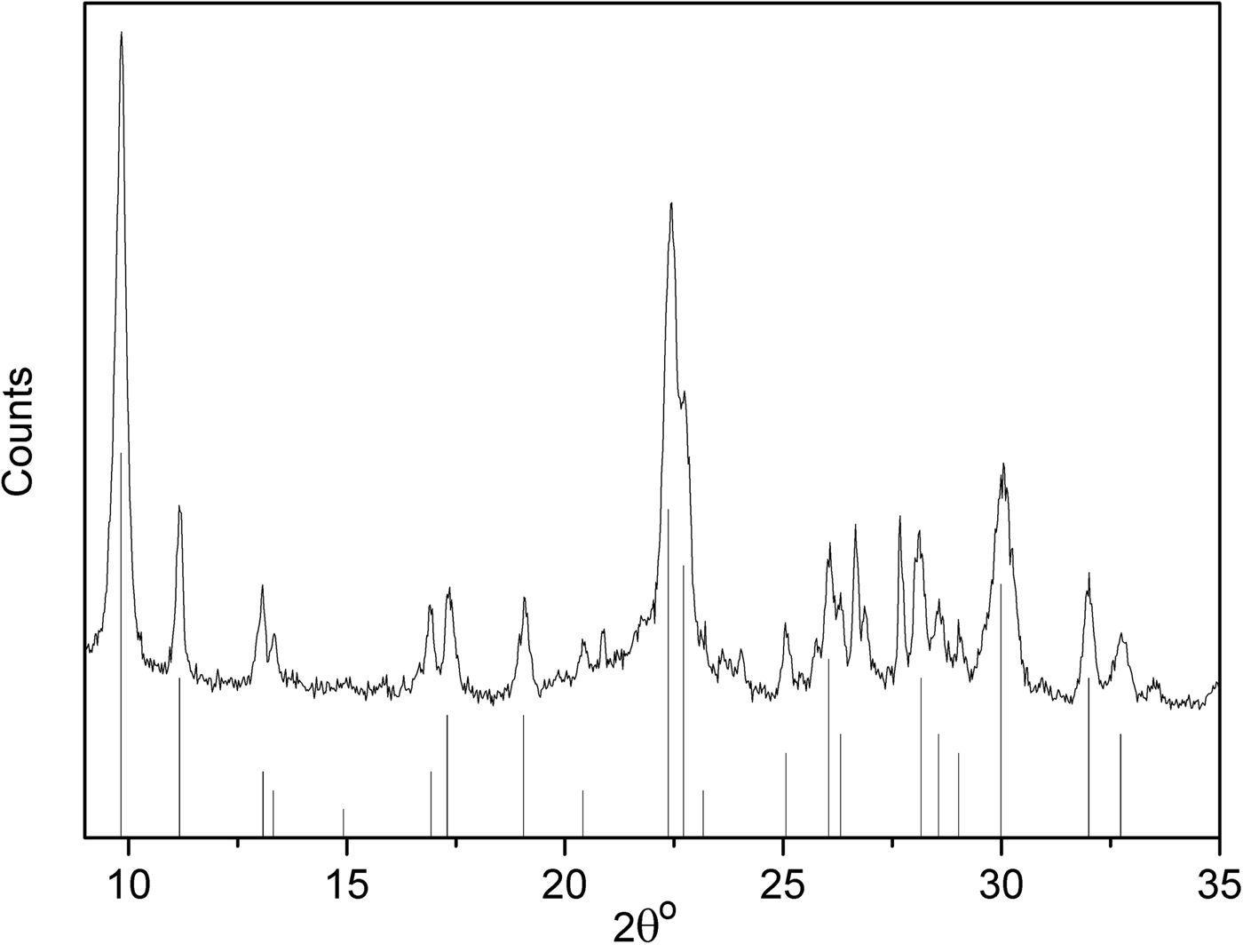
Fig. 4. XRD patterns of natural clinoptilolite. Vertical bars represent clinoptilolite patterns according to JCPDS card 25-1349.
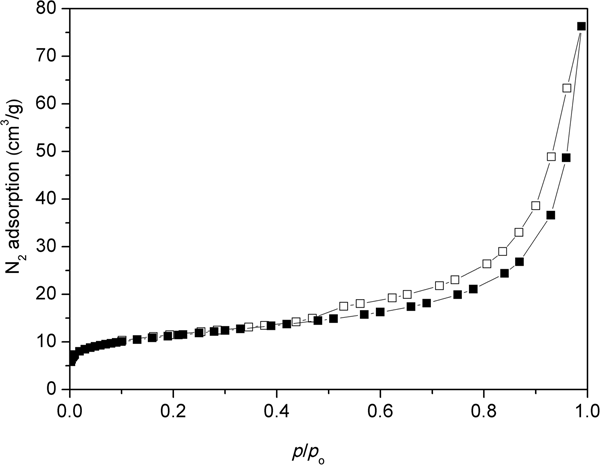
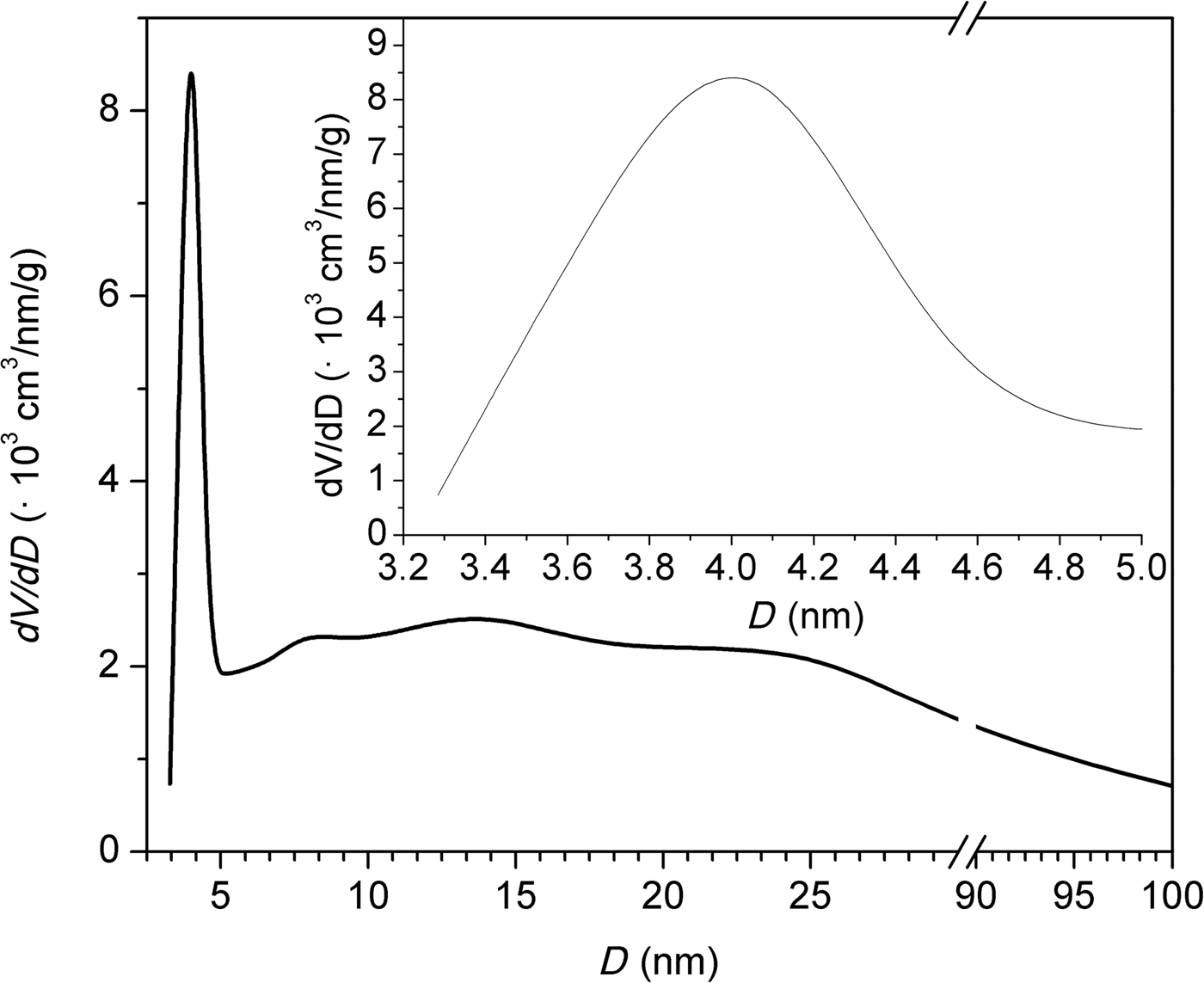
Figure 5 shows the N2 adsorption/desorption isotherm of the natural clinoptilolite at 77 K. The isotherm is of mixed types II and IV with an H3-type hysteresis loop. At low relative pressures (p/p 0 < 0.1), an initial small increase in N2 adsorption was observed. For relative pressures 0.1 < p/p 0 < 0.7, the nitrogen adsorption remained relatively constant, increasing rapidly at p/p 0 > 0.7. The desorption branch showed the presence of a hysteresis loop at 0.5 < p/p 0 < 1. Type IV isotherms are typical of mesoporous adsorbents and are irreversible (with a hysteresis loop) with a final saturation plateau, which is not observed in Fig. 5. Type II isotherms are usually reversible and represent unrestricted monolayer–multilayer adsorption. Adsorption increases without limit when p/p 0 = 1. Type II isotherms indicate physisorption of gases on nonporous or macroporous adsorbents. For an H3-type loop, the adsorption branch resembles a type II isotherm and the lower limit of the desorption branch indicates the beginning of the cavitation (Thommes et al., Reference Thommes, Kaneko, Neimark, Olivier, Rodriguez-Reinoso, Rouquérol and Sing2015). The sorption isotherms are indicative of non-rigid aggregates of plate-like particles or a pore network consisting of macropores that are not completely filled with pore condensate. Alternatively, the isotherms may indicate a mesoporous character represented by slit-shaped pores arising from the stacking of clinoptilolite crystals (Muir et al., Reference Muir, Matusik and Bajda2016). The shape of the adsorption curve above relative pressures of 0.5 indicates the presence of mesopores and macropores. The hysteresis loop is characteristic of clinoptilolite and may be attributed to multilayer adsorption and capillary condensation within the mesopore space (Kasture et al., Reference Kasture, Joshi, Soni, Joshi, Choudhari and Shiralkar1998). These findings are confirmed by pore-size distributions calculations using the BJH method (Fig. 6). The clinoptilolite displayed a dominant pore mode at ~4 nm. Both mesopores and macropores were observed. The specific surface area was 38 m2/g and the total pore volume was 0.118 cm3/g for a pore diameter of <170 nm. The micropore volume calculated by the t-plot method was very small (0.007 cm3/g).
