Introduction
Many insects can change normal plant development by causing the differentiation of tissues and formation of a gall. Galls are often defined as a specific tumor and treated like a new organ where the growing offspring find shelter and food (Stone et al., Reference Stone, Schönrogge, Atkinson, Bellido and Pujade-Villar2002). The most complex gall structures are induced by species of Hymenoptera from the family Cynipidae which specific hosts are oak trees (Quercus spp.) (Stone & Schönrogge, Reference Stone and Schönrogge2003).
There are around 1000 described species of oak gallwasp worldwide, and most of them occur in the northern hemisphere. The Palearctic region contains at least 163 species, and the genera Andricus, Cynips and Neuroterus are the richest in species (Rokas et al., Reference Rokas, Melika, Abe, Nieves-Aldrey, Cook and Stone2003). Gallwasps’ lifecycle includes an obligatory alternation between sexual generation (in spring) and parthenogenetic generation (in autumn). These two generations differ in size and morphological traits as well as shape, structure and position of galls on the host plants (Harper et al., Reference Harper, Schönrogge, Lim, Francis and Lichtenstein2004).
Since the larvae development of gall-forming insects occurs within the gall, they have to obtain increased nutritional values. Therefore, galls are treated as sinks for different nutrients (e.g., protein) and energy (Larson & Whitham, Reference Larson and Whitham1991, Reference Larson and Whitham1997), and are considered a significant drain on leaf resources (Albert et al., Reference Albert, Padhiar, Gandhi and Nityanand2011). However, the interaction between gall-makers and their host plants in the source-sink relationships are still being studied (Huang et al., Reference Huang, Huang, Chou, Lin, Chen, Chen, Chang and Yang2014, Reference Huang, Huang, Chou, Chen, Chen, Chang and Yang2015). The protein content of plant tissue poses a major nutritional challenge to phytophagous insects. It is the most common limiting nutrient for insect growth (Chen et al., Reference Chen, Gonzales-Vigil, Wilkerson and Howe2007). The attractiveness of plants as food for insects depends on the content and qualitative composition of amino acids as well as their proportion in relation to other organic compounds (e.g., carbohydrates, phenolic compounds) (Sprawka et al., Reference Sprawka, Ciepiela, Sempruch, Chrzanowski, Sytykiewicz and Czerniewicz2003). The presence of these proteins, which are not normally expressed in other plant organs, in the gall tissue is considered as a host-plant manipulation by the insects to change plant development in order to achieve gall formation (Harper et al., Reference Harper, Schönrogge, Lim, Francis and Lichtenstein2004).
Plants respond to insect and pathogen attack or elicitor treatment by activating a wide variety of protective mechanisms associated with induced resistance. Defense mechanisms include production of reactive oxygen species (ROS), accumulation of secondary metabolites and activation and/or synthesis of defense peptides and proteins involving many defense enzymes (Castro & Fontes, Reference Castro and Fontes2005; Ashry & Mohamed, Reference Ashry and Mohamed2012). Many proteins induced through acquired resistance mechanisms are classified as pathogenesis-related proteins (PR proteins).
In deciduous plants, derivatives of shikimic acid (phenols, phenolic acids, flavonoids) are of greater importance within the group of secondary metabolites that play a major role in host plant resistance to herbivores (Fürstenberg-Hägg et al., Reference Fürstenberg-Hägg, Zagrobelny and Bak2013). Plant phenols limit the insect feeding by increasing leaf toughness and reducing the nutritional value of the leaves. Quinones are formed during the oxidation of phenols catalyzed by polyphenol oxidase (PPO) and peroxidase (POD). They bind to leaf protein and make them less digestible to insects (War et al., Reference War, Paulraj, Ahmad, Buhroo, Hussain, Ignacimuthu and Sharma2012). POD is one of the PR proteins which role in resistance mechanism in plants and is associated with the synthesis of cell-wall polymers (lignin and suberin), constituting a physical barrier for insect attack (Almagro et al., Reference Almagro, Gómez Ros, Belchi-Navarro, Bru, Ros Barceló and Pedreňo2009; Jayamohan et al., Reference Jayamohan and Kumudini2011).
