Management Implications
The results of this genetic analysis identify native range origins of invasive Megathyrsus maximus (Guineagrass) in south Texas, potentially enabling identification of highly host-specific biological control agents that will attack the invasive short form of M. maximus but will not attack the desirable tall form that is a valuable forage in the neotropics.
Introduction
Guineagrass [Megathyrsus maximus (Jacq.) B.K. Simon and S.W.L. Jacobs] is a large-statured tropical grass that has been planted around the world for use as livestock forage (Burton et al. Reference Burton, Millot and Monson1973). Native to intertropical and southeast Africa, it is now considered an invasive species in parts of Asia, North and South America, Australia, and many tropical islands (CABI 2021) due to its ability to shade and outcompete other species (Rhodes et al. Reference Rhodes, Plowes, Goolsby, Gaskin, Musyoka, Calatayud, Cristofaro, Grahmann, Martins and Gilbert2021). In subtropical southern Texas, it is implicated in the long-term declines of ground-dwelling granivorous birds such as quail and the facilitation of cattle fever tick [Rhipicephalus microplus (Canestrini)] invasion (Esteve-Gassent et al. Reference Esteve-Gassent, Pérez de León, Romero-Salas, Feria-Arroyo, Patino, Castro-Arellano, Gordillo-Pérez, Auclair, Goolsby, Rodriguez-Vivas and Estrada-Franco2014; Mercadier et al. Reference Mercadier, Goolsby, Jones and Tamesse2009; Vacek et al. Reference Vacek, Goolsby, Calatayud, Le Ru, Musyoka and Kariyat2021).
Megathyrsus maximus reproduces primarily through apomixis, but some diploid and perhaps tetraploid sexual strains exist (Kaushal et al. Reference Kaushal, Paul, Saxena, Dwivedi, Chakraborti, Radhakrishna, Roy and Malaviya2015; Nakajima et al. Reference Nakajima, Komatsu, Mochizuki and Suzuki1979), and it forms an agamic complex with Megathyrsus infestus (Andersson) B.K. Simon & S.W.L. Jacobs (syn.: Panicum infestum Andersson) and Panicum trichocladum K. Schum (Muir and Jank Reference Muir and Jank2004). Hybrids of these species are found in East Africa (Savidan and Pernès Reference Savidan and Pernès1982), and perhaps elsewhere, and have made taxonomic identification difficult (Clayton and Renvoize 1982). Megathyrsus maximus has been assigned multiple scientific names over the years (see Rhodes et al. Reference Rhodes, Plowes, Goolsby, Gaskin, Musyoka, Calatayud, Cristofaro, Grahmann, Martins and Gilbert2021), and the taxonomic confusion has been compounded by introductions from multiple African sources and approximately 35 cultivars listed in the Tropical Forages website (Cook et al. Reference Cook, Pengelly, Brown, Donnelly, Eagles, Franco, Hanson, Mullen, Partridge, Peters and Schultze-Kraft2005). Both tall and short forms of M. maximus exist, with 28 and 7 cultivars listed for each, respectively. The tall form is generally 50% larger in plant height and leaf size and occurs in wetter and shadier conditions than the short form. The size differences may be due to hybridization between genotypes or differences in ploidy level (Rhodes et al. Reference Rhodes, Plowes, Goolsby, Gaskin, Musyoka, Calatayud, Cristofaro, Grahmann, Martins and Gilbert2021). In the southern United States and the neotropics, the short form of the species (abbreviated as SMm in this study) is considered invasive, while the tall form (TMm) is utilized as a forage grass, mostly in the neotropics (Rhodes et al. Reference Rhodes, Plowes, Goolsby, Gaskin, Musyoka, Calatayud, Cristofaro, Grahmann, Martins and Gilbert2021; Soti et al. Reference Soti, Goolsby and Racelis2020). The short form is considered invasive in Texas, USA, because it may better tolerate drought and can form dense stands in open pastures and disturbed areas, suppressing or displacing local plants on fertile soils in pastures (Rhodes et al. Reference Rhodes, Plowes, Goolsby, Gaskin, Musyoka, Calatayud, Cristofaro, Grahmann, Martins and Gilbert2021). Control options for invasive M. maximus include chemical applications, livestock grazing, prescribed fire, mechanical methods, and bioherbicides, but these are largely ineffective (Rhodes et al. Reference Rhodes, Plowes, Goolsby, Gaskin, Musyoka, Calatayud, Cristofaro, Grahmann, Martins and Gilbert2021). Classical biological control is also proposed for M. maximus but would require agents specific enough to manage the invasive short form but not cause significant damage to the tall form that is used as forage, especially in Mexico and Central America. Understanding the genetic diversity of the target weed in its native and introduced ranges is critical to finding the best adapted and most host-specific agents (Goolsby et al. Reference Goolsby, van Klinken and Palmer2006b; Sutton et al. Reference Sutton, Canavan, Day, den Breeyen, Goolsby, Cristofaro, McConnachie and Paterson2019).