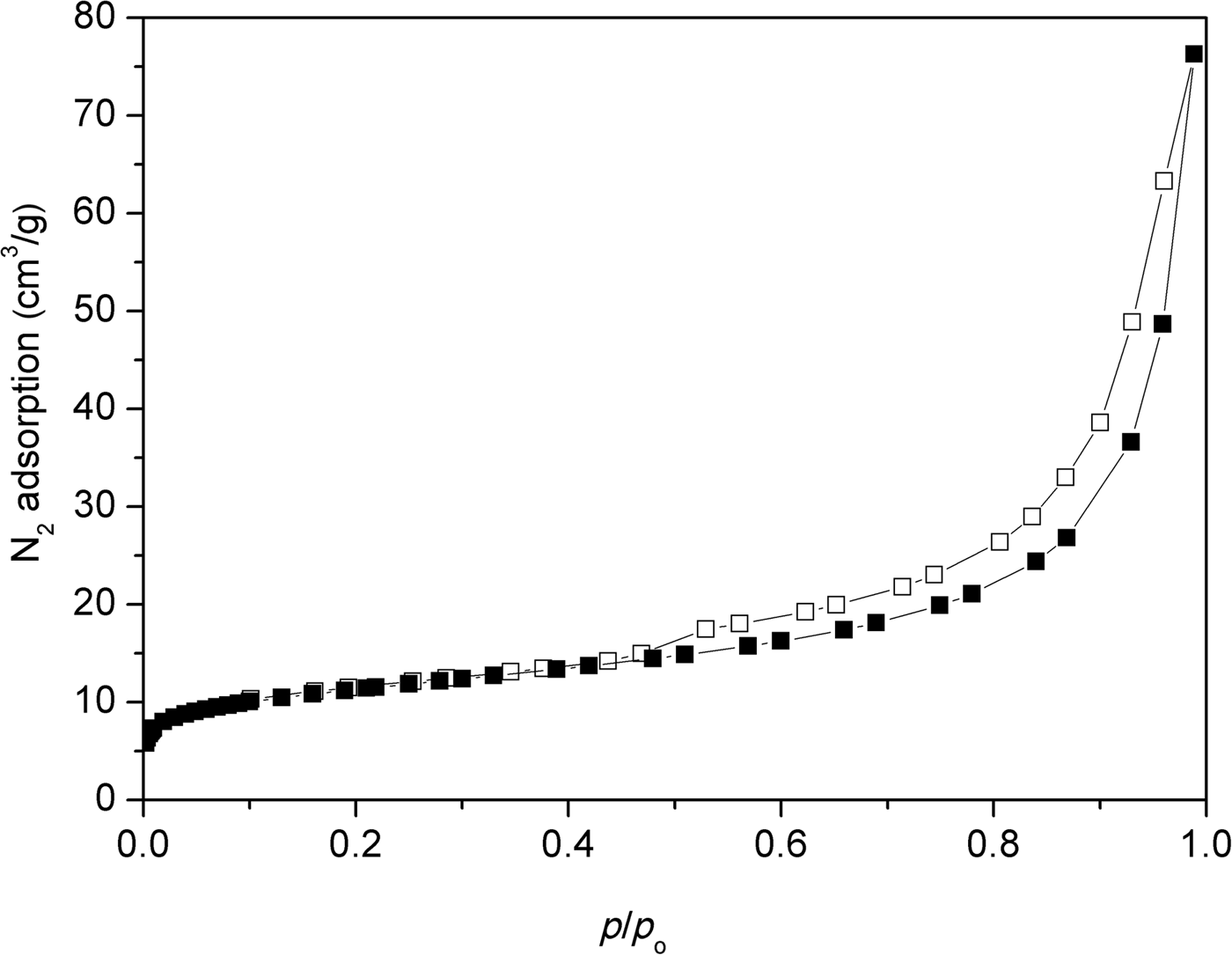
Fig. 5. N2 adsorption (solid symbols)–desorption (open symbols) isotherm of the original clinoptilolite.
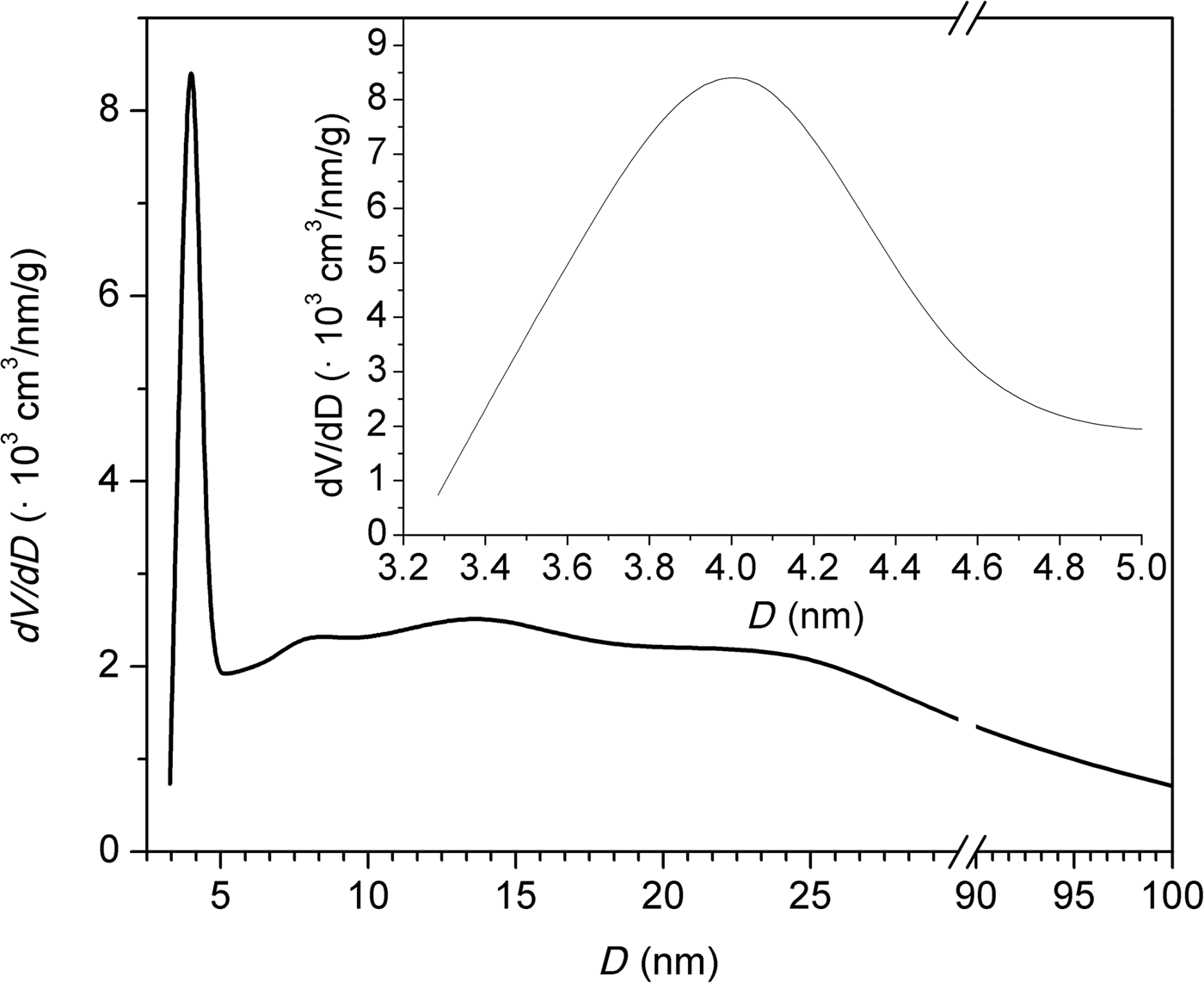
Fig. 6. Pore-size distributions calculated by the BJH method.
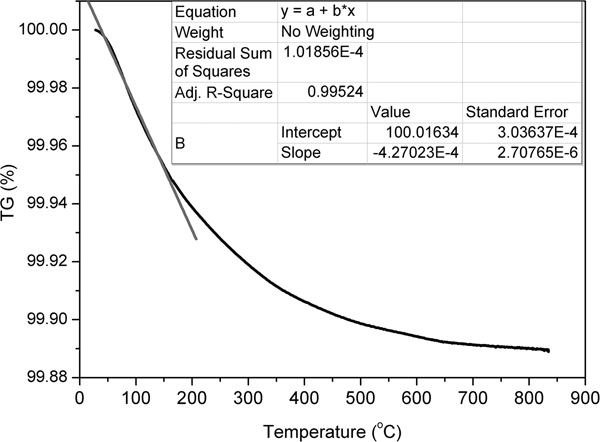
The thermal behaviour of clinoptilolite was investigated by TG analysis. The existence of various exchangeable cations in zeolites leads to different temperatures of zeolitic water release (Kasture et al., Reference Kasture, Joshi, Soni, Joshi, Choudhari and Shiralkar1998). The TG curve of original clinoptilolite is shown in Fig. 7. The fastest weight loss was observed up to 200°C and is attributed to release of zeolitic water. The inset table in Fig. 7 contains the results of linear interpolation of the initial section of the TG curve. The initial section of the TG curve may be approximated by a straight line with a slope of 0.0004. The rate of zeolitic water loss was 0.0004 wt.%/deg.