Among the defense mechanisms, less studied are other ‘pathogenesis-related’ (PR) proteins, such as chitinase and β-1,3-glucanase, enzymes that can be associated with plant resistance to insect feeding (Inbar et al., Reference Inbar, Mayer and Doostdar2003). Both of these enzymes are present in most plant tissues (stems, flowers, seeds, tubers) and they are usually constitutively expressed at low levels. Their increased production is observed in response to numerous abiotic factors (e.g., salt solutions, UV light), biotic agents (e.g., phytopathogens attacks) and contact with elicitors including ethylene (Rafit et al., Reference Rifat, Minhaj, Mahboob, Malik, Malik, Javed and Saleem2013).
So far, little study has been done on the relationship between gall-making Cynipidae and biochemical mechanisms of galls (Schönrogge et al., Reference Schönrogge, Harper and Lichtenstein2000; Allison & Schultz, Reference Allison and Schultz2005; Gailite et al., Reference Gailite, Andersone and Ievinsh2005; Rocha et al., Reference Rocha, Branco, Vilas Boas, Almeida, Protasov and Mendel2013). Presumably, the plant biochemical responses to insect feeding vary, depending on the host plant or insect species. Thus, the idea was formed to study changes in galls and foliar tissues and compare them with non-infested ones, so that to gain knowledge on the interactions between gallmakers and host plants. The present study had used galls caused by asexual generation (♀♀) of three Cynipidae species, namely Cynips quercusfolii L., Neuroterus numismalis (Fourc.) and Neuroterus quercusbaccarum L., as a model. By studying the biochemical alterations of the cynipidae-oak system, this investigation aimed at answering the following questions: can galls be treated as sinks for protein? Are biochemical changes in galls similar for three Cynipidae species or are they species-dependent? To answer these questions we have determined protein and phenolic compound contents, as well as the activity of antioxidative enzymes and PR proteins.
Methods and materials
Leaf and gall samples collection
Leaf and gall samples for the study were collected from Quercus robur L. trees growing in the forest stands in the vicinity of Lublin (Poland). Visual method was adopted to observe gall infestation. Ungalled (healthy) leaves and leaves with mature galls caused by asexual generation (♀♀) of Cynips quercusfolii L., Neuroterus numismalis (Fourc.) and N. quercusbaccarum (L.) were collected in the beginning of September. The samples (galling and intact leaves) for each gall-inducing species were detached from one tree. One sample constituted of 50 leaves collected from trees within the reach of the hand.
Plant material
In the laboratory the galls of three species were cut off from the leaves and the samples were categorized as: leaves without galls (control), leaves with galls and galls alone. Each sample had a mix of several leaves or galls to be more representative. Plant material was frozen and stored at −80°C until analysis. For guaiacol peroxidase (POD), chitinase and β-1,3-glucanase assays, 5 g of each frozen sample was homogenized with 50 ml of 0.05 M acetate buffer containing 1% (v/v) Triton X-100, (pH 5.6 for POD or pH 5.0 for chitinase and β-1,3-glucanase) and shaken for 1 h at 4°C. After that, the extracts were centrifuged for 10 min (4°C, 9000 × g). For PPO extraction, 5 g of gall and leaf tissue was homogenized in 50 ml of 0.1 M sodium phosphate buffer pH = 7.0 containing 10 mM ascorbic acid and 1% (v/v) Triton X-100, and extracted with the aid of a magnetic stirrer for 1 h. Crude extract samples were centrifuged for 10 min (4°C, 9000 × g).
Protein content
Protein content of extracts was determined using the method of Bradford (Reference Bradford1976). Six samples for each combination were used. The quantity of protein in the supernatant was calculated from the standard curve using bovine serum albumin solution as standard protein was expressed as mg g−1 FW.
