INTRODUCTION
The Chilean dolphin (Cephalorhynchus eutropia, Gray, 1846) and Peale's dolphin (Lagenorhynchus australis, Peale, 1848) are two species inhabiting the coastal waters of Chile and Argentina. Both species are sympatric on the Chilean coast form 33oS to 55oS (Goodall, Reference Goodall, Ridgway and Harrison1994; Goodall et al., Reference Goodall, De Haro, Fraga, Iniguez and Norris1997). Although that these two species are the most frequently observed of all cetaceans in southern Chile (Viddi et al., Reference Viddi, Hucke-Gaete, Torres-Florez and Ribeiro2010), our knowledge about their ecology and conservation status is limited. They are among the least known species in the dolphin family.
Due to their near-shore and restricted distribution, Chilean and Peale's dolphins are subject to direct and indirect anthropogenic threats, including incidental takes (by-catch) in local fisheries and the progressive destruction of potentially critical habitat (Hucke-Gaete et al., Reference Hucke-Gaete, Viddi and Bello2006). The Chilean dolphin, the only endemic cetacean in Chile, is listed as ‘Near Threatened’ (Reeves et al., Reference Reeves, Crespo, Dans, Jefferson, Karczmarski, Laidre, O'Corry-Crowe, Pedraza, Rojas-Bracho, Secchi, Slooten, Smith, Wang and Zhou2008) and Peale's dolphins as ‘Data Deficient’ (Hammond et al., Reference Hammond, Bearzi, Bjørge, Forney, Karczmarski, Kasuya, Perrin, Scott, Wang, Wells and Wilson2008) by the International Union for the Conservation of Nature (IUCN).
In the last five years, there has been progress in the information generated on both Chilean and Peale's dolphins, though there are still important gaps in knowledge and therefore an urgent need for research on these species. Most research until now has principally focused on distribution, habitat selection and patterns of movement (Viddi & Lescrauwaet, Reference Viddi and Lescrauwaet2005; Heinrich, Reference Heinrich2006; Ribeiro et al., Reference Ribeiro, Viddi, Cordeiro and Freitas2007; Viddi et al., Reference Viddi, Hucke-Gaete, Torres-Florez and Ribeiro2010, Reference Viddi, Harcourt, Hucke-Gaete and Field2011), while other aspects have been overlooked, including behavioural patterns. This is mainly because some researchers mistakenly believe that data on behaviour have little applicability to understanding ecological processes or because these data have little contribution to make towards the conservation and management of species. However, the truth is that behaviour of animals characterizes the relationships among species and plays a significant role in ecological processes (Dingle et al., Reference Dingle, Carroll, Loye, Clemmon and Buchholz1997). Moreover, behaviour is an important component of the adaptive complex that allows animals to function (Bartholomew, Reference Bartholomew1970) and is shaped by the interactions between the environment and the genetic make-up of individuals (Wells et al., Reference Wells, Boness, Rathbun, Reynolds III and Rommel1999). Activity patterns of animals result from a complex balance and trade-offs of energy saving and expenditure, which is related to the needs of finding food, refuge, resting, reproduction and socializing (Nielsen, Reference Nielsen1983). As this balance shifts, so do activity budgets. Consequently, behavioural patterns can indicate significant demographic or environmental alterations, resulting from natural phenomena or from human-induced activities (Parrish, Reference Parrish, Norse and Crowder2005), as the development of behaviour is directly linked to the flexibility of animals to adapt to variable ecological conditions (Hall et al., Reference Hall, Halliday, MacLannahan, Toates and Whatson1998).
Accordingly, behavioural studies can have direct application to conservation (Caro, Reference Caro1998). For instance, changes in the behavioural patterns of several cetacean species have been related to boat traffic (Lusseau, Reference Lusseau2003; Ribeiro et al., Reference Ribeiro, Viddi and Freitas2005; Dans et al., Reference Dans, Crespo, Pedraza and Degrati2008, Reference Dans, Degrati, Pedraza and Crespo2012; Lusseau et al., Reference Lusseau, Bain, Williams and Smith2009) and to climate variability (Lusseau et al., Reference Lusseau, Williams, Wilson, Grellier, Barton, Hammond and Thompson2004) and their potential negative effects on populations. Furthermore, understanding the behavioural patterns of sympatric species is crucial in order to gain insights into the mechanisms by which similar species coexist (Perri & Randall, Reference Perri and Randall1999). Different activity budgets between species may indicate differences in energy requirements, foraging strategies and different ways of responding to the characteristics of the environment.
There are many studies of cetacean behaviour, but only a few have included an interspecific comparative approach (Parra, Reference Parra2006). Regarding Chilean and Peale's dolphins, some studies have addressed behaviour (Lescrauwaet, Reference Lescrauwaet1997; Viddi & Lescrauwaet, Reference Viddi and Lescrauwaet2005; Ribeiro et al., Reference Ribeiro, Viddi, Cordeiro and Freitas2007), but none have systematically and comparatively assessed the activity budgets and behavioural patterns of both species. Considering that dolphin-watching may become an important economic activity in southern Chile, here we compared the behavioural patterns of the Chilean and Peale's dolphins in southern Chile in order to build a practical baseline framework from which to assess potential effects of anthropogenic activities.