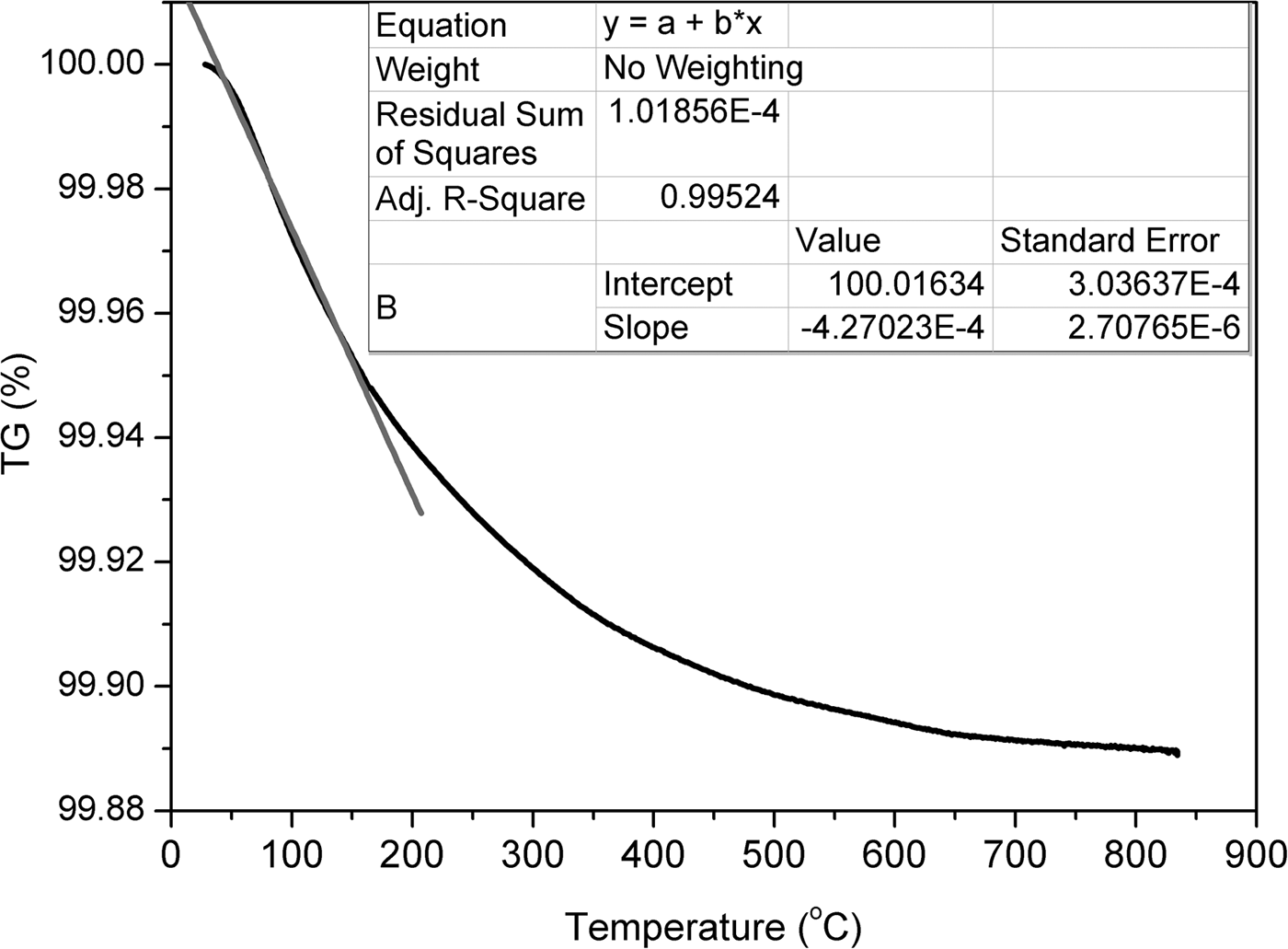
Fig. 7. TG curve of the clinoptilolite sample.
Isomerization of limonene
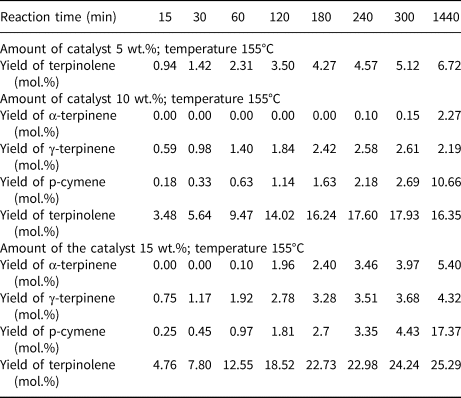
Table 2 shows the effects of reaction time and catalyst content (clinoptilolite) on the yields of the main products of isomerization of limonene at 155°C.
Table 2. The influence of reaction time and catalyst content on the yields of the main products of the isomerization of limonene at 155°C.
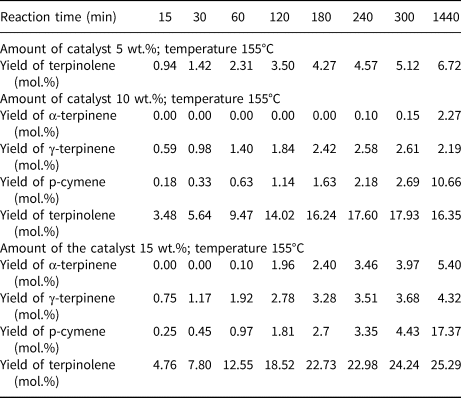
The low temperature of the isomerization/dehydroaromatization process (155°C) did not produce a high yield of the resulting products. At the lowest catalyst content (5 wt.%), the only reaction product is terpinolene, with a relatively small yield (6.72 mol.%) over the range of reaction times studied. At higher catalyst contents (10 and 15 wt.%), the products obtained were terpinolene, α-terpinene, γ-terpinene and p-cymene. At 15 wt.% catalyst content, the product yields were significantly higher compared to the yields for lower catalyst contents.
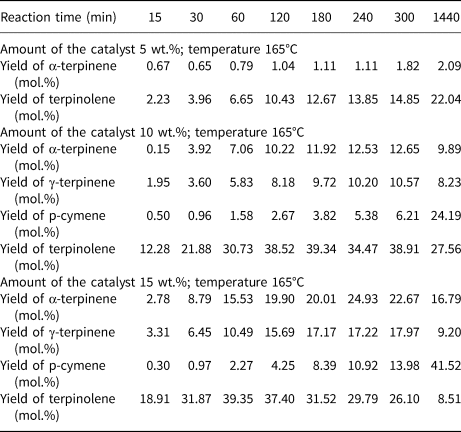
Table 3 presents the influence of reaction time and catalyst content on the yields of the main products of the isomerization of limonene at 165°C. At low catalyst contents (5 wt.%), only two products were obtained: α-terpinene and terpinolene. At 10 and 15 wt.% catalyst contents, three isomerization products and one dehydroaromatization product were obtained. At this temperature, high yields of terpinolene and p-cymene were obtained. With 15 wt.% catalyst content, a significantly higher yield of p-cymene (41.52 mol.%) was obtained compared to the yield of this product at 10 wt.% catalyst content (24.19 mol.%) for the reaction time of 1440 min.
Table 3. The influence of reaction time and catalyst content on the yields of the main products of the isomerization of limonene at 165°C.
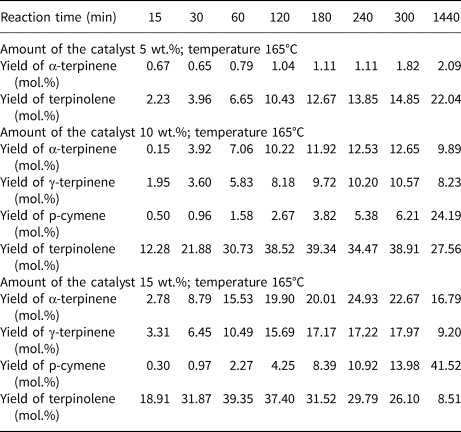
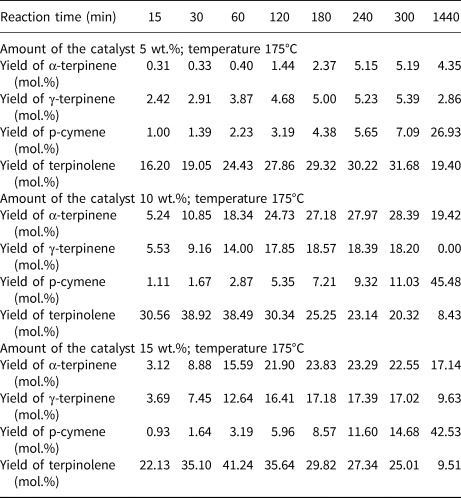
Table 4 lists the influence of reaction time and catalyst content on the yields of the main products of the isomerization of limonene at 175°C. Even at a low catalyst content (5 wt.%), all four products were obtained in the post-reaction mixtures. Although at 150°C and 165°C the yields of the products obtained increased with increasing catalyst content (Tables 2, 3), at 175°C, increasing the catalyst content from 10 to 15 wt.% did not have a significant impact on the product yields.
Table 4. The influence of reaction time and catalyst content on the yields of the main products of the isomerization of limonene at 175°C.
