INTRODUCTION
In parasites that spend part of their life off-host (non-permanent parasites) the environment where they leave the host strongly affects their survival and reproductive success (Price, Reference Price1980; Poulin, Reference Poulin2007). The parasite's fitness is low when leaving the host in environments with unfavourable abiotic conditions, high risk of natural enemies, or low host availability. Therefore, it is expected that in non-permanent parasites mechanisms have evolved that optimize fitness by controlling the separation from the host. In addition, the location where parasites leave their hosts unavoidably determines their dispersal, particularly in species with low intrinsic mobility. Parasite dispersal is recognized to be one of the most important factors affecting its host specificity (i.e. the degree to which a parasite occurs in association with a single host species), parasite and disease dynamics, and the evolution of reciprocal adaptations in host-parasite interactions (Price, Reference Price1980; Boulinier et al. Reference Boulinier, McCoy, Sorci, Clobert, Danchin, Dhondt and Nichols2001; Poulin, Reference Poulin2007). The dispersal capability of a parasite is shaped by the behavioural and ecological characteristics of both the host (e.g. host vagility, behaviour and social structure) and the parasite (e.g. intrinsic mobility) (McCoy et al. Reference McCoy, Boulinier, Tirard and Michalakis2003; Poulin, Reference Poulin2007). In ectoparasites with low intrinsic mobility, dispersal often takes place in association with host movements (McCoy et al. Reference McCoy, Boulinier, Tirard and Michalakis2003; Dick and Patterson, Reference Dick and Patterson2007; Poulin, Reference Poulin2007). Here the cues that determine the timing of the parasite's separation from the host play a crucial role (Matuschka et al. Reference Matuschka, Richter, Fischer and Spielman1990).
Here we study the circadian detachment rhythm as well as the variation in detachment times of 2 widespread ixodid ticks throughout Europe, Ixodes ricinus L. (the sheep tick) and Ixodes arboricola Schulze & Schlottke (the tree-hole tick) in the great tit (Parus major L.), a diurnal active passerine host shared by both tick species (Kluyver, Reference Kluyver1957; Gosler, Reference Gosler1993; Hillyard, Reference Hillyard1996; Literak et al. Reference Literak, Kocianova, Dusbabek, Martinu, Podzemny and Sychra2007). Ixodid ticks are a common group of haematophagous ectoparasites that vector disease-causing agents to humans and animals throughout the world (Sonenshine, Reference Sonenshine1993; Hillyard, Reference Hillyard1996; Jongejan and Uilenberg, Reference Jongejan and Uilenberg2004). They typically take a single bloodmeal lasting several days before detachment and moulting to the next development stage, and thus spend most of their life off-host (Hillyard, Reference Hillyard1996). Consequently, each life stage experiences the dual pressures of survival on and off the host. It is well documented that ticks have low intrinsic dispersal capability when separated from their hosts (Milne, Reference Milne1950a, Reference Milneb; Gray, Reference Gray1985; Falco and Fish, Reference Falco and Fish1991; Carroll and Schmidtmann, Reference Carroll and Schmidtmann1996). Therefore ticks depend on host movement for transportation to sites where they subsequently develop and contact the next host.
Although I. ricinus and I. arboricola may co-occur in the same macro-habitat, they have different ecologies, micro-habitat requirements and host specificities. Ixodes arboricola is a nidicolous tick with its entire life cycle restricted to natural tree holes. This tick species mainly parasitizes birds breeding and roosting in tree holes, such as P. major (Hudde and Walter, Reference Hudde and Walter1988; Hillyard, Reference Hillyard1996). On the other hand, I. ricinus is a non-nidicolous tick with a broad variety of vertebrate hosts (mammals, birds and even lizards). Immature instars (larvae and nymphs) frequently infest the P. major individuals of different European populations (Heylen et al. Reference Heylen, Madder and Matthysen2009). Unfed I. ricinus ticks typically climb to some vantage point in the lower vegetation (‘questing’) from where they contact passing vertebrate hosts. All stages of I. ricinus are very sensitive to desiccation (Knülle and Rudolph, Reference Knülle, Rudolph, Obenchain and Galun1982; Kahl and Knülle, Reference Kahl and Knülle1988; Gray, Reference Gray1998), which limits their vertical distribution in the vegetation (Lees, Reference Lees1948; Mejlon and Jaenson, Reference Mejlon and Jaenson1997). Especially the immature instars, which mainly infest small mammals and birds, are susceptible to low humidity and hence restricted to habitats with good vegetation cover, often near the surface of the litter layer in deciduous woodland (Gray, Reference Gray1998). Adult females typically infest larger mammals (Hillyard, Reference Hillyard1996).