MATERIALS AND METHODS
Data collection
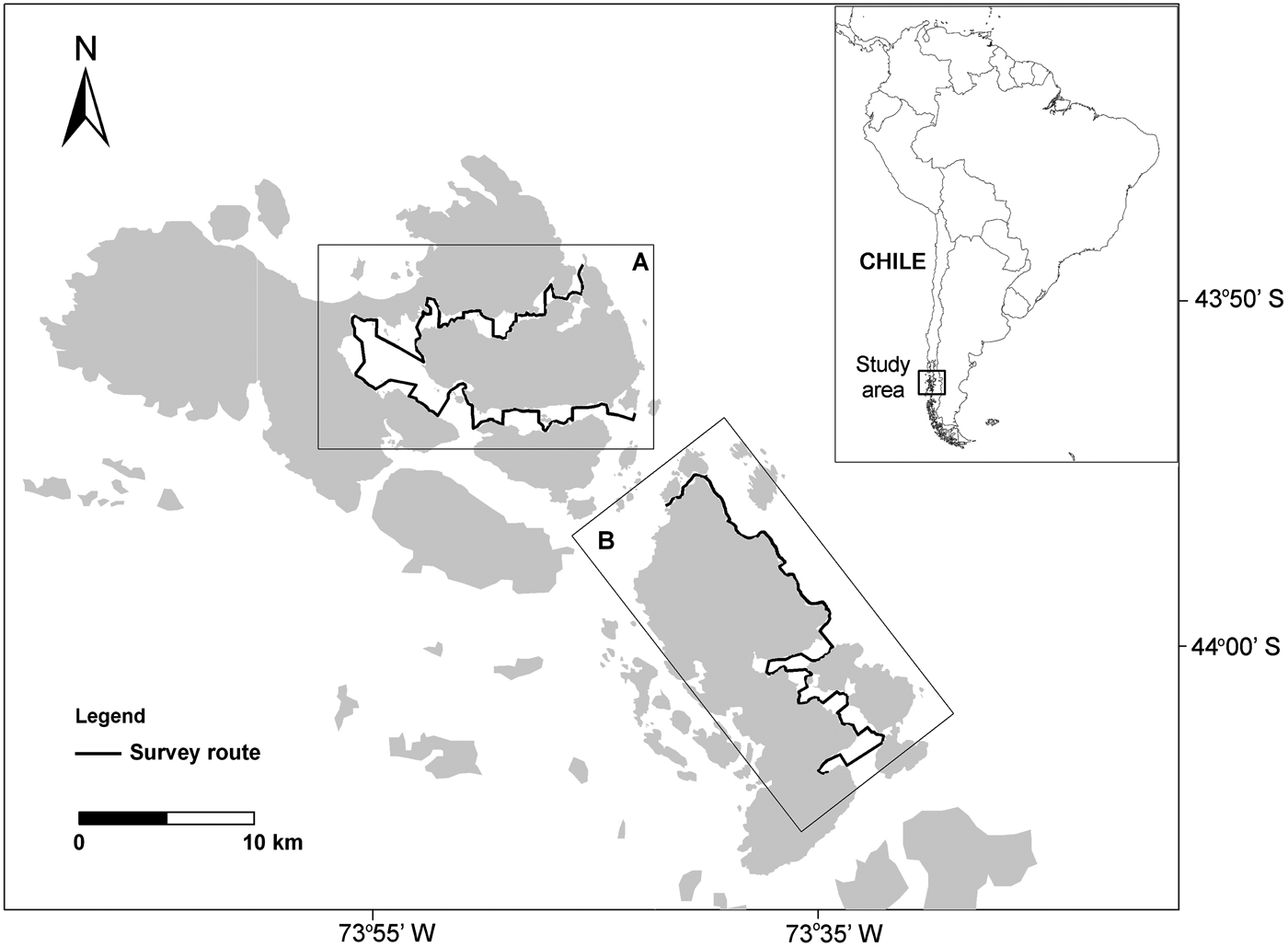
Boat-based surveys were conducted during January–April 2007, 2008 and 2009 in the waters off Guaitecas Archipelago in the Chilean Northern Patagonian fjords (43°52′S 73°45′W, Figure 1). Surveys were undertaken by two or three observers on-board a rigid-hulled inflatable boat 5 m long with an outboard four-stroke 25 hp engine. Observers looked for dolphins with naked eye or 7 × 50 binoculars, covering a strip of about 300 m ahead and 90° to each side, at a searching speed of 8 knots. Upon sighting a group of dolphins, search effort was interrupted in order to record dolphin behavioural states, group size and group dispersion. Data on weather conditions (sea state, cloud cover, wind direction and relative strength) were also recorded.

Fig. 1. Study area in Southern Chile, showing an inset of the location of Guaitecas Archipelago and the two sub-areas: (A) Puquitin; (B) Leucayec. Main boat routes also shown.
A group of dolphins was defined by spatial proximity. Animals within a radius of 50 m were considered to be part of the same group. Under this definition, dolphins are part of a group as long as they are within this radius. This ‘distance measure’ definition of a group was chosen over a ‘coordinated activity’ definition for its simplicity and because it does not rely on any explicit or implicit assumptions about the behaviour of a group's member (Mann, Reference Mann1999).
Dolphin behavioural states were assessed by ‘group-follow’ protocol and ‘scan sampling’ method (Martin & Bateson, Reference Martin and Bateson1993; Mann, Reference Mann1999). Instantaneous scan samples were taken at 2 min intervals of all individual dolphins on the surface. A behavioural state for the group was assigned as the predominant behaviour in which most of the individuals were engaged (more than 50% of the animals).
Behavioural states recorded included:
• Foraging/feeding (F): frequent dives characterized by steeply downward movements sometimes preceded by fluking up or peduncle arches. Dolphins may be seen swimming in circles, or swimming in parallel formation, with fast, directional and synchronized movements. Dolphins may be observed engaging in rapid chases of fish, or observed with fish in their mouth. Fish may be seen leaping out of the water.
• Milling (M): animals move slowly, with repeated unsynchronized dives in different directions in a determined location. Movement has no apparent direction, short dives at different intervals.
• Travelling (T): directional and persistent movement at speed with regular patterns of surfacing. Dive angles shallow.
• Resting (R): very slow movements with no direction, or staying still at the surface from several seconds to minutes.
• Socializing (S): inter-individual interactions within a group, in frequent physical contact, rubbing, chasing each other, with frequent vigorous movements.
Group dispersion was defined by inter-individual spatial proximity, or spread between dolphins in groups, and was also recorded by instantaneous sample at 2 min intervals. Mode nearest neighbour distance in adult dolphin body lengths was classified as (a) tight: animals <1 body length apart; (b) intermediate: 1–3 body lengths apart; and (c) disperse: >3 body lengths apart.