It is possible that biological control agents of M. maximus may not be host specific enough to attack only one form and not the other, but arthropod and microbial biological control agents of weeds can be, in some cases, specific below the host-species level: for example, leaf galling mites on the fern Lygodium microphyllum (Cav.) R. Br. (Goolsby et al. Reference Goolsby, De Barro, Makinson, Pemberton, Hartley and Frohlich2006a), leafhopper insects (Cicadellidae) on Gulf coast cordgrass (Spartina alterniflora Loisel.) (Garcia-Rossi et al. Reference Garcia-Rossi, Rank and Strong2003), and the rust fungus Puccinia chondrillina Bubak & Sid on rush skeletonweed (Chondrilla juncea L.) (Burdon et al. Reference Burdon, Groves and Cullen1981). The goal of our work is to identify locations in the native range (Africa) that contain plants that are most similar genetically to the invasive short form of M. maximus found in the United States. These plants should be examined in the native range as a source of highly host-specific biological control agents.
Materials and Methods
We obtained fresh, silica-dried leaf material from 198 Megathyrsus plants from Florida and Texas in the United States (n = 34 short form; n = 9 tall form) and Africa (n = 155; Figure 1). All U.S. plants were weedy accessions from pastures, ditches, or roadway edges. The African populations were collected randomly from batches along roads or ways, and the species identification of the plant was confirmed by comparison with specimens from the East African herbarium in Nairobi and by using the key of Maitland and Hubbard (Reference Maitland and Hubbard1927). Due to uncertainty in distinguishing tall and short forms in Africa, we did not morphologically identify at the subspecific level. We selected one disease-free young leaf from each plant, and plants sampled were at least 5 m apart. We performed DNA sequencing (n = 144) and amplified fragment length polymorphism (AFLP) analysis (n = 198) on these plants (Supplementary Material, Data File 1). We extracted genomic DNA from approximately 20 mg of silica-dried leaf material using a modified hexadecyltrimethylammonium bromide (CTAB) method (Hillis et al. Reference Hillis, Moritz and Mable1996). For DNA sequencing we followed Gaskin and Wilson (Reference Gaskin and Wilson2007). We sequenced the chloroplast tRNA-Leu (trnL) gene and trnL-trnF intergenic spacer using the primer pair “c” and “f” of Taberlet et al. (Reference Taberlet, Geilly, Pautou and Bouvet1991). All unique sequences (GenBank accessions MT327722 to MT327739) were aligned with Clustal W (980-bp length alignment) and constructed into a bootstrap (1,000 repeats) consensus phylogenetic tree using maximum parsimony under default parameters, all using MEGA X (Kumar et al. Reference Kumar, Stecher, Li, Knyaz and Tamura2018). Our AFLP method followed Vos et al. (Reference Vos, Hogers, Bleeker, Reijans, van de Lee, Hornes, Frijters, Pot, Peleman and Kuiper1995) with modifications as in Gaskin and Kazmer (Reference Gaskin and Kazmer2009). All 15 selective primer combinations of MseI + CAA, CAC, CAT, CTA, or CTA and EcoRI + AAG, ACC, or ACT were prescreened for PCR product quality and number of variable loci using eight plant samples, and the two most polymorphic primer pairs were chosen (MseI + CAC/EcoRI + ACC and MseI + CAC/EcoRI + ACT). We generated all AFLP data on an Applied Biosystems 3130 Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA 02451, USA), and omitted any individuals that did not produce a typical electropherogram pattern (i.e., noise >20 relative fluorescence units or failure to produce a sufficient number of peaks). We used NTSYS-pc v. 2.1 software (Rohlf Reference Rohlf1992) (SIMQUAL program) to calculate the Dice (Reference Dice1945) similarity coefficient. Principal coordinates analysis (PCoA) was performed on Dice similarity coefficients using the DCENTER and EIGEN modules of NTSYS.