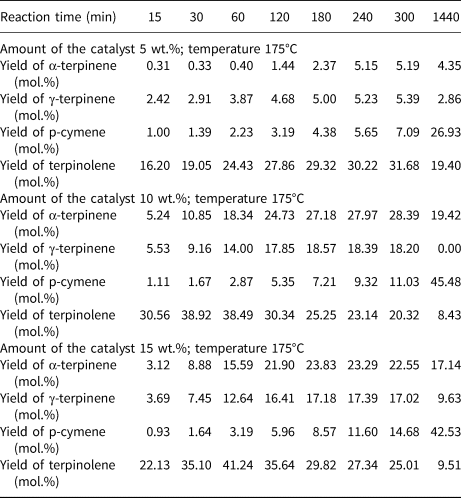
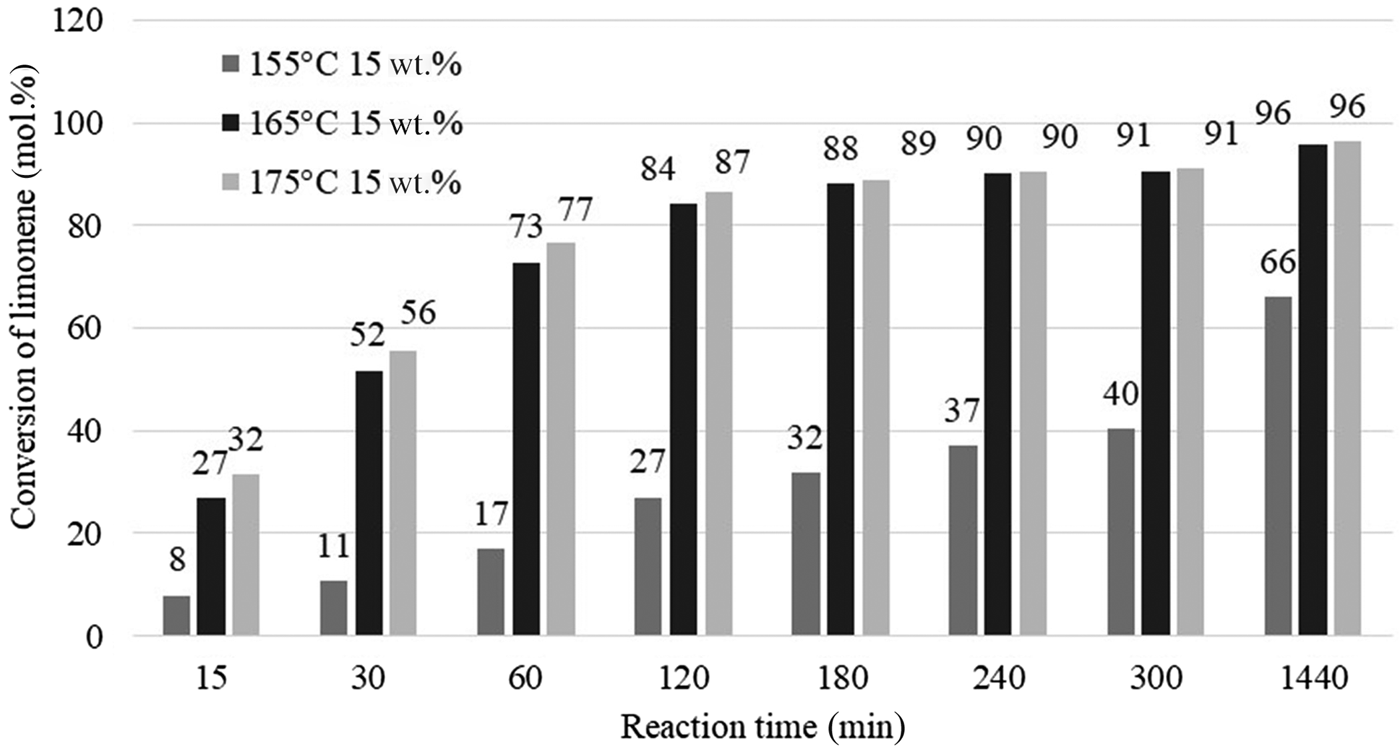
Figure 8 shows the influence of temperature and reaction time on the conversion of limonene for the catalyst content of 0.75 g (15 wt.% in relation to the mass of limonene) at 155°C, 165°C or 175°C and reaction times of 15–1440 min. The temperature of the isomerization/dehydroaromatization process has a significant effect on the conversion of the substrate. At high catalyst contents (15 wt.%) and at higher process temperatures (165°C and 175°C), the substrate conversions were similar over the range of reaction times studied.
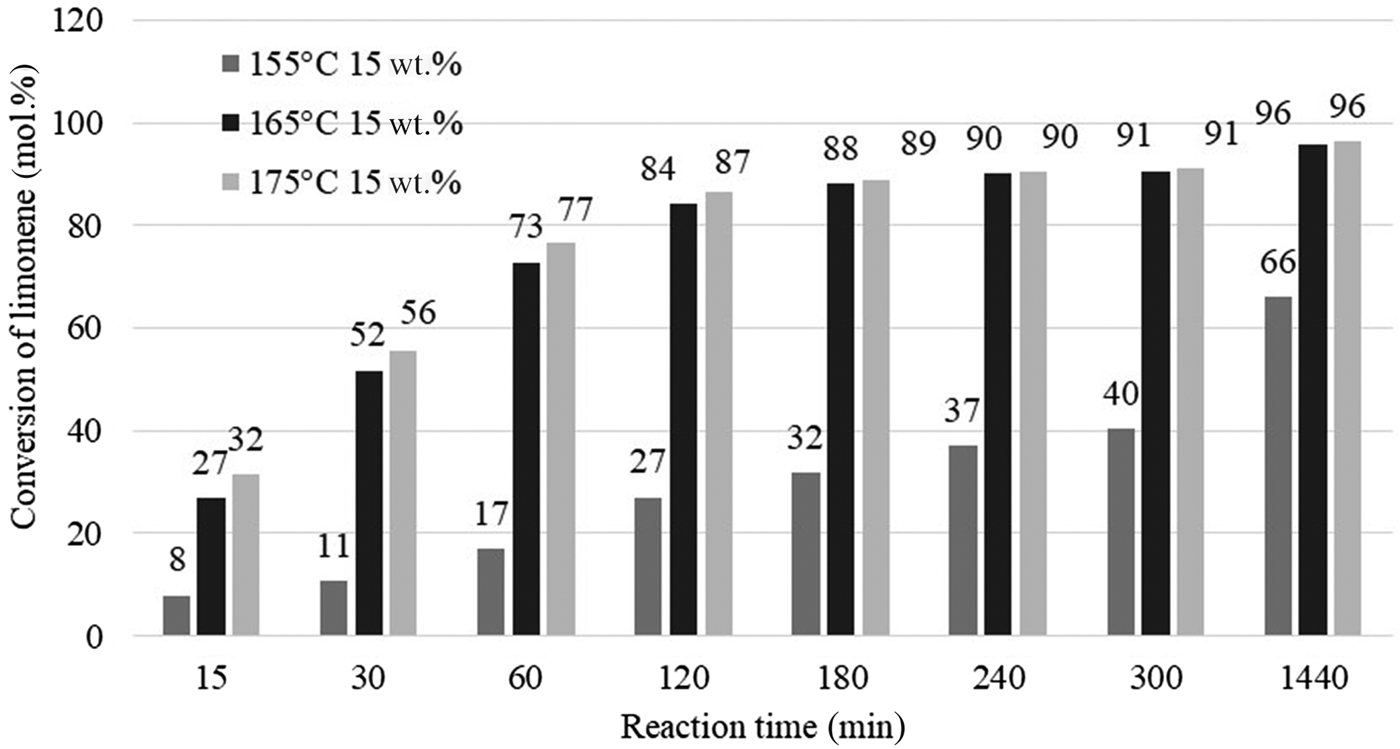
Fig. 8. Influence of temperature and reaction time on the conversion of limonene.
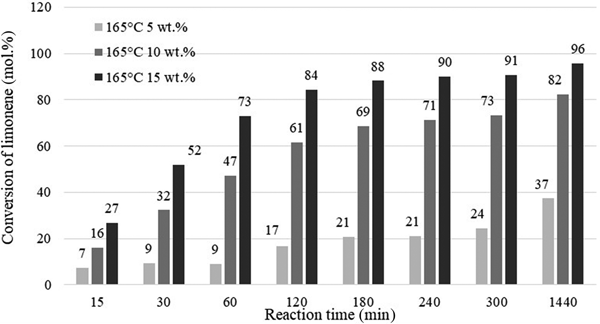
Figure 9 presents the influence of catalyst content and reaction time on the conversion of limonene for catalyst contents of 5, 10 or 15 wt.% (in relation to the mass of limonene) at 165°C and for reaction times of 15–1440 min. At 15 wt.% catalyst content, a substrate conversion rate of >90% was achieved after 240 min of reaction, whereas with lower catalyst contents, such limonene conversion was not achieved within the time period studied.