Because group size in both Chilean and Peale's dolphins is relatively small (medians 2 and 4, respectively) and individual animals are hard to identify for both species, scan sampling on group follows was chosen as the best method to record behavioural data (Mann, Reference Mann1999; Whitehead, Reference Whitehead2004). Group-follow was chosen over a survey protocol, as dolphin group encounter rate is higher than the rate of change of behavioural state (Whitehead, Reference Whitehead2004).
Focal group behavioural samples were designed to maximize time with dolphins, rather than collect a specific time with a single group and then continue the track in search of another new group. This rule was undertaken because sighting rate was, in general, low (Viddi, Reference Viddi2009). Dolphin follows were conducted as long as the group was in sight, so long as weather and light conditions permitted. To minimize disturbance of dolphins, and, therefore, reduce any bias due to the presence of the research boat, a four stroke engine was used and an average distance of 250 m was kept from the dolphins. Under many situations, the engine was in fact kept turned off. Finally, only groups which were followed more than 30 min and did not appear to react to the boat (e.g. bow-riding) were included in the analysis.
Data analysis
Sequences of behavioural states for both species were assessed by transition probabilities and Markov chains. In this study the main interest was to understand the change in the likelihood that when a dolphin group was in a ‘behavioural state A’ they would be in ‘behavioural state B’ two minutes later (at the next sampling interval). In this case, it was assumed that the succeeding behavioural state was only dependent on the preceding state, thus the analysis on behaviour transition was based on first order Markov chains (Caswell, Reference Caswell2001; Lusseau, Reference Lusseau2003).
Transition matrix analysis assumes that the transition probabilities remain constant across time, an assumption called stationarity (Martin & Bateson, Reference Martin and Bateson1993; Lehner, Reference Lehner1996). To assess stability over time, a three-way contingency table of 5 × 5 × 4 was designed (preceding behavioural state versus succeeding behavioural state versus season). Season included four time blocks: January–February 2007, March–April 2007, January–February 2008 and March–April 2008 (data from year 2009 were not included into transition matrices as sample size for this year was too small). If the transition probability was stable over time, season had no effect on the outcome of the transition. The effect of season on transitions was analysed by log-linear models of the three-way contingency table. The fully saturated model was compared to the model with all two-way interactions using G 2 statistics (Quinn & Keough, Reference Quinn and Keough2002).
By pooling data from all seasons, a two-way 5 × 5 contingency table was developed for each species, which included the frequencies of preceding behavioural state versus succeeding behavioural state. From these, the transition probabilities were calculated as:
 $${\,p_{ij}} = \displaystyle{{{a_{ij}}} \over {\sum\limits_{\,j = i}^5 {{a_{ij}}} }}\quad {\rm and} \quad \sum\limits_{j = i}^5 p_{ij\, =\, 1}$$
$${\,p_{ij}} = \displaystyle{{{a_{ij}}} \over {\sum\limits_{\,j = i}^5 {{a_{ij}}} }}\quad {\rm and} \quad \sum\limits_{j = i}^5 p_{ij\, =\, 1}$$where p ij is defined as the transition probability in the Markov chain from behavioural state i to behavioural state j, a ij is the number of 2 min intervals in which state i was followed by state j (i and j ranges from 1 to 5 because there are five behavioural states).
To determine if the transition probabilities differed significantly from a random transition model, a χ2 test was performed (Lehner, Reference Lehner1996). A significant difference would indicate that the behavioural patterns observed were in fact sequentially dependent (Martin & Bateson, Reference Martin and Bateson1993). The difference in the transition probability matrix between species was compared through a Pearson's χ2 two-sample test for equality of proportions with continuity correction (Newcombe, Reference Newcombe1998).
Markov chains have ergodic properties because the transitions between all states are possible (Caswell, Reference Caswell2001). A group of dolphins could be involved in a transition from any state to another, as there are no biological restrictions preventing dolphins from changing between one state and the others (Lusseau et al., Reference Lusseau, Bain, Williams and Smith2009). Hence, the probability of observing a specific activity at a specific time for each species was calculated from the left eigenvector, v , of the matrix. This vector represented the stationary distribution of a Markov chain, and its components vi summed up to 1. Here, v represents the behavioural budget of dolphins and each, vi, represents the proportion of time spent in activity, i. The left eigenvector was obtained with PopTools 3.1 (available from http://www.cse.csiro.au/cdg/poptools). The difference between the behavioural budgets between Chilean and Peale's dolphins was assessed by Pearson's χ2 two-sample test for equality of proportions with continuity correction.
The association between behavioural states and group size was assessed by Kruskal–Wallis rank sum test. The association between behavioural states and group dispersion, as well as frequency of events was evaluated by correspondence analysis (Lehner, Reference Lehner1996).
All statistical analyses were made in R v.2.9.1 (R Development Core Team, 2009).
RESULTS
Survey effort, data summary and temporal patterns
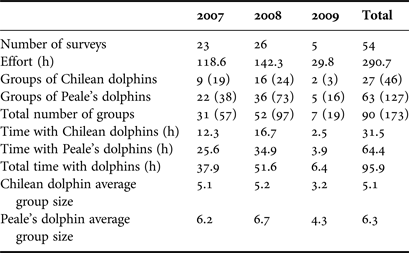
Over the three fieldwork summer seasons of 2007, 2008 and 2009, a total of 54 surveys were conducted, which encompassed 290 h on effort searching for dolphins. 173 dolphin groups were encountered for which groups follows were attempted. However, only 90 groups were analysed, which were followed for more than 30 min, representing 95.9 h of total net time following dolphins. Mean duration of group follows was 82.8 min (standard deviation (SD) = 51.31) for Chilean dolphins, ranging from 30 to 240 min, whereas for Peale's dolphin mean duration time was 67.7 min (SD = 36.39) ranging from 30 to 196 min. The difference in time following dolphins was not significant between species (t-test, t = −1.34, d.f. = 34.5, P = 0.189). Number of dolphin groups sighted, time on effort, time with dolphins and dolphin group size all varied between species and are summarized in Table 1.