Figure 1. Map positions of the Megathyrsus samples collected in sub-Saharan Africa. Country codes are followed by number of samples collected. Red circles indicate ≥80% genetic match (amplified fragment length polymorphism [AFLP]) to U.S. short-form Megathyrsus maximus (SMm); blue circles indicate ≥80% match to U.S. tall-form M. maximus (TMm). No circle indicates <80% match to U.S. samples. Country codes: BE, Benin; BT, Botswana; CR, Cameroon; DC, Democratic Republic of Congo; ET, Ethiopia; GH, Ghana; IC, Ivory Coast; KY, Kenya; MZ, Mozambique; NI, Nigeria; SA, South Africa; TA, Tanzania; TO, Togo; UG, Uganda; ZI, Zimbabwe; ZM, Zambia.
Results and Discussion
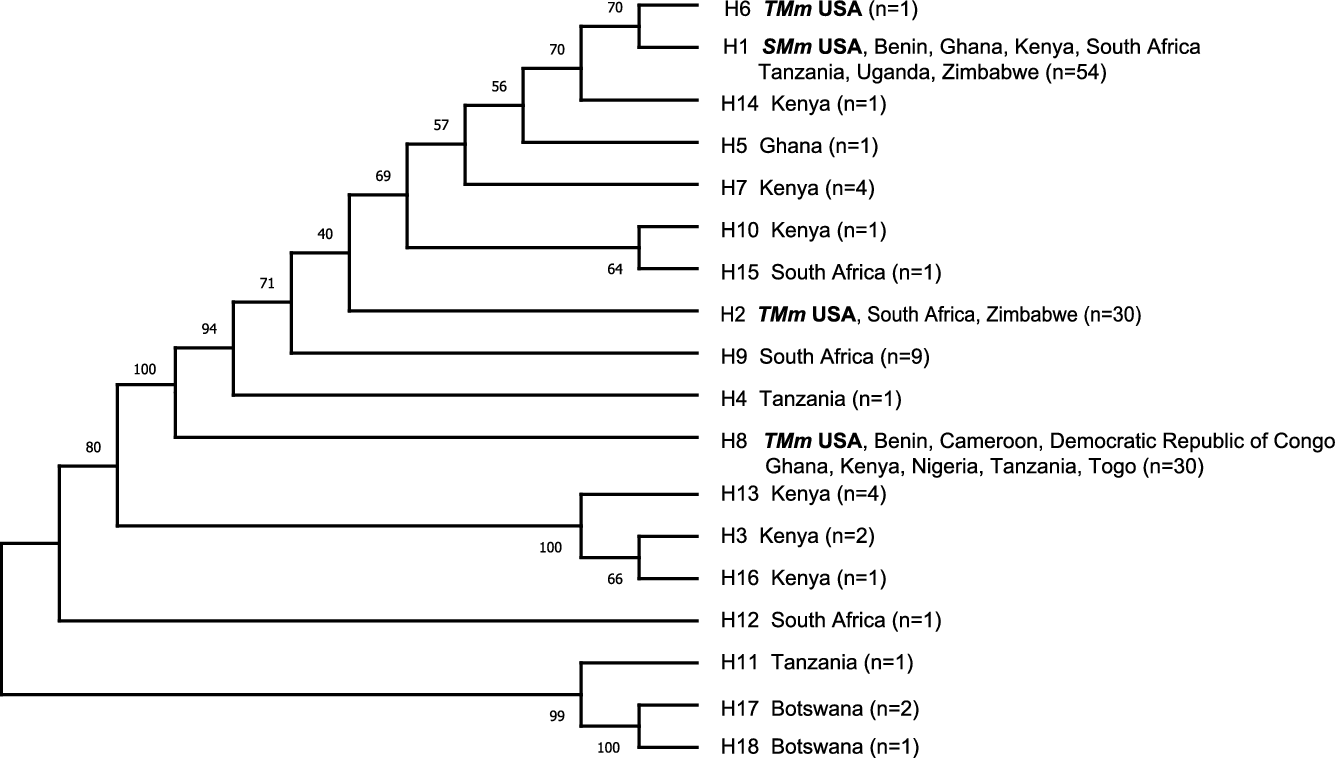
Materials from sites in the United States (nonnative) and African (native) ranges were genetically characterized with DNA sequences from the trnc-f cpDNA region in an attempt to find the geographic origins of this invasion in the United States. Eighteen haplotypes were recovered (Figure 2; Supplementary Material, Data File 2), with haplotype H1 found broadly from East Africa to South Africa providing a match for the short form (SMm) from Texas and Florida. The tall-form (TMm) haplotypes (H2 in Texas; H6 