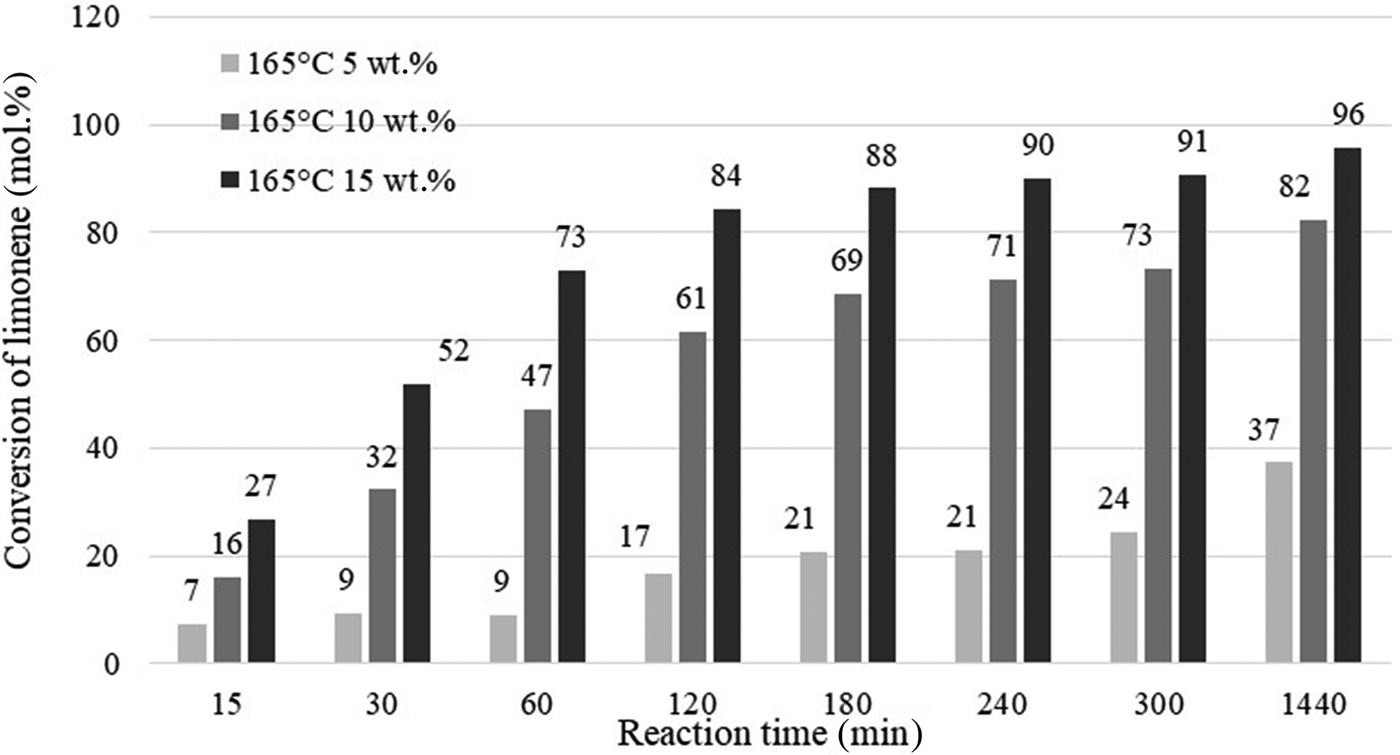
Fig. 9. Influence of the amount of catalyst and reaction time on the conversion of limonene at 165°C.
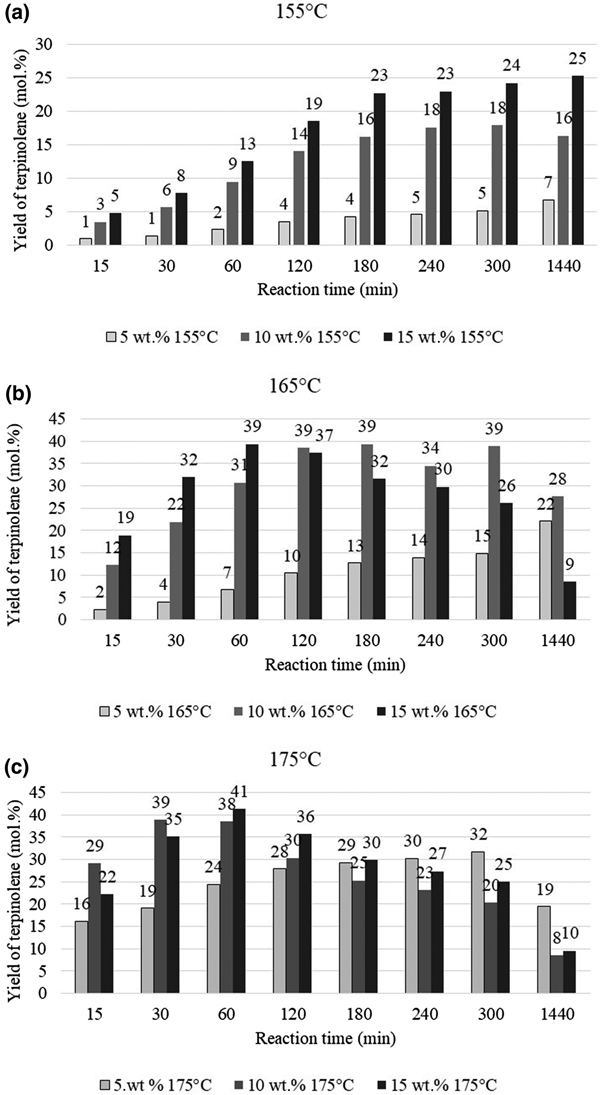
The main product of isomerization of limonene over clinoptilolite is terpinolene, and the yield of this compound increases with increasing catalyst content. This dependence is particularly evident at a lower temperature (155°C) (Fig. 10a). At 165°C and 175°C, the yield of the main isomerization product (terpinolene) is less dependent on the catalyst content. For catalyst contents of 10 and 15 wt.%, the yield of terpinolene is similar over the whole range of reaction times studied (Fig. 10b,c)
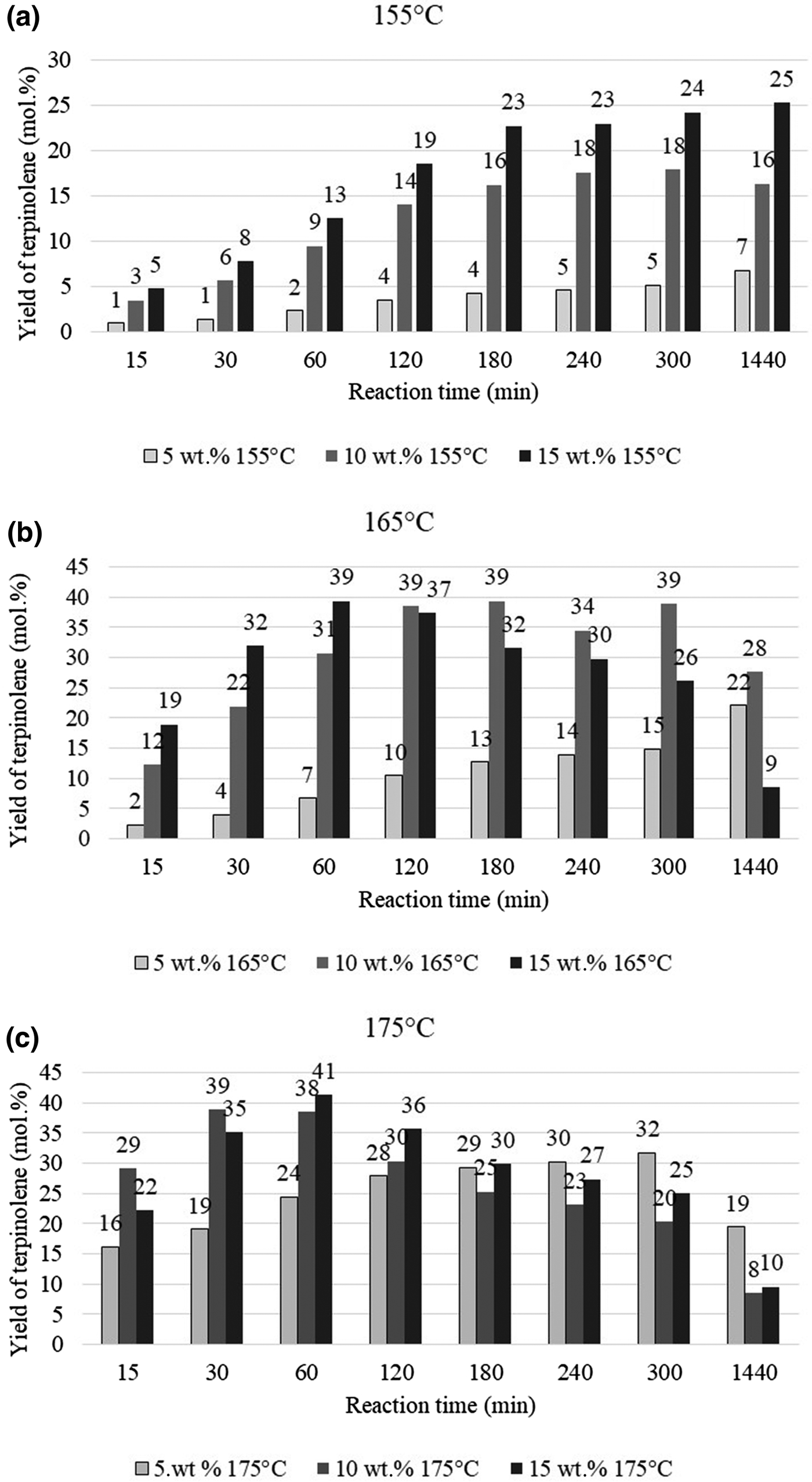
Fig. 10. Influence of the amount of catalyst and reaction time on the yield of terpinolene at (a) 155°C, (b) 165°C and (c) 175°C.