Because ticks should detach from hosts in environments that are suitable to reproduce and survive, we expect that detachment strategies in I. arboricola and I. ricinus strongly differ. We predict that I. arboricola individuals detach nocturnally which maximizes their chance to end up in a tree-hole where they can continue their life cycle. On the other hand, I. ricinus will optimize its fitness when detaching close to relatively humid soils, outside the tree holes, since the relative humidity inside tree holes may become too low for this extremely hydrophilic tick species, and since I. ricinus adults require larger mammals that are mostly available outside tree holes. Therefore we predict that I. ricinus detaches diurnally from P. major. To test our predictions, we experimentally infested birds with different developmental stages (larvae, nymphs and adult females) of either I. ricinus or I. arboricola in controlled conditions, and registered the time of tick detachment.
MATERIALS AND METHODS
Study design
Timing of detachment was studied by executing an experiment according to a randomized, 2×2 factorial design, with factors ‘tick species’ and ‘moment of infestation’ (2 levels: ‘dawn’: 08.00 h a.m., and ‘dusk’: 08.00 h p.m.). The combination of both factors led to 4 experimental groups in which birds were infested either with I. ricinus or with I. arboricola, either at dawn or at dusk. If the timing of tick detachment were pre-programmed and independent of the birds' circadian activity, we expect that in each tick species equal numbers of ticks detach during day and night. If tick detachment were adjusted to the birds' circadian activity, we expect that the I. ricinus prefers to detach during the day, and I. arboricola during the night (see Introduction section). Birds were infested in separate experiments with either larvae, nymphs or adult ticks, with numbers of ticks within the range of natural conditions (Hudde and Walter, Reference Hudde and Walter1988; Literak et al. Reference Literak, Kocianova, Dusbabek, Martinu, Podzemny and Sychra2007; Heylen and Matthysen, Reference Heylen and Matthysen2008).
Experimental infestation
In the autumn of 2008, P. major individuals were captured at 2 sites near Antwerp, Belgium with mist nets under licence from the Belgian Ringing Scheme (Brussels). During the total length of the experiments, birds were maintained with a photo-phase beginning at 08.00 h a.m. and a scotophase beginning at 08.00 h p.m. Temperature varied with the daily ambient outdoor temperature. Birds received food and water ad libitum. After a habituation period of 7 days, birds were artificially infested with ticks at either 08.00 h a.m. or 08.00 h p.m. (‘moment of infestation’).
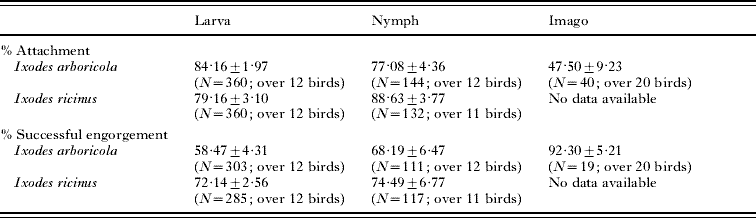
In the experiment using larvae and nymphs, respectively 24 and 23 birds were randomly assigned to the 4 treatments as described above (see Table 1 for details). Birds infested with larvae, received 30 ticks of a single species, while birds infested with nymphs received 12 ticks of a single species. Since we have never observed infestations of P. major with I. ricinus adults in more than 2000 captures (Dieter Heylen, unpublished data), we studied the detachment rhythm of adult female ticks only with respect to I. arboricola. For the latter experiment, 20 birds were randomly assigned to the ‘moment of infestation’ and each bird received 2 I. arboricola females. Using tweezers, ticks were put underneath the feathers on the head of the bird. Immediately afterwards birds were kept for 2 h in an air-permeable cotton bag (sized: 20 cm by 15 cm) inside a darkened cage which kept them inactive (Heylen and Matthysen, Reference Heylen and Matthysen2008). All ticks that did not attach and remained in the bag were counted after experimental infestation (see Table 1). The proportion of ticks that successfully attached was not related to the tick species, the moment of infestation, or their interaction in any of the developmental stages (all P⩾0·06; based on a Generalized estimation equation (GEE) for each of the immature instars; based on a Fisher-exact test for the adult females).