and H8 in Florida) were found across a broader range in Africa. SMm and TMm haplotypes differed by one to seven mutations, with H1 and H6 being most similar (Figure 2). Seven additional accessions were obtained and sequenced earlier by the European Biological Control Laboratory (EBCL) (Bon et al. Reference Bon, Goolsby, Mercadier, Le Bourgeois, Poilecot, Jeanneau and Kirk2011) for the trnL-trnF intergenic spacer only (436 bp of the larger region that we sequenced; Supplementary Material, Data File 2), and accessions from Mali, Central African Republic, Cameroon, Benin, and French Guiana (South America) were identical to H8, and accessions from Djibouti and Mozambique were identical to H1, H2, H6, H7, H9, and H14 for this shorter intergenic region.
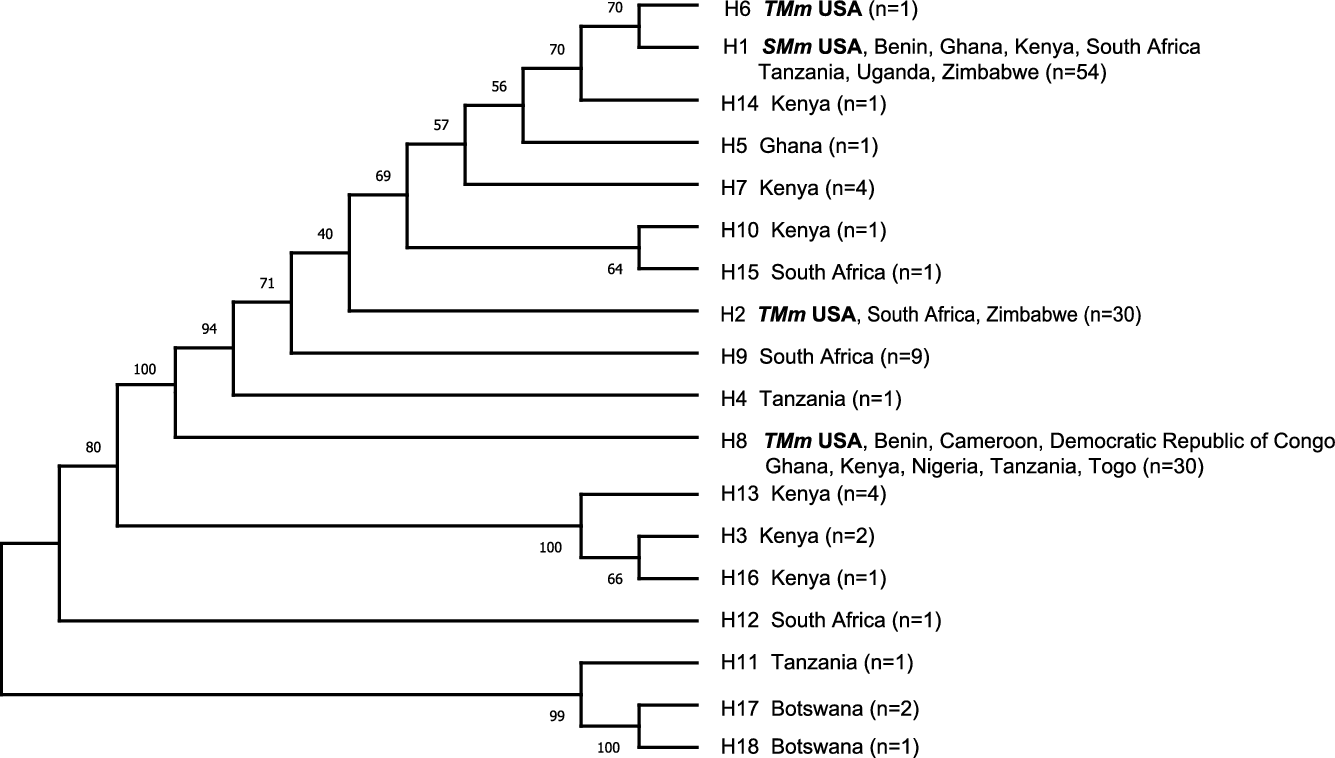
Figure 2. Consensus maximum parsimony phylogeny of 18 trnc-f chloroplast region DNA haplotypes (980 bp) found in 144 Megathyrsus samples from United States and Africa. Bootstrap values (1,000 replicates) are shown next to the branches. SMm, short-form Megathyrsus maximus; TMm, tall-form M. maximus.