Table 1. Total number of surveys, hours of effort, number of dolphin groups followed and analysed (number in parenthesis are the total number of groups encountered), time with dolphins and group size for each species for each field season.

Markov chains: behavioural sequence and budget
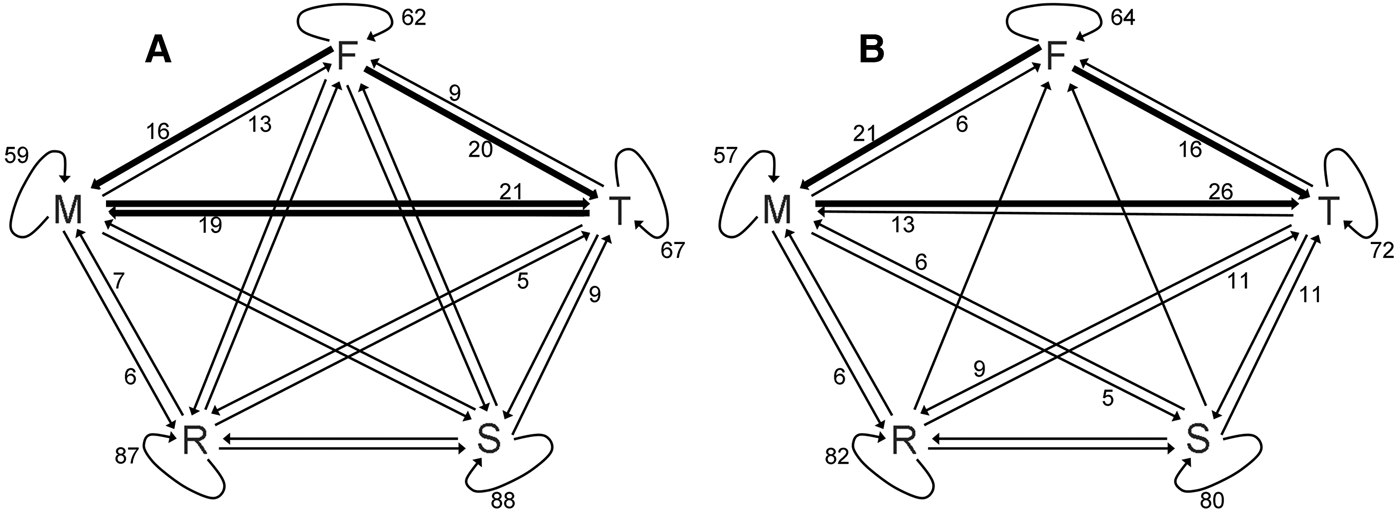
The transition probability matrices for both Chilean and Peale's dolphins were stable over time (G 2 = 59.95, d.f.= 48, P = 0.116 and G 2 = 58.87, d.f. = 48, P = 0.135, respectively). The transition probabilities for both species were significantly different from a random transition model (Chilean dolphins, χ2 = 1934.8, d.f. = 16, P < 0.001; Peale's dolphin, χ2 = 3504.4, d.f. = 16, P < 0.001), indicating that the behavioural patterns showed by these species are sequentially dependent (Figure 2).

Fig. 2. Markov chains representing probabilities of transition in behavioural states: (A) Chilean dolphins; (B) Peale's dolphins. Values represent round percentages. Values less than 5% have been omitted. Thicker arrows represent values over 15% and the absence of arrows represents lack of transition between any two particular behavioural states.
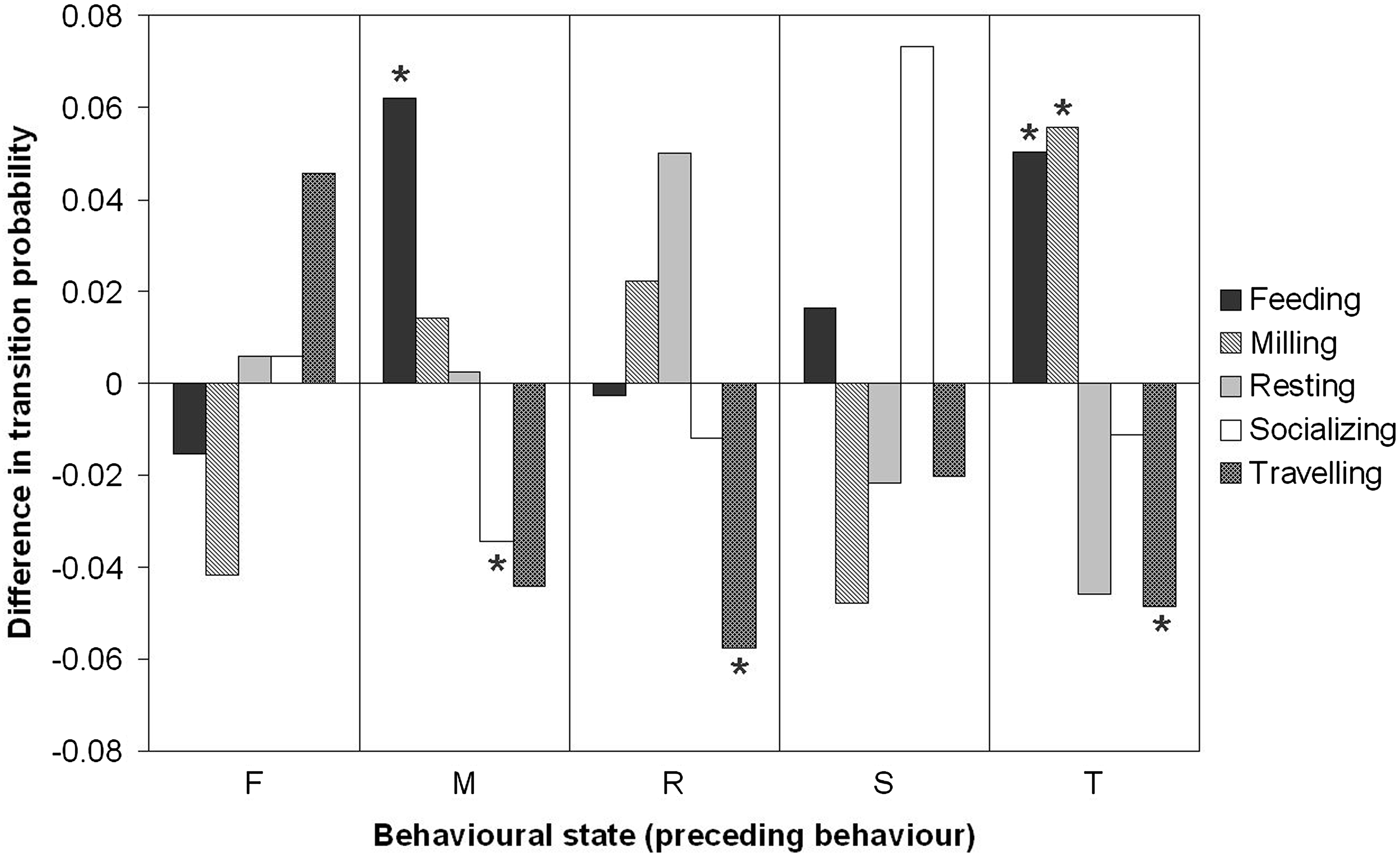
From the 25 behavioural transitions, only six were significantly different between species. The transition probabilities M → F, T → F and T → M were higher for Chilean dolphins, whereas the transitions M → S, R → T and T → R were higher for Peale's dolphins (Figure 3).