Table 1. Mean infestation parameters per bird (±s.e.) of the developmental stages of Ixodes arboricola and Ixodes ricinus fed on Parus major
(N represents the total number of ticks on which the calculation of the infestation parameter has been based.)

All I. arboricola ticks were obtained in the spring of 2008 from nest boxes in which P. major nestlings were infested with ticks. Engorged I. arboricola individuals typically climb to the ceiling of the nest box upon host detachment (personal observation) where they can easily be collected by removing the nest box lid. Engorged individuals were kept at 25°C and 83% relative humidity until moulting to the next developmental stage, or until the emergence of larvae from the eggs of adult females. Subsequently all individuals were kept at 20°C:10°C temperature cycle and 83% relative humidity (in the dark). All I. ricinus larvae were obtained from eggs laid by 5 engorged females, isolated from 2 cats (Felis catus). Females were kept individually in tubes at 25°C and more than 90% relative humidity until larvae emerged from the deposited eggs. Emerged larvae were kept in a climate room at 90% relative humidity and 12 h:12 h (light:dark photoperiod; 20°C:10°C temperature cycle) until infestation. Ixodes ricinus nymphs were caught by dragging a white flannel flag over suitable vegetation. Ixodes ricinus nymphs were kept in the same climatic conditions as those of the larvae (see above).
Study of detachment
After tick exposure, birds were placed individually in a cage with a wire-mesh floor (40 cm×80 cm). Below the wire-mesh floor was a removable plastic tray containing damp filter paper and edges streaked with Vaseline to prevent ticks from escaping. The engorged ticks that dropped through the mesh cage were collected twice a day: the morning check started at 06.30 h a.m., the evening check around 06.30 h p.m. Each check took on average 1 to 1.5 h. Trays were removed, and checked in good light conditions outside the room where all birds were caged. Consequently, the birds were kept in the dark until all trays had been examined. Some immature ticks were presumably lost because they could not be found amongst the faeces and food remains beneath the wire-mesh or because they were eaten by the hosts before detachment. Each tick was rinsed with purified water, blotted on dry and clean filter paper, and weighed individually on an electronic microbalance to the nearest 0·01 mg. Engorged I. arboricola individuals were kept individually in tubes at 25°C and 83% relative humidity until moult to the next development stage was completed. The detachment of engorged ticks was followed up for a maximum period of 20 days after infestation. Ticks that stayed on the birds were removed with tweezers. All birds were released after all ticks had detached or had been removed. The proportion of ticks that had successfully engorged, was not related to tick species, the moment of infestation, or their interaction in any developmental stage (all P⩾0·06; based on a GEE for each of the immature instars, Fisher-exact for the adult females; see Table 1).
Statistical analysis
Generalized estimation equations (GEE) were fitted (logit-link, and binomial distributed residuals) when modelling the proportion of ticks that detached, taking into account the statistical dependence of measurements on the same bird. The proportion of engorged ticks that nocturnally detached was modelled against the moment of infestation, the tick species and their interaction. We used methods of survival analysis (time-to-event data) for modelling the duration until tick detachment (see Cox and Oakes, 2004 for general information). The duration until detachment (in days) was modelled by a marginal cox proportional hazards model for clustered data (Shu and Klein, Reference Shu and Klein1999) with tick species and the moment of infestation added into the model. Those ticks that were removed at the end of the experiment were handled as right-censored data. Data are represented by Kaplan-Meier curves. Since only a small number of I. arboricola adults successfully engorged in the experiment, a Fisher-exact test was executed on the pooled dataset to test if nocturnal detachment was related to the moment of infestation. All data manipulations and statistical analyses were done in SAS v 9.1 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Larvae
Of the larvae that successfully engorged, the majority of I. arboricola detached by night (87·13±3·04% per bird) while this was rarely observed in I. ricinus (2·14±4·31% per bird; logit(I.a – I.r.)=4·12±0·48; χ2=72·40; d.f.=1; P<0·0001; see Fig. 1a). In both tick species, nocturnal detachment occurred significantly less often from birds that were infested in the morning (43·92±12·56% per bird) compared to the evening (55·34±10·95% per bird; logit(dusk-dawn)=1·05±0·49; χ2=4·7; d.f.=1; P=0·03). There was no significant interaction between ‘tick species’ and ‘moment of infestation’.