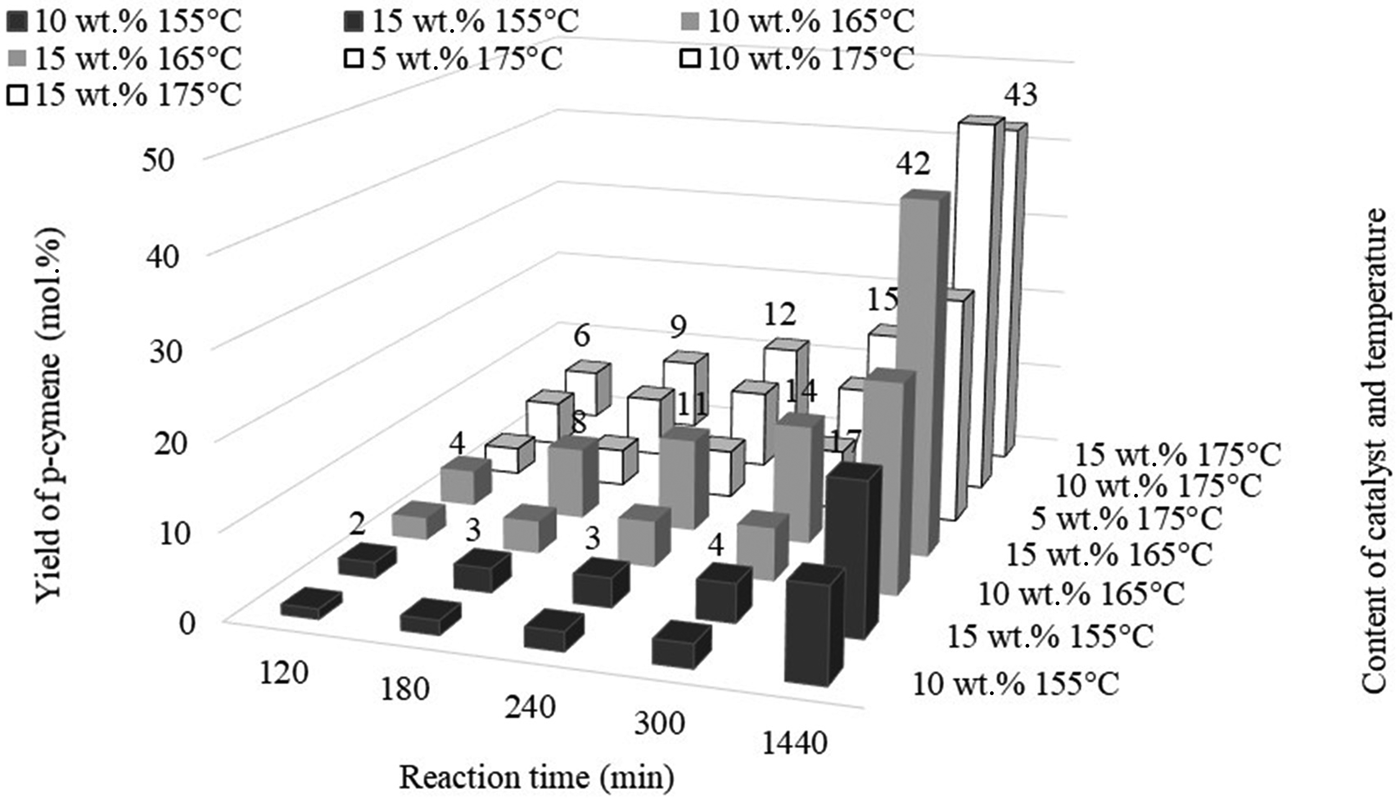
The greatest yield of terpinolene (41 mol.%) was obtained after 60 min at 15 wt.% catalyst content. However, a comparable yield of this compound (39 mol.%) was obtained after 30 min of reaction at 175°C and with 10 wt.% catalyst content. The yield of p-cymene, which is the product of the dehydroaromatization of limonene and its isomers, is heavily dependent on the amount of catalyst and the process temperature (Fig. 11).
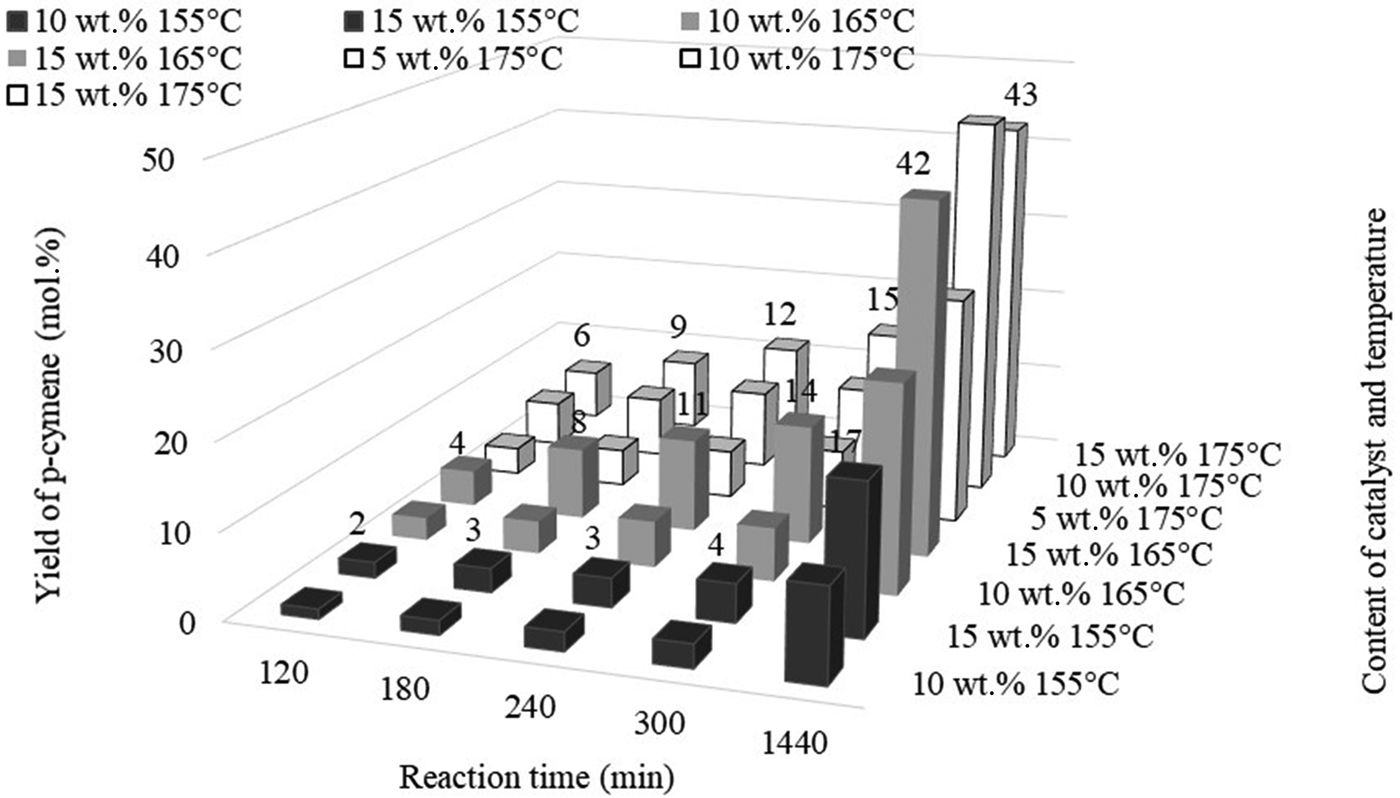
Fig. 11. Influence of the amount of catalyst and reaction time on the yield of p-cymene at 155°C, 165°C and 175°C.
Figure 11 shows that obtaining a dehydroaromatization product requires a longer reaction time. At low catalyst contents (5 wt.%), p-cymene is not obtained even after 1440 min of reaction. At a higher catalyst content (10 wt.%), it was possible to obtain p-cymene at 155°C; however, the most favourable conditions for obtaining significant amounts of p-cymene are 165°C for catalyst contents of 15 wt.% and 175°C for catalyst contents of 10 wt.%. At 175°C, a higher catalyst content (15 wt.%) produced almost the same yield of p-cymene as a catalyst content of 10 wt.%.
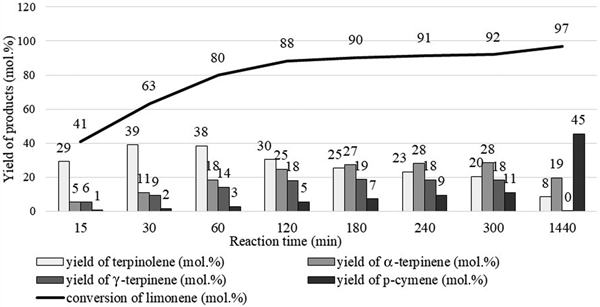
The dependence of product yield on reaction time is shown in Fig. 12. The limonene isomerization/dehydroaromatization products appeared in a specific order. Initially, the yield of terpinolene increased significantly, at which time the yield of α-terpinene and γ-terpinene also increased. The yield of terpinolene reached its highest value and then decreased. During this time, the yield of α-terpinene and γ-terpinene slowly increased up to a certain point. With the decrease in the yield of limonene isomers, the yield of p-cymene increased, confirming the validity of the assumed mechanism of this reaction. The optimum temperature for the isomerization of limonene over clinoptilolite is 175°C for catalyst contents of 10 wt.% because it allows high yields of the main isomerization products to be obtained over the shortest time. The use of greater catalyst contents (15 wt.%) did not improve the reaction yield significantly.