Fig. 3. Differences in transitions probabilities in behavioural states between Chilean and Peale's dolphins (p ij(Chilean dolphins) – p ij(Peale's dolphins)). Negative values indicate that the transition probability for behavioural states for Chilean dolphins is lower than for Peale's dolphins. The six transitions with a significant difference (P < 0.05) are denoted by (*).
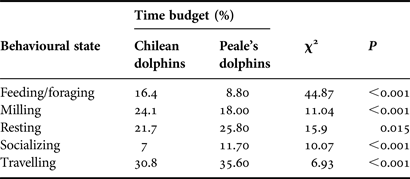
The Markov chain model predicts that Chilean dolphins invest most of their time in travelling and less in socializing (30.8% and 7%, respectively). Peale's dolphins also invest more time in travelling, but less in feeding/foraging (35.6% and 8.8%, respectively, Table 2). For all behavioural states, Chilean and Peale's dolphins differed significantly in the time allocated to each behavioural state (behavioural budget). The Markov chain model predicts that Chilean dolphins invest more time in feeding/foraging and milling than Peale's dolphins, whereas the latter invest more time in resting, socializing and travelling when compared with Chilean dolphins (Table 2)
Table 2. Differences in time budget of behavioural states for Chilean and Peale's dolphins.

Behavioural states versus group size, group dispersion
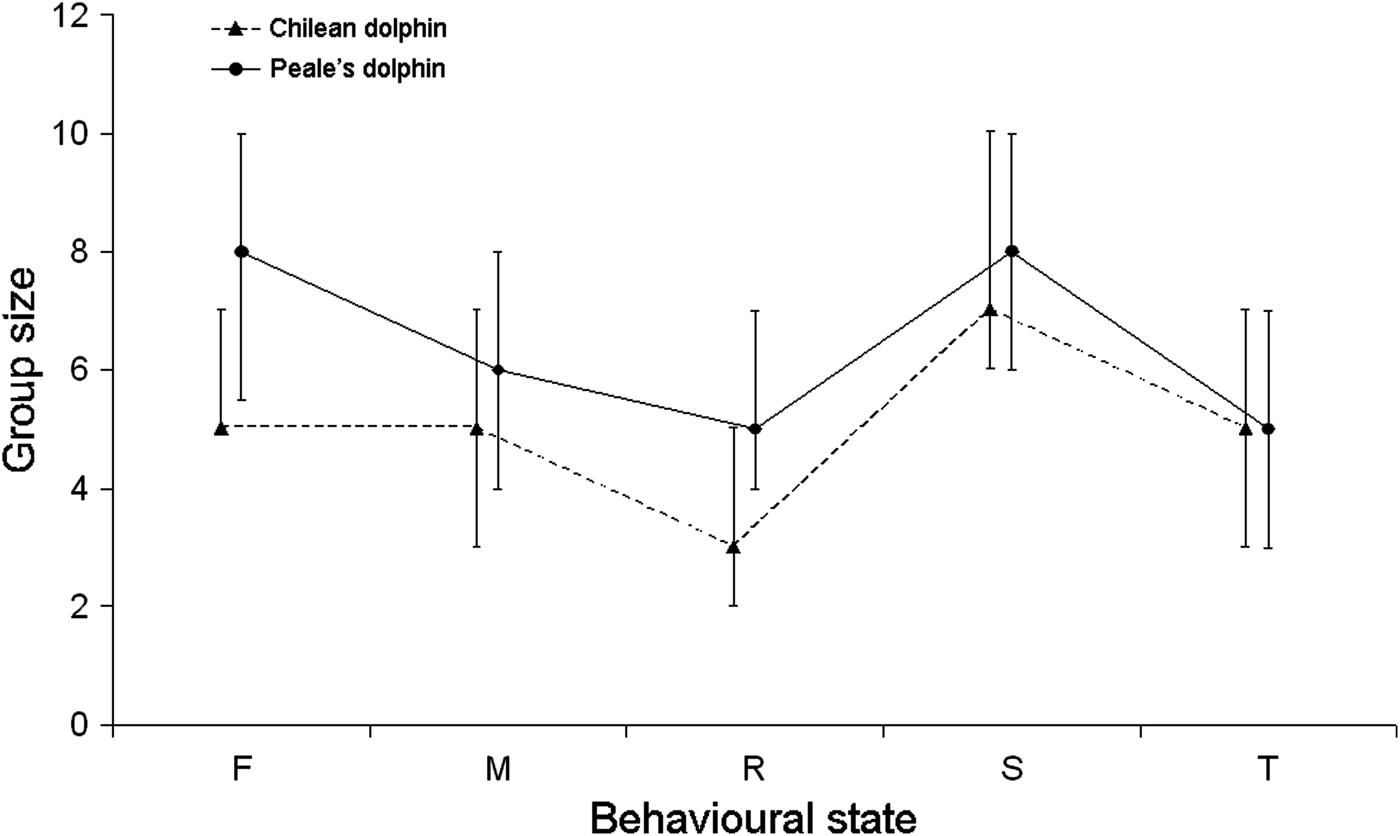
For Peale's dolphins, bigger groups engaged mostly in socializing and feeding/foraging while small groups were more associated with resting (Kruskal–Wallis test, K-W = 216.4, d.f. = 4, P < 0.001). Chilean dolphins bigger groups were also significantly more associated with socializing, and as Peale's dolphins, small groups were related with resting (Kruskal–Wallis test, K-W = 205.9, d.f. = 4, P < 0.001; Figure 4).