To develop a higher-resolution estimate of the closest African match for the U.S. samples, we used 68 variable AFLP loci. For the U.S. samples, we found 8 SMm AFLP genotypes in 34 samples and 3 TMm AFLP genotypes in 9 samples (Figure 3). The range of genetic similarity (AFLPs) between TMm and SMm was from 40% to 69%. Similarity within U.S. SMm was 68% to 100%, and 60% to 100% within U.S. TMm. Genetic similarity of African plants to SMm ranged from 0% to 81%. Plants that were most similar to SMm (≥80%) were from Mkuze and Durban, South Africa. Similarity of African plants to TMm ranged from 0% to 85%. Plants that were genetically (AFLP) most similar to TMm (≥80%) were from Kenya, Uganda, Ivory Coast, and Zambia. Highest similarities were from Kenya (85%) (Supplementary Material, Data File 3). Variation in ploidy (e.g., Collins and Müller-Schärer Reference Collins and Müller-Schärer2012), as well as nuclear DNA content in plants of the same ploidy (e.g., Hopkins et al. Reference Hopkins, Taliaferro, Murphy and Christian1996), can potentially alter phenotype, life form, and/or demographics of plants. These differences may affect biological control efficacy, thus it would be wise to investigate the chromosome numbers and DNA content of invasive M. maximus accessions and their genetically similar accessions from Africa.