Fig. 1. Total number of Ixodes arboricola (black fill colour) and I. ricinus (grey fill colour) larvae (a), nymphs (b) and females (c) that have detached during the night and during the day. No data are available for the adult females of I. ricinus.
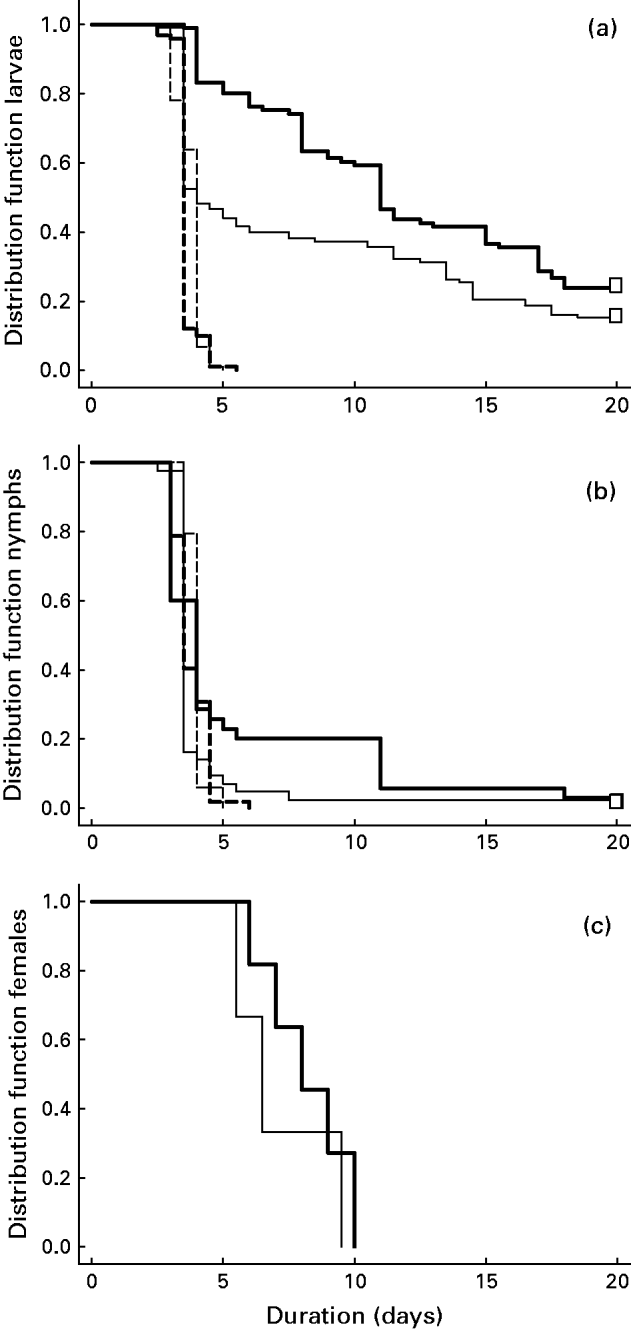
After a time-period of 20 days, on average 3·5±0·62 I. arboricola larvae per bird were still attached, despite visual signs of successful engorgement (personal observations). Those that did detach, stayed on average 8·38±0·78 days. In contrast, I. ricinus larvae stayed on average only 3·67±0·04 days, and all detached within 5.5 days after infestation (see Fig. 2a).

Fig. 2. Kaplan-Meier curves of the detachment of Ixodes arboricola (solid lines) and I. ricinus (dashed lines) larvae (a), nymphs (b) and adult females (c). Bold lines and thin lines represent the distribution functions of detached ticks that have been administered respectively at 8 h a.m. and at 8 h p.m. Open boxes represent censored ticks (see main text for details).
The estimated hazard for I. arboricola larvae to detach was, on average, 89·4% (95%-confidence interval: 62·2%–97·1%) lower than in I. ricinus (P<0·0001) at any given time-point. The I. arboricola larvae of the birds infested at dusk stayed significantly shorter on the birds than the larvae of the birds infested at dawn (hazard ratio=1·38; χ2=6·03; d.f.=1; P=0·014; see Kaplan Meier curves Fig. 2a). In contrast, no effect of ‘moment of infestation’ was found in I. ricinus. The average within-individual variance of the duration until detachment was considerably higher in I. arboricola (18·79±3·04 days) compared to I. ricinus (0·19±0·03 days) (Wilcoxon test: S=222, exact P<0·0001).