Fig. 4. Behavioural state of Chilean and Peale's dolphins relative to group size. Dots and triangles indicate average, bars represent standard deviation.
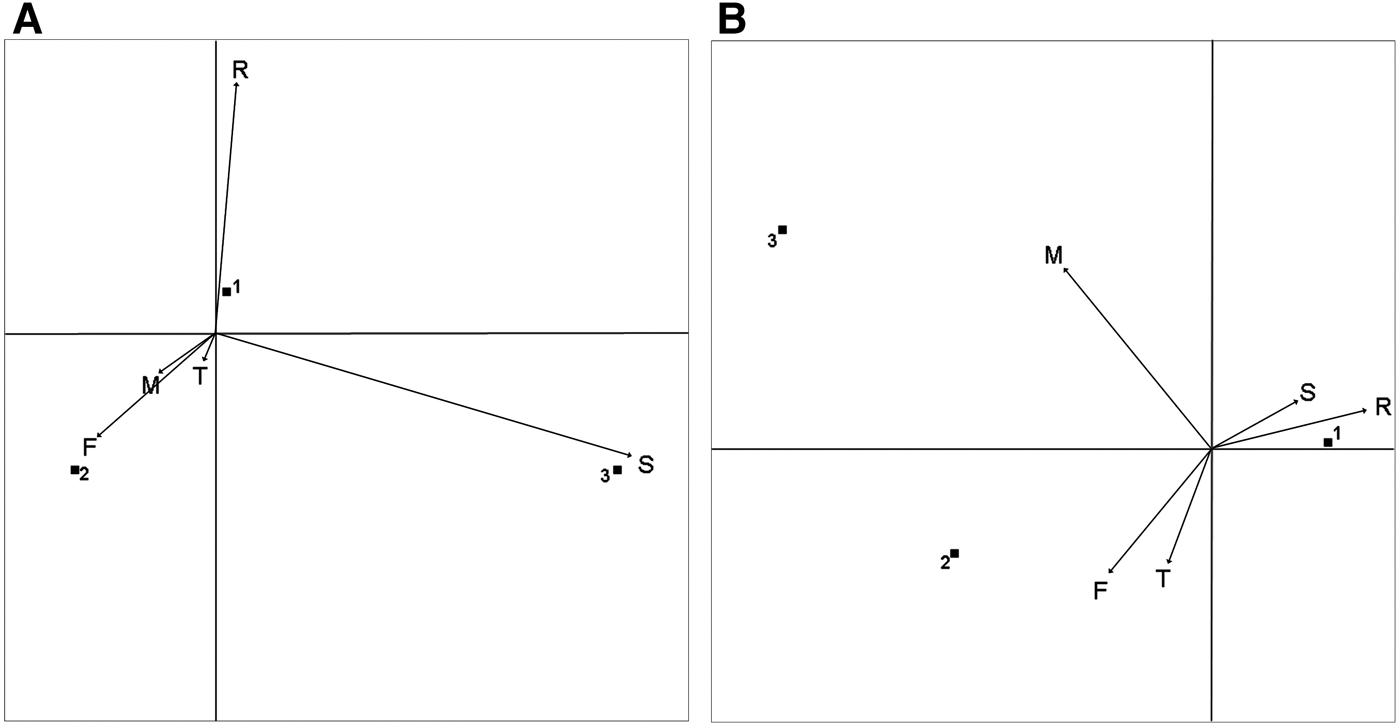
Correspondence analysis showed that group dispersion and behavioural state for Peale's dolphins was significantly associated (χ2 = 190.41, d.f. = 8, P < 0.001). Two eigenvectors were extracted which explained 100% of the total inertia (88.5% and 11.5%, respectively). Socializing and resting were associated with tight groups, feeding/foraging and travelling with intermediate dispersion and milling was more associated with dispersed groups (Figure 5). For Chilean dolphins, the association between group dispersion and behavioural state was also significant (χ2 = 118.01, d.f. = 8, P < 0.001). Two eigenvectors were extracted which explained 100% of the total inertia (72.5% and 27.5%, respectively). Resting was associated with tight groups, feeding/foraging, travelling and milling were associated with intermediate dispersion and socializing was related strongly with dispersed groups (Figure 5).

Fig. 5. Correspondence analysis joint plot. Behavioural states in relation to group dispersion for: (A) Chilean dolphins; (B) Peale's dolphins. Group dispersion category: (1) tight; (2) intermediate dispersion; (3) dispersed. F, foraging/feeding; M, milling; T, travelling; R, resting; S, socializing.