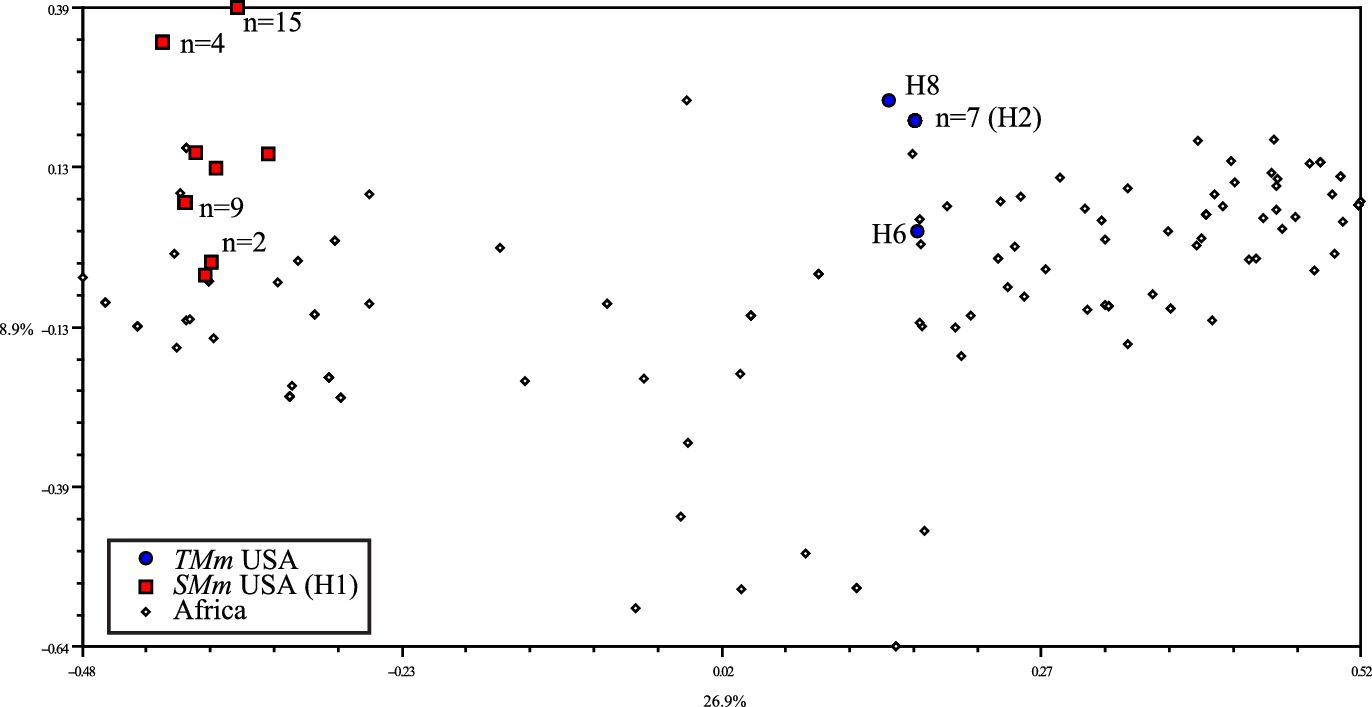
Figure 3. Principal coordinates analysis (PCoA) of amplified fragment length polymorphism (AFLP) data from 68 polymorphic loci for 198 U.S. and African Megathyrsus samples. If not indicated otherwise for tall-form M. maximus (TMm) or short-form Megathyrsus maximus, n = 1. Chloroplast DNA haplotypes (e.g., H1) are indicated for U.S. AFLP genotypes (red and blue symbols).
The chloroplast sequences are useful to unravel interspecific divergences but are not fine-scale enough to distinguish origins of U.S. genotypes. As we found the invasive SMm cpDNA haplotype in seven African countries, we used AFLPs to obtain a higher resolution of genetic diversity and narrow down origins of SMm to South Africa. Multiple AFLP genotypes of both SMm and TMm in the United States confirm that multiple introductions have occurred for both forms, or multiple genotypes in fewer introductions, and point to the need to carefully evaluate each form or cultivar for invasive potential before introduction for agriculture. Though we did not perform in-depth population sampling, we did sample multiple plants (n = 3) at nine U.S. SMm locations; within four of these locations, the plants had identical AFLP genotypes (see pairwise Dice values, Supplementary Material, Data File 3), suggesting clonal reproduction. The remaining SMm locations contained multiple genotypes, suggesting that there is some outcrossing occurring, or multiple genotypes were introduced; this does not exclude clonal reproduction such as tillering. Outcrossing is well documented in M. maximus (Nakajima et al. Reference Nakajima, Komatsu, Mochizuki and Suzuki1979); hybridization has been extensively used in breeding programs; and there is evidence of reproductive plasticity, including facultative apomixis (see Rhodes et al. Reference Rhodes, Plowes, Goolsby, Gaskin, Musyoka, Calatayud, Cristofaro, Grahmann, Martins and Gilbert2021). This complex situation suggests that the predominant reproductive mode of the invasion should be further investigated with more in-depth sampling, ploidy analysis, and fine-scale genetic analysis. Knowledge of a plant’s reproductive mode can help determine which guild of biological control agents will be most effective at significantly impacting the physiology of this invasive grass.