Nymphs
As in the larvae, most I. arboricola nymphs detached during the night (95·57±1·93% per bird) compared to a minority of I. ricinus nymphs (28·38±5·44% per bird; logit(I.a−I.r.)=3·83±0·62; χ2=37·49; d.f.=1; P<0·0001; see Fig. 1b). Nocturnal detachment was not affected by the moment of infestation and the interaction between ‘moment of infestation’ or ‘tick species’ (all Ps⩾0·36). Only 2 I. arboricola nymphs did not detach within 20 days. The I. arboricola nymphs that did detach stayed, on average, 4·44±0·38 days compared to 3·84±0·05 days for I. ricinus. All I. ricinus nymphs detached within 5·5 days after infestation (see Fig. 2b).
In contrast to the findings in the larvae, the estimated hazard to detach did not differ between the tick species (hazard ratio=0·93; χ2=0·08; d.f.=1; all P=0·8; see Kaplan Meier curves Fig. 2b). In neither of the tick species did the ‘moment of infestation’ have an effect on the time to detachment (all P⩾0·46). The average within-individual variance of time to detachment had a tendency to be higher in I. arboricola (6·66±3·63 days) than in I. ricinus (0·28±0·08 days) (Wilcoxon test: S=110, exact P=0·09).
Adult females
All adult females of I. arboricola that successfully engorged detached during the night (see Fig. 1c). The average time to detach in I. arboricola females was 7·50±0·46 days per bird. The ‘moment of infestation’ did not affect the duration until detachment (0·93; χ2⩽2·66; d.f.=1; all P⩾0·10; see Fig. 2c).
DISCUSSION
To our knowledge this is the first study that compared the detachment rhythms of different tick species parasitizing the same natural avian host. In line with our expectations, most I. arboricola individuals detached during the night whereas most I. ricinus detached during the day from a diurnally active passerine bird. In addition we observed a substantially higher variance in time to detachment in I. arboricola larvae, and also a longer overall time to detachment compared to I. ricinus. Ixodes arboricola larvae that were administered at dusk detached more rapidly than those administered at dawn.
From an evolutionary perspective, we can interpret the contrasting circadian detachment rhythms as adaptations that are related to ecological differences between the two tick species. For I. ricinus, the detachments during the daylight hours from diurnally active songbirds optimize the chance to end up in a habitat where ticks may continue their life-cycle, outside the dry tree holes where songbirds roost. In a related experiment, Matuschka et al. (Reference Matuschka, Richter, Fischer and Spielman1990) infested different hosts of I. ricinus and found that in other non-mammalian hosts (Lacerta agilis L. and Turdus merula L.) ticks detached during the daylight hours when hosts are most active, indicating that these types of hosts may be very important in the dispersal over large distances of I. ricinus (Matuschka et al. Reference Matuschka, Richter, Fischer and Spielman1990). Furthermore, the diurnal detachments from those hosts may result in a scattering of the ticks over the entire host home range, which bring the ticks into contact with many potential host species, and therefore may favour the reduction in host specificity.
On the other hand, the nocturnal detachment of the I. arboricola can be recognized as an adaptation towards a tree hole existence. Also the negative phototropism by I. arboricola (personal observation) is a typical adaptation to the dark and sheltered microhabitats where several nidicolous tick species live (Sonenshine, Reference Sonenshine1993). Studies on different mammalian hosts have also reported that in nest-dwelling ticks detachment rhythms tend to be co-ordinated with rhythms of activity of the hosts, in order to end up in the nest where the hosts shelter (reviewed by Belozerov, Reference Belozerov, Obenchain and Galun1982). Since tree holes are visited by only a limited number of vertebrate hosts, these behavioural characteristics probably shape the narrow host range observed in I. arboricola (Hillyard, Reference Hillyard1996). In the same way, host specificity in other tick species may be basically interpreted as a consequence of the ticks' ecological specificity (Klompen et al. Reference Klompen, Black, Keirans and Oliver1996). Furhermore, the detachments in physically isolated habitats may drive the formation of host races, as the result of limited gene flow among ticks that parasitize different host species with distinct tree hole requirements (Magalhaes et al. Reference Magalhaes, Forbes, Skoracka, Osakabe, Chevillon and McCoy2007). The exceptionally high variation in the morphology of I. arboricola (Haarlov, Reference Haarlov1962) could be an indicator of the existence of different host races, which still needs to be investigated.