DISCUSSION
In this study, Chilean and Peale's dolphin behavioural states did not show any association with season or time of the day. Many studies have demonstrated that other dolphin species exhibit daily and seasonal patterns in their behaviour, which is suggested to be in response to variations in prey abundance and distribution, predator avoidance, reproduction and breeding (Bearzi et al., Reference Bearzi, Politi and Di Sciara1999; Mann et al., Reference Mann, Connor, Barre and Heithaus2000; Markowitz, Reference Markowitz2004). For instance, studies of bottlenose dolphin (Tursiops truncatus), have shown that there is a trend towards increased feeding in the morning and again later around dusk, increased socializing in the afternoon and increased travelling in the afternoon and evening (Hansen & Defran, Reference Hansen and Defran1993; Bearzi et al., Reference Bearzi, Politi and Di Sciara1999). Bearzi et al. (Reference Bearzi, Politi and Di Sciara1999) found extreme variability in bottlenose dolphin behaviour in the Adriatic Sea, particularly with travelling and feeding, and they associated this with prey availability.
The lack of temporal patterns in behaviour for both Chilean and Peale's dolphins may be a response to relatively constant prey availability, also suggested for bottlenose dolphins (Hansen & Defran, Reference Hansen and Defran1993) and dusky dolphins, Lagenorhynchus obscurus (Degrati et al., Reference Degrati, Dans, Pedraza, Crespo and Garaffo2008), in Argentina. This may be the case for Peale's dolphins, as they rely strongly on kelp beds (Viddi & Lescrauwaet, Reference Viddi and Lescrauwaet2005; Viddi et al., Reference Viddi, Harcourt, Hucke-Gaete and Field2011), which may sustain a more constant prey availability. In addition, a lack of temporality may be also be explained by patterns of prey availability other than circadian, such as tidal cycle, which is not constant during the day and changes from one day to the next. Certainly, it has been determined that Chilean dolphins are highly associated with tide regime (Ribeiro et al., Reference Ribeiro, Viddi, Cordeiro and Freitas2007; Viddi, Reference Viddi2009).
However, the results presented in this study come from a restricted period of time (only from January to April), so true seasonal patterns through the year are unknown. Reproductive and breeding seasonality is known for Chilean and Peale's dolphins (De Haro & Iñiguez, Reference De Haro and Iñiguez1997; Lescrauwaet, Reference Lescrauwaet1997). Accordingly, seasonal variation in activity budgets might occur if energy requirements differ through the year, for example if reproduction is temporally concentrated, as has been observed for dusky dolphins in New Zealand (Markowitz, Reference Markowitz2004) and bottlenose dolphins in the Adriatic Sea (Bearzi et al., Reference Bearzi, Politi and Di Sciara1999).
All behavioural budgets differed significantly between Peale's and Chilean dolphins, and within species, behaviours were not performed evenly. Peale's dolphins spent most time travelling (>35%), followed by resting, milling, socializing and foraging/feeding. Chilean dolphins, on the other hand, also spent most of their time travelling (>30%), but followed by milling, resting, foraging/feeding and socializing. The proportion of time that Chilean dolphins spent travelling has also been observed in other studies, but the total proportion that this species is engaged in foraging/feeding is below the time it has been documented previously (Ribeiro et al., Reference Ribeiro, Viddi, Cordeiro and Freitas2007). However, the results of this study support the notion that Chilean dolphins spend only a small fraction of time in social activities (Heinrich, Reference Heinrich2006; Ribeiro et al., Reference Ribeiro, Viddi, Cordeiro and Freitas2007).
Peale's dolphins spending more time on travelling is consistent with the observations by Heinrich (Reference Heinrich2006), but inconsistent with those by Viddi & Lescrauwaet (Reference Viddi and Lescrauwaet2005). These authors found that Peale's dolphins spent most of their time in feeding and foraging behaviours, followed by travelling. Difference in behavioural patterns within the same species has also been observed in the eastern fjords. Peale's dolphins seem to spend more time milling and Chilean dolphins more time feeding and socializing (F. Viddi, unpublished data). It seems reasonable to suggest that activity budgets may vary with habitat and foraging strategies, as different ecological processes would influence energy requirements, as well as the abundance, distribution and quality of prey. Differences in behavioural patterns within species in different habitats have been observed in dusky (Degrati et al., Reference Degrati, Dans, Pedraza, Crespo and Garaffo2008), bottlenose (Bearzi et al., Reference Bearzi, Politi and Di Sciara1999) and humpback dolphins, Sousa chinensis (Karczmarski & Cockcroft, Reference Karczmarski and Cockcroft1999). Spending less time foraging/feeding, and also more time resting and socializing, as is the case for Peale's dolphins, is a potential indication that they may be preying on high quality items. The opposite would be the case for Chilean dolphins, as they spend more time in foraging activities and less time in socializing and resting. The relationship between prey quality and behavioural budgets has been the focus of research in several other species (Estes et al., Reference Estes, Jameson and Rhode1982; Doran, Reference Doran1997; Cruz-Rivera & Hay, Reference Cruz-Rivera and Hay2000).
The difference in behavioural patterns between sympatric species may indicate differences in foraging tactics, selection for different habitat and prey of dissimilar quality, distribution and abundance of prey, as well as specific energy requirements (Ewald & Bransfield, Reference Ewald and Bransfield1987; Perri & Randall, Reference Perri and Randall1999). Indeed, Shane (Reference Shane, Leatherwood and Reeves1990) pointed out that dolphin behaviour can be influenced by a number of ecological variables, and behavioural responses can differ considerably depending on the habitat.