POD assay
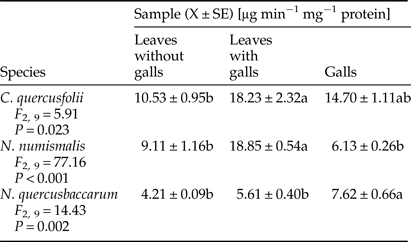
The activity of POD was assayed using the method of Chance & Maehly (Reference Chance and Maehly1955) with some modifications. Four samples for each combination were used. The reaction mixture contained 0.5 ml of 20 mM, 0.95 ml of 50 mM acetic buffer, pH 5.6, and 0.05 ml enzyme extract. The reaction was started with addition of 0.5 ml of 60 mM H2O2. Increase in absorbance was spectrophotometrically measured at 470 nm for 1 min. One unit (1U) of POD activity was defined as 0.001 ΔA470 in min and was expressed as specific activity as units of enzyme activity per mg protein (U mg−1 protein).
PPO assay
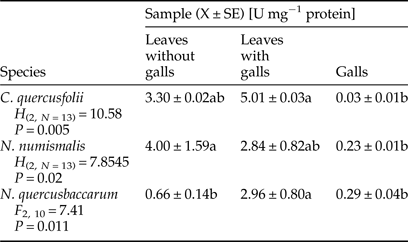
The activity of PPO was determined according to the method of Wisserman & Lee (Reference Wisserman and Lee1980) with minor modifications. Four samples for each combination were used. The reaction mixture contained 1.4 ml 10 mM catechol as the substrate in 100 mM phosphate buffer (pH 7.0) and 0.1 ml enzyme extract. The reference cuvette contained buffer instead of enzyme and the change in absorbance was spectrophotometrically measured at 420 nm. One unit (1U) of PPO activity was defined as 0.001 ΔA410 in min and was expressed as specific activity as units of enzyme activity per mg protein (U mg−1 protein).
Chitinase assay
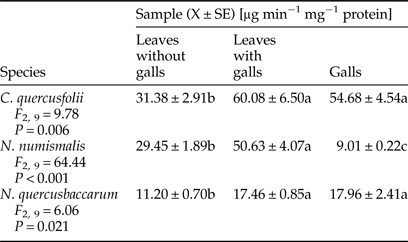
The activity of chitinase was assayed colorimetrically as described by Boller et al. (Reference Boller, Gehri, Mauch and Vögeli1983). Four samples for each combination were used. The assay mixture contained the 0.1 ml enzyme extract, 0.1 ml of colloidal chitin and 0.2 ml of 50 mM acetic buffer, pH 5.0 was incubated at 40°C for 1 h. The amount of liberated N-acetyl-glucosamine was determined by Miller (Reference Miller1959) method as glucose equivalent (mg ml−1). For this purpose after incubation the reaction was stopped by adding 1 ml of 3,5-dinitrosalicylic acid and heating in a boiling water bath for 10 min. The mixture was then made up to 12 ml with double distilled water. The chitinase activity was defined as equivalent of glucose (μg min−1) and was expressed as specific activity as units of enzyme activity per mg protein (μg min−1 mg−1 protein).
β-1,3-Glucanase assay
The activity of β-1,3-glucanase was determined according to the method of Vázquez-Garcidueňas, Leal-Morales & Herrera-Estrella (Reference Vázquez-Garcidueňas, Leal-Morales and Herrera-Estrella1998) with minor modifications. Four samples for each combination were used. The assay mixture contained the 0.1 ml enzyme extract, 0.9 ml of laminarin (0.5% in 50 mM acetic buffer, pH 5.0), 0.1 ml of 50 mM acetic buffer, pH 5.0 was incubated at 40°C for 30 min. The amount of liberated reducing sugars was determined by Miller (Reference Miller1959) method as glucose equivalent (mg ml−1). For this purpose after incubation the reaction was stopped by adding 1 ml of 3,5-dinitrosalicylic acid and heating in a boiling water bath for 10 min. The mixture was then made up to 12 ml with double distilled water. The β-1,3-glucanase activity was defined as equivalent of glucose (μg min−1) and was expressed as specific activity as units of enzyme activity per mg protein (μg min−1 mg−1 protein).