Populations in Africa that contain SMm genotypes most similar to those in the United States (two localities in South Africa; Figure 1) should be more intensively surveyed for potential coevolved biological control agents. Potential agents should also be assessed for efficacy on all U.S. SMm genotypes and for lack of significant development of or damage to U.S. TMm genotypes identified in this study. This fine-scale identification of origins helps identify coevolved natural enemies that may be safer and more efficacious biological control agents (Gaskin et al. Reference Gaskin, Bon, Cock, Cristofaro, De Biase, De Clerck-Floate, Ellison, Hinz, Hufbauer, Julien and Sforza2011; Harms et al. Reference Harms, Cronin, Diaz and Winston2020).
Three candidate agents have undergone preliminary screening on SMm and TMm and the closely related North American grass, switchgrass (Panicum virgatum L). A stem-boring moth (Buakea kauae Moyal), known only from SMm in Kenya was imported, but researchers attributed the lack of success in rearing this agent to the genetic mismatch between SMm in Kenya and Texas, USA (Vacek et al. Reference Vacek, Goolsby, Calatayud, Le Ru, Musyoka and Kariyat2021). Two eriophyoid mites collected from SMm, Abacarus sp. (Eriophyidae) from Mpala, Kenya, and Diptacus sp. (Diplomatidae) from Hluhluwe, South Africa (a high genetic match of 79% with SMm from Texas), were imported, but both were able to develop on SMm and TMm (JAG, unpublished data), so are not specific enough to be used as biological control agents of the SMm form of M. maximus. Additional exploration is needed in South Africa and other places in Africa that closely match SMm genotypes to discover and evaluate potential biological control agents of invasive M. maximus in the United States.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2022.7
Acknowledgments
We acknowledge the financial support of Lee and Ramona Bass Foundation, which enabled the foreign exploration and Africa-wide collections of M. maximus for genetic characterization. We would like to give special thanks to all plant collectors; Alan Kirk and Guy Mercadier (EBCL), Georg Goergen (IITA, Benin), Rose Ndemah (IITA, Cameroon), Onésime Mubenga (University of Kisangani, Democratic Republic of Congo), Eric Ntiri (Togo), Régis Babin (CIRAD, Ivory Coast), Tomás Chiconel, Francisco Munguambe and Domingos Cugala (University of Eduardo Mondlane, Mozambique), Johnnie Van Den Berg (North West University, South Africa), Bonoukpoè M. Sokame and Boaz Musyoka (icipe, Kenya), Richard Molo (National Agricultural Research Laboratories, Uganda), Muluken Goftishu Muleta (Haramaya University, Ethiopia), Chapwa Kasoma and Gilson Chipabika (Zambia Agricultural Research Institute, Zambia), Reyard Mutamiswa and Casper Nyamukondiwa (Botswana International University of Science and Technology, Botswana), Francesca Marini and Francesca di Cristina (BBCA, Rome, Italy). We thank Iain Paterson and Guy Sutton (Rhodes University, Grahamstown, South Africa) for their input on this project, and we also thank Melanie Jeanneau (EBCL) and Kim Mann and Jeannie Lassey (USDA-ARS Sidney, MT, USA) for DNA sequencing and AFLP data. This article reports results of research only, and mention of a proprietary product does not constitute an endorsement or recommendation by the USDA for its use. USDA is an equal opportunity provider and employer. No conflicts of interest have been declared.