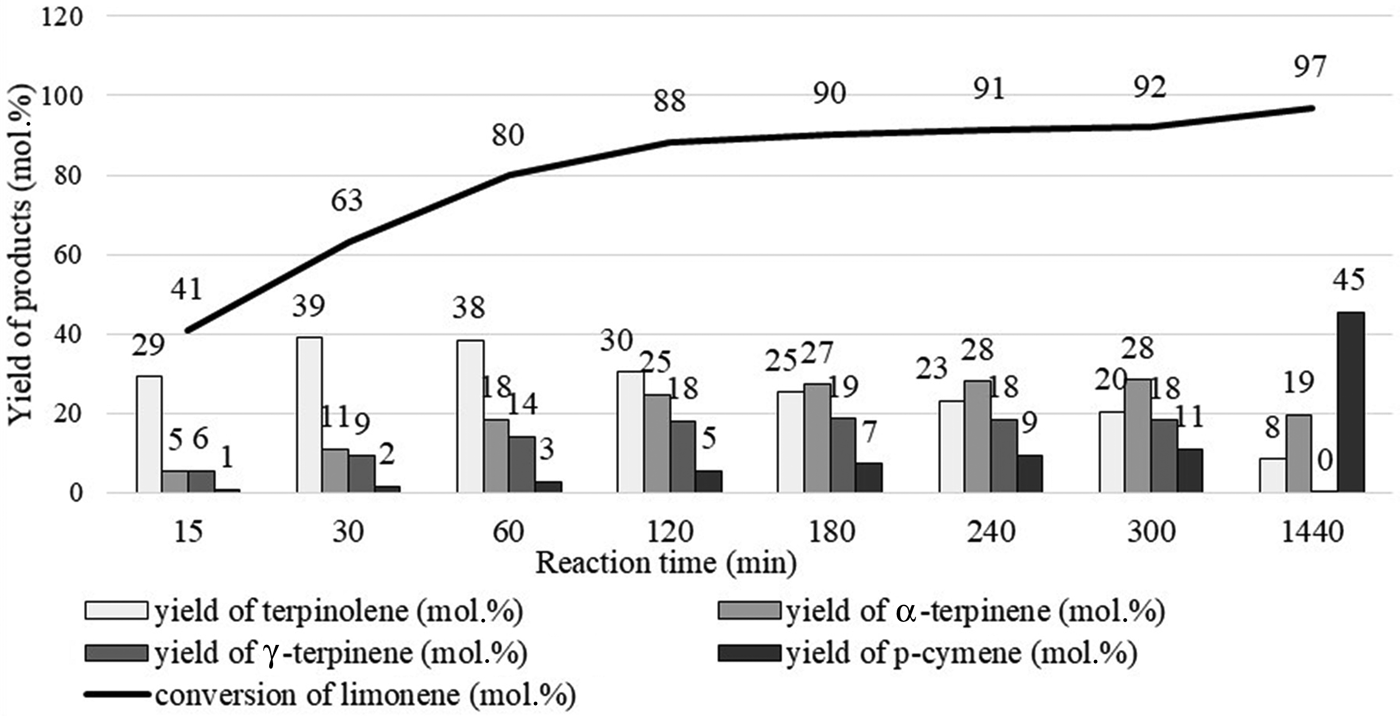
Fig. 12. Influence of reaction time on the yields of products at 175°C for 10 wt.% catalyst content.
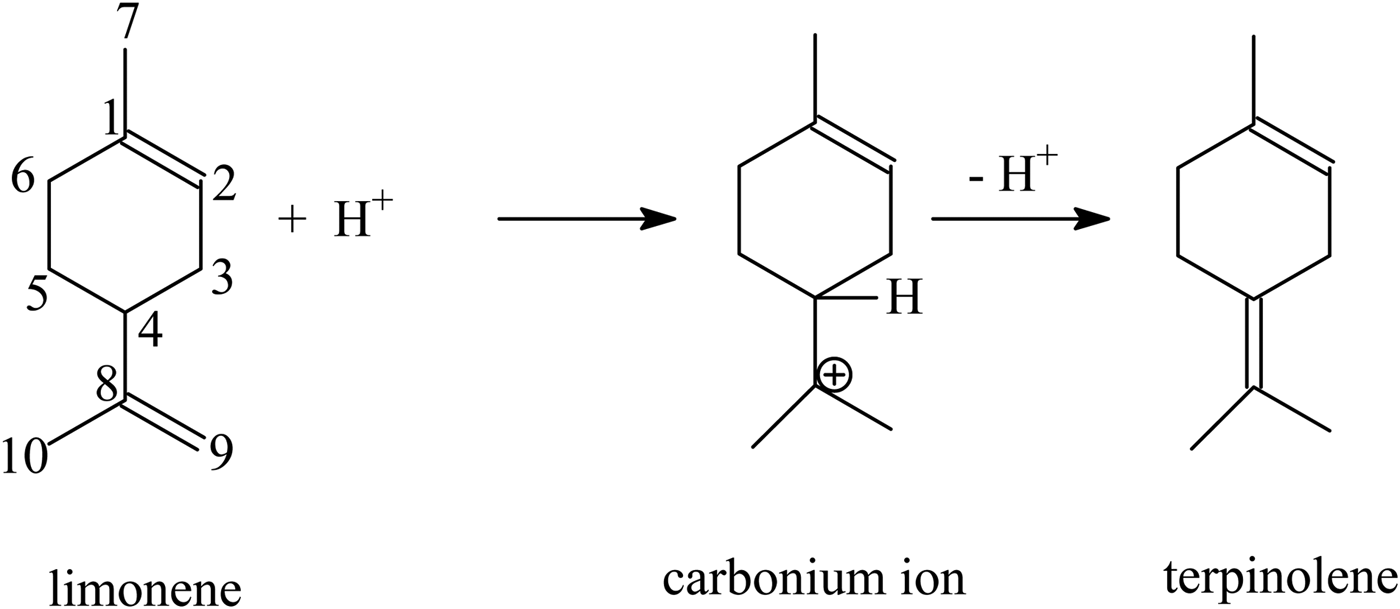
Based on the results of this work and previous reports (Stanislaus & Yeddanalpalli, Reference Stanislaus and Yeddanalpalli1972; Detrekoy et al., Reference Detrekoy, Jacobs, Kallo and Uytterhoeven1973; Grau et al., Reference Grau, Zgolicz, Gutierrez and Taher1999), the following mechanism of formation of terpinolene, α-terpinene, γ-terpinene and p-cymene may be proposed. In the first step, a proton derived from a strongly acidic site (Brønsted acidic site) attaches to the double bond in position 8–9 of the limonene molecule and simultaneously a carbonium ion at the C8 atom is formed. Next, the removal of a proton from the C4 atom of the limonene molecule leads to the formation of terpinolene (double-bond formation at position 4–8; see sketch below).
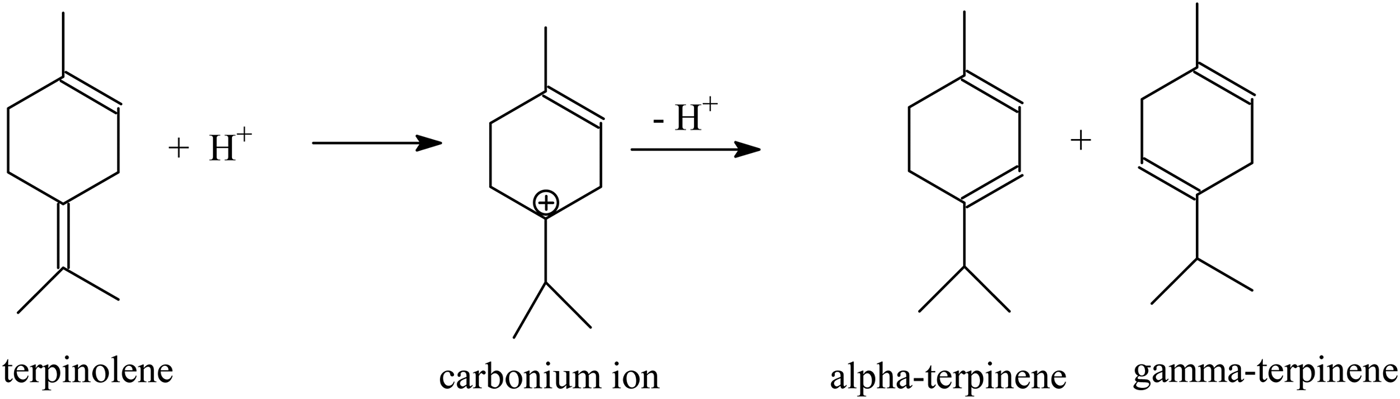
α-Terpinene and γ-terpinene may be formed from terpinolene by further double-bond isomerization. A Brønsted acidic site also participates in this reactions. Next, the removal of the proton from the C3 or C5 atom in the limonene molecule leads to the formation of α-terpinene (double-bond formation at position 3–4) or γ-terpinene (double-bond formation at position 4–5; see sketch below).
p-Cymene may be also formed from α- and γ-terpinenes with the help of a Brønsted acidic site. In the first step, a proton derived from a Brønsted acidic site attaches to the double bond in position 3–4 of the limonene molecule and simultaneously a carbonium ion at the C4 atom is formed. Next, this carbonium ion reacts with the molecule of γ-terpinene, and p-menthene and carbonium ions are formed at the C3 atom. Finally, the removal of the proton from the carbonium ion (the positive charge on the C3 atom) leads to the formation of p-cymene (see sketch below).