Extraction and analysis of phenolic compounds
Lyophilized plant tissue (0.5 g) was ground with a mortar and pestle with 5 ml of 50% (v/v) methanol and the phenolic compounds were extracted for 20 min at room temperature, then centrifuged at 9000 g for 30 min – this procedure was repeated three times and the supernatants were combined. For C. quercusfolii three samples for each combination were used, while for N. numismalis and N. quercusbaccarum six samples for each combination were used. The amount of total phenolics was determined using Folin-Ciocalteau reagent (Singleton et al., Reference Singleton, Orthofer and Lamuela-Raventos1974). To 0.5 ml of the sample, 0.5 ml H2O, 2 ml Folin-Ciocalteau reagent (1:5 H2O) was added, after 3 min, 10 ml of 10% (w/v) Na2CO3 and the contents were mixed and allowed to stand for 30 min. Absorbance at 725 nm was measured in a UV-Vis spectrophotometer. The amount of total phenolic was calculated as gallic acid equivalent (GAE) in mg g−1 of dry weight (DW).
Data analysis
The obtained data were analyzed using Statistica for Windows v. 9.1 (2009); P < 0.05 was used as the threshold of significance. Student t-test or non-parametric Mann–Whitney U test was used to compare the percentage of changes in the content/activity of biochemical parameters between the control and leaves with galls as well as control and galls. One-way ANOVA with Tukey's Simultaneous Test (HSD) or Kruskal–Wallis test, as a non-parametric alternative, was used to compare the biochemical alterations induced in galls of three cynipid species. All data are presented as means with standard error values (±SE).
Results
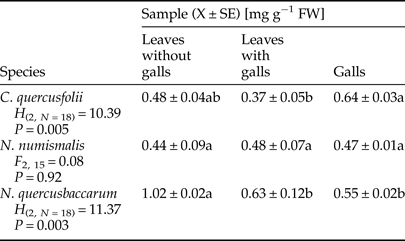
Quantification of total soluble protein in non-galled and galled tissues is shown in Table 1, and it is clear that the protein content was highly variable and depended on gall maker species. It was reduced by 22.92 and 38.24% in leaves with galls of C. quercusfolii and N. quercusbaccarum, respectively, when compared with ungalled leaves. Further, the protein content was higher in leaves with N. numismalis galls than in control and even higher than that of galls themselves. On the other hand, the content of these biomolecules in galls of N. quercusbaccarum was significantly lower than in the control leaves.
Table 1. Total soluble protein content in galled and ungalled tissues of oak leaves.
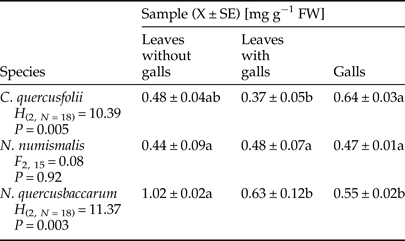
Values followed by the same letter in the same line do not differ significantly at P < 0.05.
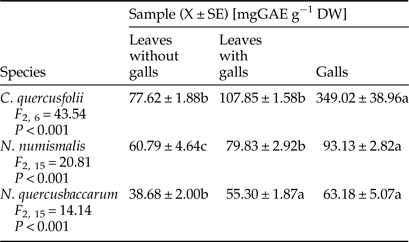
The pattern of phenolic compound contents was similar in relation to particular gall wasp species. In general, galled leaves and galls of three Cynipidae species contained significantly higher content of soluble phenols than the control tissues. Galls of C. quercusfolii were characterized by extremely high phenols levels of 349.02 mg GAE g−1 DW, which was 3.7-fold and 5.5-fold higher than in galls of N. numismalis and N. quercusbaccarum, respectively (Table 2).
Table 2. Total phenols content in galled and ungalled tissues of oak leaves.

Values followed by the same letter in the same line do not differ significantly at P < 0.05.