An unpredicted outcome of our comparative experiment is the longer time to detachment, and the considerably larger variation in the time to detachment of I. arboricola, particularly in the larvae. Several ticks did not detach for an extended period of time, even when visual signs of successful engorgement were apparent at an early stage in the experiment (personal observation). Since engorgement weights even increased with attachment duration (Dieter Heylen, unpublished data), there is no indication that the long feeding durations are caused by the birds' resistance mechanisms (Varma et al. Reference Varma, Hellerhaupt, Trinder and Langi1990; Rechav, Reference Rechav1992). In addition, the moulting success of the larvae over the total duration of the experiment was high (80·9%), though with a tendency to decrease with the duration until spontaneous detachment (logit: −0·10±0·04/day; P=0·04). This indicates that there may be a cost for the larvae when delaying their detachments, e.g. due to grooming of the host. Still, 75% of the larvae that detached after 14 days successfully moulted, as well as the 62·5% of the larvae that were removed with the tweezers. We suggest that delayed detachments may be an adaptation to effective transmission of I. arboricola among tree holes which is only possible through the host. In soft ticks Argas spp. and Ornithodoros capensis with long-feeding larvae, a similar dispersal strategy by passive transportation of feeding stages on birds has been proposed (see Balashov, Reference Balashov1972 and references herein). We hypothesize that by staying longer on the host, ticks increase the chance that the host switches to a different roost site, e.g. because of competition over roost sites or disturbance (Kempenaers and Dhondt, Reference Kempenaers and Dhondt1991; Gosler, Reference Gosler1993). The ability to disperse may optimize an animal's fitness in different ways, e.g. by reducing the competition between conspecifics for the same resources (Hamilton and May, Reference Hamilton and May1977), by avoiding inbreeding (Gandon, Reference Gandon1999), and by making it possible to move in response to temporal and spatial heterogeneities in the environment (Clobert et al. Reference Clobert, Danchin, Dhondt and Nichols2001). The high variance in detachment time in I. arboricola can be considered as a dispersal mechanism leading to the distribution of offspring over a wider range of environments, and could therefore be seen as a form of risk-spreading (Hopper, Reference Hopper1999; Clobert et al. Reference Clobert, Danchin, Dhondt and Nichols2001).
Several proximate mechanisms have been proposed that explain how ticks may synchronize the detachment rhythms to their environment. In some tick species the circadian detachment pattern is determined by the rhythm that is endogenous to the tick and that is mainly entrained by the external light/dark cycles immediately following attachment. In other species the activity rhythm of the host overrules those endogenous rhythms, whereby physiological changes in the host may serve as exogenous cues that ‘set the clock’ of the detachment process (Mather and Spielman, Reference Mather and Spielman1986). The mechanisms involved in the perception of external stimuli leading to detachment of the ticks are still poorly understood. Our study shows that I. arboricola and I. ricinus differ in circadian detachment rhythms when infesting the same type of host, and therefore the two tick species process external stimuli in a different way in order to adjust their detachment to the birds' circadian activity. Further insights into the cues that trigger the ticks to detach may be obtained by infesting nocturnally active birds that roost in tree holes, e.g. Tawny owls (Strix aluca L.). If the birds' physiological activity is the most important cue we expect that detachments from nocturnal hosts should take place in I. arboricola during the daylight when owls are roosting in tree holes, while in I. ricinus detachments should occur during the night (cf. nocturnally active mammals infested with I. ricinus (Matuschka et al. Reference Matuschka, Richter, Fischer and Spielman1990)). Also, the mechanisms underlying the delayed detachment of I. arboricola when administered at dawn remain to be elucidated. Some authors have suggested that the conditions following attachment affect the long-lasting feeding phase at the beginning of the tick infestation, and hence eventually determine the total feeding duration (Mather and Spielman, Reference Mather and Spielman1986).
In conclusion, our experiments demonstrate that a simple behavioural characteristic, the moment of leaving the host, may strongly differ between congeneric parasites on the same host, and may be one of the proximate mechanisms maintaining host specificity in ticks. Both habitat requirements and host preference, as such, may have driven the evolution of the observed adaptive behavioural responses in ticks towards a common host. This model system offers excellent opportunities for further studies of the exact cues used by ticks for leaving the host. Further research will reveal to what extent detachment strategies, including variation in detachment time, may be part of a dispersal strategy associated with the specificity and spatial occurrence of suitable hosts.
ACKNOWLEDGMENTS
We are grateful to Joris Elst and Frans Fierens for their technical assistance.