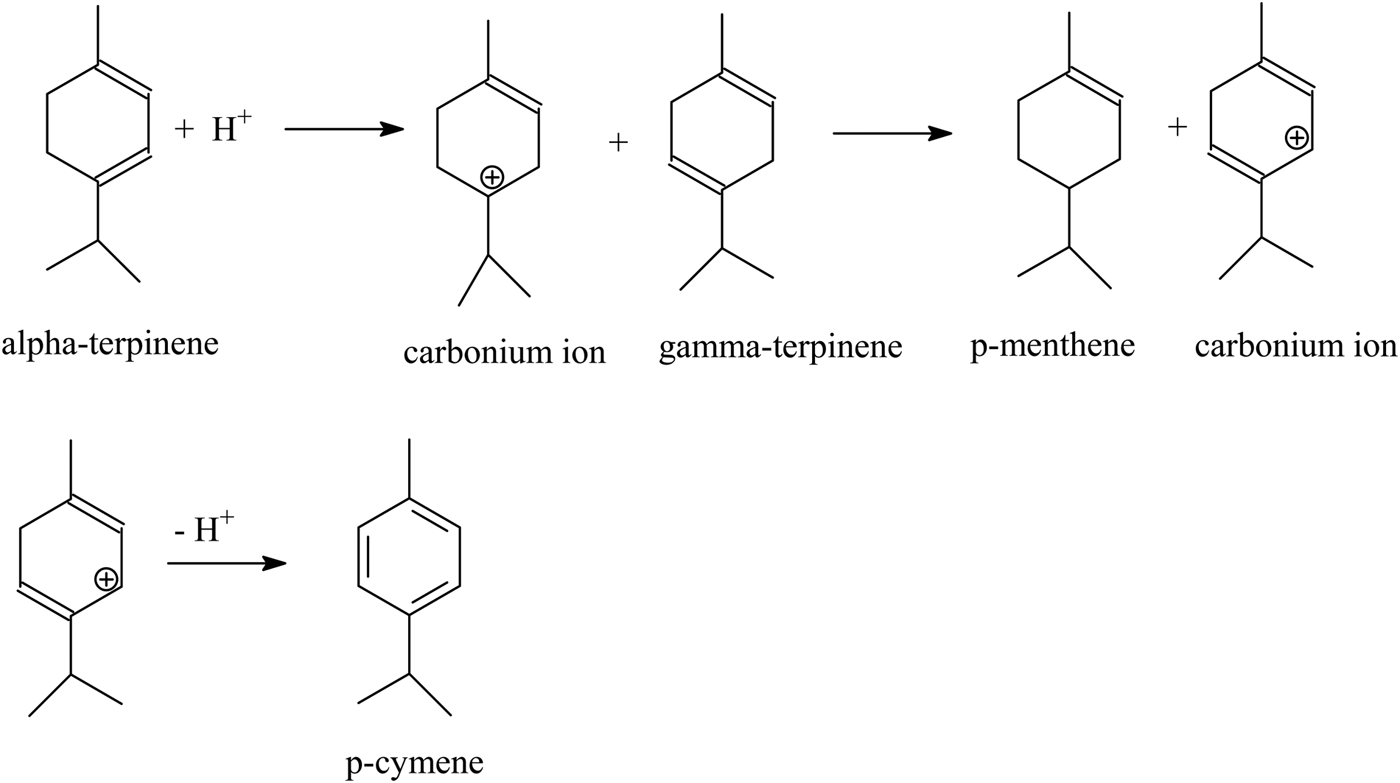
The p-cymene formation may also be based on the direct dehydrogenation of α-terpinene at the acid site of aluminium (Lewis acid site; see sketch below).
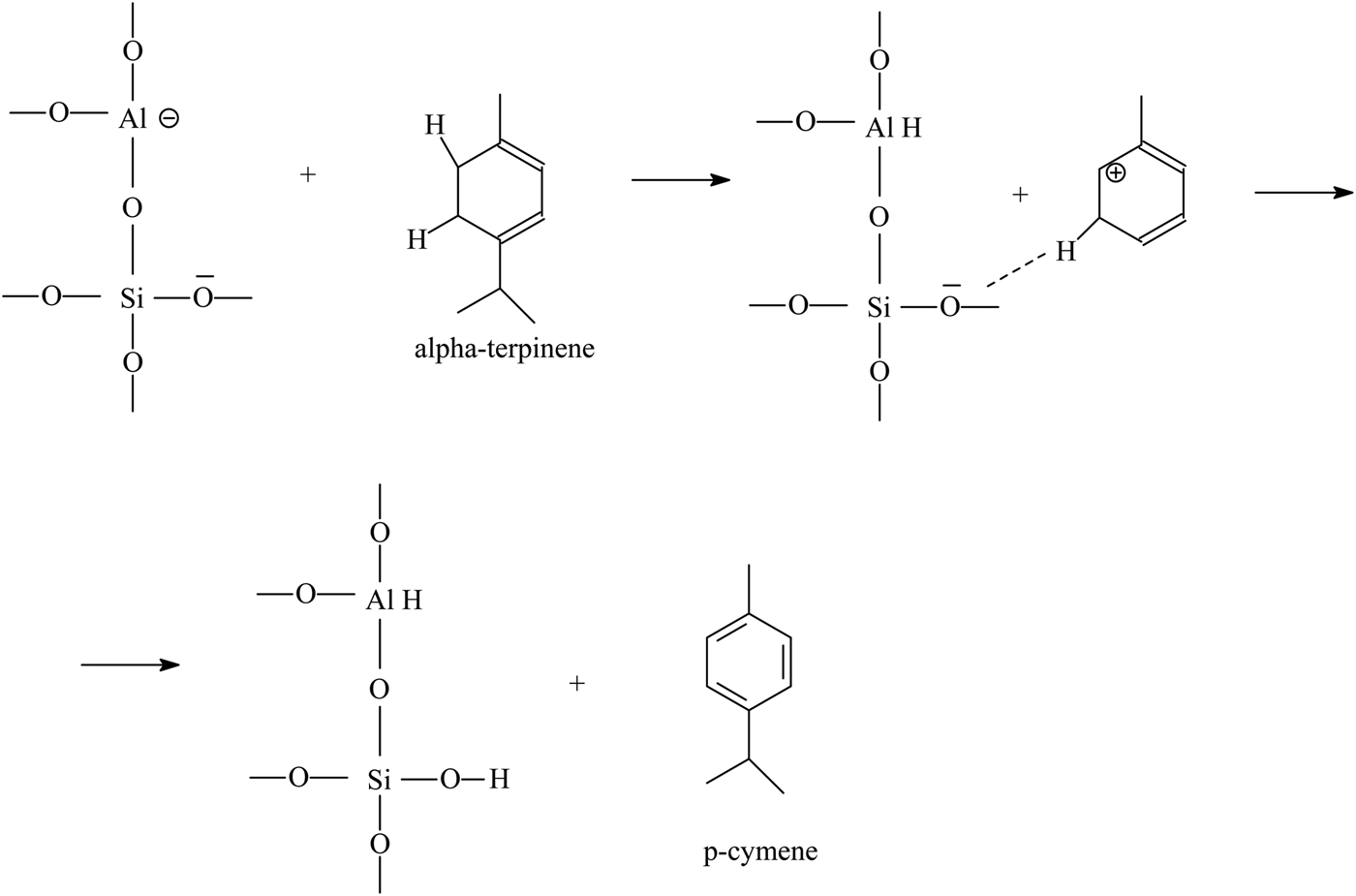
The increase in the amount of terpinolene and p-cymene may be achieved by increasing the number of strong Brønsted acidic sites. The main process of improving the catalytic activity of clinoptilolite is acid treatment or preparation of the H-form of clinoptilolite after heat treatment of the NH4+-form.
Comparison of the results presented here with previous work using an anatase catalyst (Johnson, Reference Johnson1985) showed that it is possible to obtain as high a selectivity of terpinolene in the presence of clinoptilolite (~70 mol.%) as with over anatase. Over clinoptilolite, the isomerization of limonene in the direction of terpinolene requires higher temperatures (155–175°C) and shorter reaction times (mainly 15–180 min), but at ~70 mol.% selectivity of limonene, the maximum conversion of limonene is lower (~43 mol.% for a temperature of 175°C, a reaction time of 15 min and 10 wt.% clinoptilolite). On the other hand, the isomerization of limonene over clinoptilolite is easier because it does not require a nitrogen atmosphere or the presence of a buffer.
The isomerization of limonene performed over bentonites yielded a lower selectivity of terpinolene compared to this work (only 20 mol.%) in experiments conducted at similar temperatures (150°C) and short reaction times (25 min; Catrinescu et al., Reference Catrinescu, Fernandes, Castilho and Breen2006). In addition, the studies conducted with bentonites were carried using a solvent (n-dodecane), which increased the cost of recycling and has a greater environmental impact than the solution used in this study.
Similar isomerization studies of limonene (Martin-Luengo et al., Reference Martin-Luengo, Yates, Rojoa, Arribas, Aguilar and Hitzkya2010) also yielded considerably lower values of selectivity of terpinolene (28 mol.%) compared to this work. Isomerization of limonene was performed at 210°C for 5 min using microwave heating. The present work has shown that, for isomerization performed using clinoptilolite, temperatures >175°C are not beneficial because the reaction mixtures undergo polymerization.
Isomerization of limonene over mesoporous titanium silicate catalyst Ti-SBA-15 yielded a lower selectivity of terpinolene compared with clinoptilolite (maximum value 57 mol.% at the conversion rate of limonene of ~14 mol.%) at 160°C, 5 wt.% catalyst content and a reaction time of 240 min (Retajczyk & Wróblewska, Reference Retajczyk and Wróblewska2017). Taking into account the fact that the Ti-SBA-15 catalyst is synthesized at hydrothermal conditions and its synthesis uses high-cost raw materials and energy, the utilization of clinoptilolite – a material of natural origin – is more cost efficient. The high selectivity of terpinolene decreases the cost of separation of this product from the post-reaction mixture, thus decreasing the cost of production of this compound.
Conclusions
The use of a clinoptilolite catalyst allowed for the isomerization of limonene to several products with many applications. By selecting an appropriate reaction time the, formation of the appropriate products may be tuned, especially terpinolene and p-cymene. An increase in the amount of the catalyst increased the yield of the products obtained and simultaneously reduced the reaction time necessary to obtain optimum products. Moreover, with the increase in the amount of p-cymene in the reaction mixture, a decrease in the amount of terpinolene, α-terpinene and γ-terpinene was observed. This confirms the limonene isomerization mechanism suggested here.
The limonene isomerization is a useful process for obtaining terpinolene and p-cymene, and is a process which may be performed without the use of any solvents and with the natural catalyst, clinoptilolite. Orange peel is a renewable source (biomass) of natural limonene. This study has demonstrated a low-cost and environmentally friendly process where orange peel waste can be used. The proposed process is more cost effective than similar approaches using the Ti-SBA-15 catalyst synthesized in the laboratory under hydrothermal condition.
Author ORCIDs
Agnieszka Wróblewska, 0000-0002-9713-259X