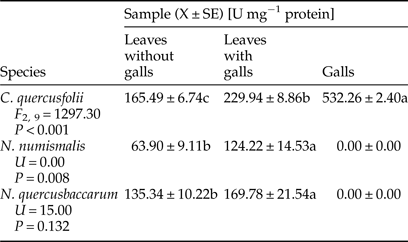
A significant increase in the activity of POD was observed in galled leaves of C. quercusfolii and N. quercusbaccarum compared with ungalled leaves. The sharpest, almost fivefold higher, increase was recorded in N. quercusbaccarum (Table 3). A significant reduction in the activity was observed in galls of all Cynipidae species. The level of POD activity in C. quercusfolii galls was the lowest and hardly detectable.
Table 3. Activity of guaiacol peroxidase (POD) in galled and ungalled tissues of oak leaves.
Values followed by the same letter in the same line do not differ significantly at P < 0.05.
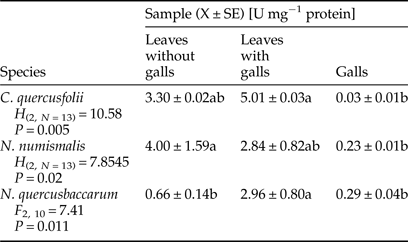
Galled leaves and galls of C. quercusfolii were characterized by significantly higher PPO activity compared with the control samples, as a 3.2-fold difference between gall tissues and control was measured (Table 4). PPO activity was significantly higher in leaves with N. numismalis and N. quercusbaccarum galls compared with ungalled leaves. However, this enzyme showed no activity in the galls of both species.
Table 4. Activity of polyphenol oxidase (PPO) in galled and ungalled tissues of oak leaves.
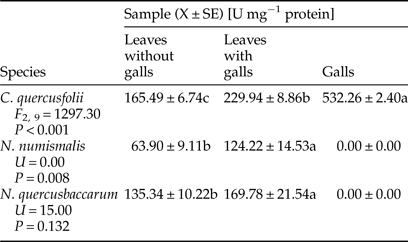
Values followed by the same letter in the same line do not differ significantly at P < 0.05.
Chitinase activity profile varied depending on the gall maker species (Table 5). A significant induction in this enzyme activity was observed in the leaves with galls and galls of both, C. quercusfolii and N. quercusbaccarum in comparison with control samples. The presence of N. numismalis galls on the leaves had a significant effect on the increase in chitinase activity. However, its activity in galled tissues was significantly decreased by 70% when compared with control.
Table 5. Activity of chitinase in galled and ungalled tissues of oak leaves.
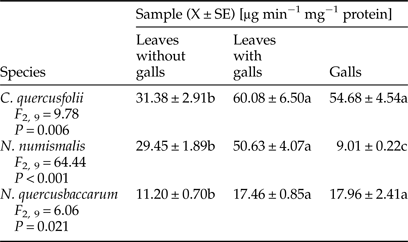
Values followed by the same letter in the same line do not differ significantly at P < 0.05.
A trend similar to chitinase activity was found for β-1,3-glucanase. Only gall tissues of N. numismalis demonstrated decreased activity of β-1,3-glucanase when compared with ungalled host plant leaves (Table 6). A significantly higher activity of this enzyme was found in the leaves with galls of C. quercusfolii (73.12%) and N. numismalis (106.92%) as well as in galls of N. quercusbaccarum (81%) compared with ungalled leaves.
Table 6. Activity of β-1,3-glucanase in galled and ungalled tissues of oak leaves.
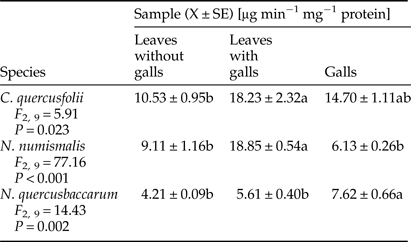
Values followed by the same letter in the same line do not differ significantly at P < 0.05.
Discussion
Gall-making species provoke the action of mechanical and chemical stimuli, which leads to the formation of new tissues, namely galls (Mukherjee et al., Reference Mukherjee, Lokesh, Aruna, Sharma and Sahay2016). Their morphological and anatomical structure is characteristic of the gall-maker species even on the same host plant. However, factors that stimulate specific plant response to this insects feeding are not known (Hartley, Reference Hartley1998). In the cynipid-oak system, which we studied, the chemical changes of leaves with galls and galls of all cynipid species were not strikingly convergent. This confirms the hypothesis that the biochemical features of galled tissues are determined by the species of galling herbivores (Isaias et al., Reference Isaias, Oliveira, Carneiro, Kraus, Fernandes and Santos2014).