As expected, the probability of a particular behaviour being followed by the same behaviour was high for all behavioural states for both Peale's and Chilean dolphins. From the 25 behavioural sequences, Chilean and Peale's dolphins differed only in six. In general, Chilean dolphins showed a greater probability of transition from milling and travelling to foraging/feeding than Peale's dolphin. This pattern, which may be an important functional component of Chilean dolphins, may be associated with the spatial distribution of prey. The association between travelling and feeding, in which animals move rapidly over areas that are poor in resources and stay longer in feeding grounds, has been widely reported in cetaceans and other species (Bergman et al., Reference Bergman, Schaefer and Luttich2000; Mårell et al., Reference Mårell, Ball and Hofgaard2002; Stevick et al., Reference Stevick, McConnell, Hammond and Hoelzel2002; Austin et al., Reference Austin, Bowen, McMillan and Iverson2006; Viddi et al., Reference Viddi, Harcourt, Hucke-Gaete and Field2011; Degrati et al., Reference Degrati, Dans, Garaffo and Crespo2012). Peale's dolphins, on the other hand, showed higher probabilities of transition from milling to socializing, resting to travelling and travelling to resting. This pattern is consistent with the behavioural budgets observed for this species, supporting the fact that this species spends only a small fraction in foraging/feeding activities. Indeed, the dissimilar behavioural patterns shown by both Chilean and Peale's dolphins is consistent with the difference found on their habitat preference patterns. Chilean dolphins prefer more turbid waters, areas close to rives and influenced by tide regimes, while Peale's dolphins prefer shallow and clearer waters and are very associated with kelp beds (Viddi, Reference Viddi2009). Interspecific variation on habitat selection and behavioural patterns are a good indicator of difference that dolphins may pose regarding prey quality and abundance consumed, preying tactics and predator avoidance.
Chilean and Peale's dolphin group size differed significantly in relation to behavioural states. For Peale's dolphins, travelling and resting were mostly carried out by small groups, while socializing and foraging/feeding was performed by bigger groups. For Chilean dolphins, groups also tended to be small when resting and bigger when socializing. These results are consistent with the observations by Heinrich (Reference Heinrich2006) at Chiloe Island and by Viddi & Lescrauwaet (Reference Viddi and Lescrauwaet2005) in the Magellan Strait, southern Chile. Patterns of association between group size and behavioural states are well documented for dolphin species. It is suggested that grouping in dolphins decreases predation risk, enhances foraging efficiency (Würsig, Reference Würsig, Schuster, Thomas and Wood1986) and increases the probabilities of finding reproductive mates while socializing (Connor, Reference Connor and Hoelzel2002). However, as proposed by Connor (Reference Connor and Hoelzel2002), the size of prey schools may restrict the size of cooperative groups. An ideal group size must optimize energy intake (Baird & Dill, Reference Baird and Dill1996; Wells et al., Reference Wells, Boness, Rathbun, Reynolds III and Rommel1999); thus, for example, cooperative feeding in large groups of dolphins has been linked to large schools of prey (Connor, Reference Connor and Hoelzel2002). When prey is dispersed, patchy or in low abundance, dolphins have a propensity to forage in small groups or individually (Würsig, Reference Würsig, Schuster, Thomas and Wood1986). Grouping also results from protection against predation risk, which has been documented for several cetacean species (Heithaus & Dill, Reference Heithaus and Dill2002). Group size and behaviour are closely related regarding the cost and benefits of associations between individuals, which are linked to functional ecological processes of the life history of the species: predation risk, foraging, information exchange, access to mates, access to helpers to raise infants, disease transmission and thermoregulation (Lee, Reference Lee, Slater and Halliday1994). More specifically, for marine mammals, group size and behaviour have been shaped by three major ecological characteristics which include where animals give birth, where they forage and what they eat (Connor, Reference Connor and Hoelzel2002).
Group cohesiveness was highly associated with specific behavioural patterns for both Peale's and Chilean dolphins. For both species, tight groups were mostly observed during resting, and intermediate dispersion during travelling and foraging/feeding. The major difference between these species was for socializing, in which Peale's dolphins mostly formed tight groups whilst in this behavioural state, while Chilean dolphins formed dispersed groups. To our knowledge, there is no other precedent documenting loose group cohesion in dolphins while socializing. However, due to the low proportion of time that Chilean dolphins were engaged in a social context, these observations are not conclusive for the species.
Tight cohesiveness in dolphin species is generally related to social behaviours (Wells et al., Reference Wells, Boness, Rathbun, Reynolds III and Rommel1999; Markowitz, Reference Markowitz2004), as seen with Peale's dolphins. Tight formation is also a common tactic as a ‘defensive posture’ against predators (Wells et al., Reference Wells, Boness, Rathbun, Reynolds III and Rommel1999; Connor, Reference Connor and Hoelzel2002; Markowitz, Reference Markowitz2004). Moreover, grouping pattern and cohesion differ relative to habitat, demography and geographical isolation, with a tendency towards smaller populations (communities) having larger parties (groups). In other words, the smaller the population, the higher the cohesion between society members (Karczmarski et al., Reference Karczmarski, Würsig, Gailey, Larson and Vanderlipb2005).
Habitat and ecological processes in general may play an important role in shaping the behavioural patterns of species. However, differences in behavioural patterns in sympatric species, as observed for Chilean and Peale's dolphins, may reflect differences in foraging strategies, habitat and/or energy requirements, or even social differences. Understanding interspecific differences in behavioural patterns may reveal essential ecological relationships that allow these species to co-exist. In addition, the information generated in this study will assist in contributing to the development of better and holistic predictive models on how animals are responding or will respond to a rapidly changing environment and the potential effects of human activities.
ACKNOWLEDGEMENTS
We are grateful to all volunteers who assisted in the field. Iain Field, Ryan Portner and Joanna Wiszniewski provided insightful comments which greatly improved earlier drafts of this manuscript. F.A.V. held the International Postgraduate Research Scholarship and the International Macquarie University Research Scholarship while undertaking his PhD candidature at Macquarie University. The study was conducted with the approval of the Macquarie University Committee for the Care and Use of Animals in Research.
FINANCIAL SUPPORT
Financial support was provided by the Rufford Small Grant Foundation, Wildlife Trust, Macquarie University Postgraduate Research Fund, Society for Marine Mammalogy, Cetacean Society International and Tecnomar Ltda (Chile).