The infested and control leaves exhibited different protein profiles depending on the gall cynipid species. The highest protein concentration was observed in galls of C. quercusfolii, which was consistent with the results of Gailite et al. (Reference Gailite, Andersone and Ievinsh2005). Nutrients level around the larva is usually enhanced, but chemical defense is decreased (Isaias et al., Reference Isaias, Oliveira, Carneiro, Kraus, Fernandes and Santos2014). Ni et al. (Reference Ni, Quisenberry, Heng_Moss, Markwell, Sarath, Klucas and Baxendale2001) suggested that feeding of piercing-sucking insects could induce local accumulation of proteins, which indicated that infested plants were likely to exhibit enzymatic response. Own studies indicated that this cannot be applied generally to the whole Cynipidae, which are chewing insects, because feeding of N. quercusbaccarum significantly decreased protein content compared with control. The decrease in protein biosynthesis during the stress condition resulted from the drain of amino acids (Khattab, Reference Khattab2007) and the whole translation machinery shifted to produce defense-related proteins (Singh et al., Reference Singh, Dixit, Verma and Kumar2013). Leaves with N. numismalis galls contained higher levels of proteins than leaves with galls of other gall-making species when compared with control, but the rate of protein synthesis declined under stress. In our study, three gall making cynipid species modified the protein levels of surrounding tissues, but galls cannot be treated as protein sinks serving as a rich food source for gall-inducing agent (Isaias et al., Reference Isaias, Oliveira, Carneiro, Kraus, Fernandes and Santos2014). This is also consistent with the study of Schönrogge et al. (Reference Schönrogge, Harper and Lichtenstein2000).
Galled leaves and galls of all cynipid species contained significantly higher levels of phenols than the control tissues. Generally, the content of phenolic compounds increased as a consequence of defense reaction to the infestation (Biswas et al., Reference Biswas, Chakraborty and Baidyanath2014). According to Inbar et al. (Reference Inbar, Mayer and Doostdar2003), a high level of phenolics in gall tissues can be treated as insect manipulation of the host plants aimed at reducing the risk of natural enemy attacks or pathogen infection. In turn, degradation of phenolic compounds in infested plants makes the host plant more susceptible. It has been earlier reported by Barbehenn et al. (Reference Barbehenn, Cheek, Gasperut, Lister and Maben2005); Lattanzio et al. (Reference Lattanzio, Lattanzio, Cardinali and Imperato2006); War et al. (Reference War, Paulraj, Ahmad, Buhroo, Hussain, Ignacimuthu and Sharma2012).
PODs can have diverse roles in plant response to insect feeding. On the one hand, they are involved in the efficient removal of ROS (Gulsen et al., Reference Gulsen, Eickhoff, Heng-Moss, Shearman, Baxendale, Sarath and Lee2010), on the other, in generation of highly reactive quinones, which are toxic for insects (Shivashankar et al., Reference Shivashankar, Sumathi and Ranganath2012). The increase of POD activity due to insect feeding has been recorded in different plant species (Gailite et al., Reference Gailite, Andersone and Ievinsh2005; Taggar et al., Reference Taggar, Gill, Gupta and Sandhu2012; Golan et al., Reference Golan, Rubinowska and Górska-Drabik2013; Kot et al., Reference Kot, Kmieć, Górska-Drabik, Golan, Rubinowska and Łagowska2015) and can be used with POD as a biochemical marker of resistant cultivars (Wei et al., Reference Wei, Zhikuan and Qingfang2007; Gulsen et al., Reference Gulsen, Eickhoff, Heng-Moss, Shearman, Baxendale, Sarath and Lee2010; Soffan et al., Reference Soffan, Alghamdi and Aldawood2014). Enhance POD activity indicates that the stress was induced and the secondary metabolites were released as a defensive mechanism (Biswas et al., Reference Biswas, Chakraborty and Baidyanath2014). In our study, higher activity of this enzyme was found in galled leaves compared with control leaves with the exception of N. numismalis. In turn, the activity of POD was low in galled tissues of all Cynipidae species, which was consistent with our working hypothesis that the gall-maker species manipulate biochemistry of host plants for their own needs.
PPO is a key secondary metabolism enzyme and its enhanced activity may increase ROS scavenging capacity (He et al., Reference He, Chen, Chen, Lv, Deng, Fang, Liu, Guan and He2011). In our research, the activity of PPO was significantly increased in the leaves with galls of all species, which may indicate a defensive reaction of plants. The highest PPO activity observed in galled tissue of C. quercusfolii did not corroborate the observations of Gailite et al. (Reference Gailite, Andersone and Ievinsh2005). This enzyme showed no activity in the galls of N. numismalis and N. quercusbaccarum. This may be related to the action of PPO on phenolic compounds that changes a variety of cellular constituents, including proteins, leading to the reduction of their nutritional value for the insect (Shivashankar et al., Reference Shivashankar, Sumathi and Ranganath2012). In turn, Allison & Schultz (Reference Allison and Schultz2005) suggested that measurement of PPO activity depends to a large extent on the species of the plant as well as PPO may be difficult to extract from some plant tissues. The lack of activity of PPO modified dietary proteins for the gall-maker's needs. However, according to Musser et al. (Reference Musser, Cipollini, Hum-Musser, Williams, Brown and Felton2005), insects have the ability to suppress plant defense response through signal interactions.
It has been well documented that PR proteins (e.g., β-1,3-glucanase, chitinase) are associated with plant defense mechanism against pathogen infections, because of their ability to degrade fungal cell walls (El-Khallal, Reference El-Khallal2007; Ashry & Mohamed, Reference Ashry and Mohamed2012). However, it has also been proven that chitinase and β-1,3 glucanase affect Bemisia argentifolia (Mayer et al., Reference Mayer, Inbar, McKenzie, Shstters, Borowicz, Albrecht, Powell and Doostdar2002), Bemisia tabaci (Antony & Palaniswami, Reference Antony and Palaniswami2006), Myzus persicae (Zhao et al., Reference Zhao, Zhang, Xuel and Zhang2015) and galling aphids (Inbar et al., Reference Inbar, Mayer and Doostdar2003), as phloem-feeding insects. In our study, chitinase activity was highly induced in the leaves with galls of all cynipid species, especially those infested with C. quercusfolii. The same results were obtained by Inbar et al. (Reference Inbar, Mayer and Doostdar2003) in aphid galls on Pistacia atlantica, and these authors suggested that plant chitinases showed limited effects on insect fitness. The activity of β-1,3-glucanase demonstrated a similar trend as the activity of chitinase. Insect galls are exposed to the attack of pathogenic microorganisms, especially fungi, due to the breach of plant tissues structures. According to Sampson & Gooday (Reference Sampson and Gooday1998), PR proteins, such as chitinase and β-1,3-glucanase, induced locally by galling aphids, may protect the galls and aphids from pathogenic infections. On the other hand, chitinases can negatively affect insect growth and development through the ability to damage the chitin-based peritrophic membrane of insects. In the case of β-1,3-glucanase (which are molecules that are known as signal to other resistance reaction in plants), to release of oligosaccharides, from the plant cell wall has been postulated as its role in the insect resistance (Zhao et al., Reference Zhao, Zhang, Xuel and Zhang2015). These two PR-proteins are regulated in different ways and are vulnerable to genetic manipulation (McCollum et al., Reference McCollum, Doostdar, McDonald, Shapiro, Mayer, Timmer and Sonoda1995).
In conclusion, the gall-making Cynipidae can induce the defensive response in host plants and manipulate the biochemical machinery of the galls for their own needs. However, the direction and intensity of changes in the activity of certain defense enzymes are insect species dependent.
Acknowledgements
The study was financed by the University of Life Sciences in Lublin, Poland (Project OKE/DS/2 in 2013–2017).