Nowadays, the first option of the native mitral valve surgery remains repair. This is particularly important in children where there is still no implantable material with proven growth potential. However, in some patients, the mitral valve replacement becomes the only surgical option, although there is no ideal prosthesis for children – both mechanical and biological valves have limitations. Observations in small children show that mitral valve replacement is associated with a complicated postoperative course, as well as with high early and late mortality rate.Reference Sawan, Brink and Soquet 1 Also, the need of reoperation is high in this group of children because of a structural valve degeneration, pannus formation, infective endocarditis complications, and outgrowth of the valve.Reference Trezzi, Cetrano, Iacobelli and Carotti 2 The Edwards Inspiris Resilia® valve (Edwards Lifesciences, Irvine, California, United States of America) was primarily designed as aortic valve, but because of availability of small size it could be potentially used in mitral position in children.
Case description
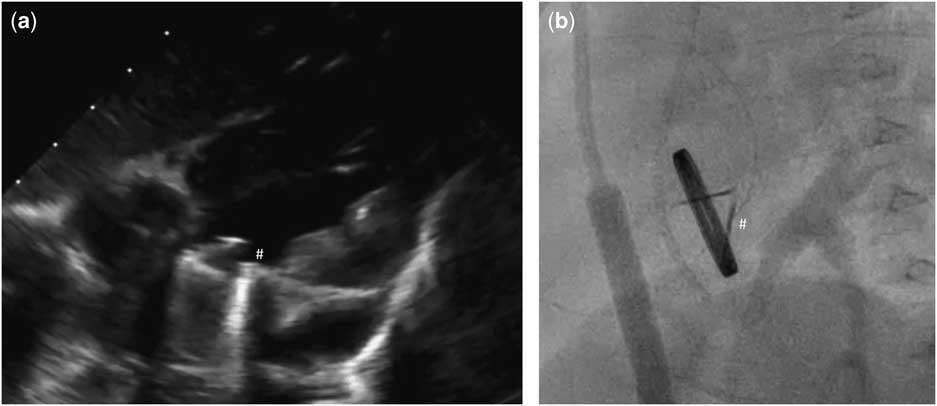
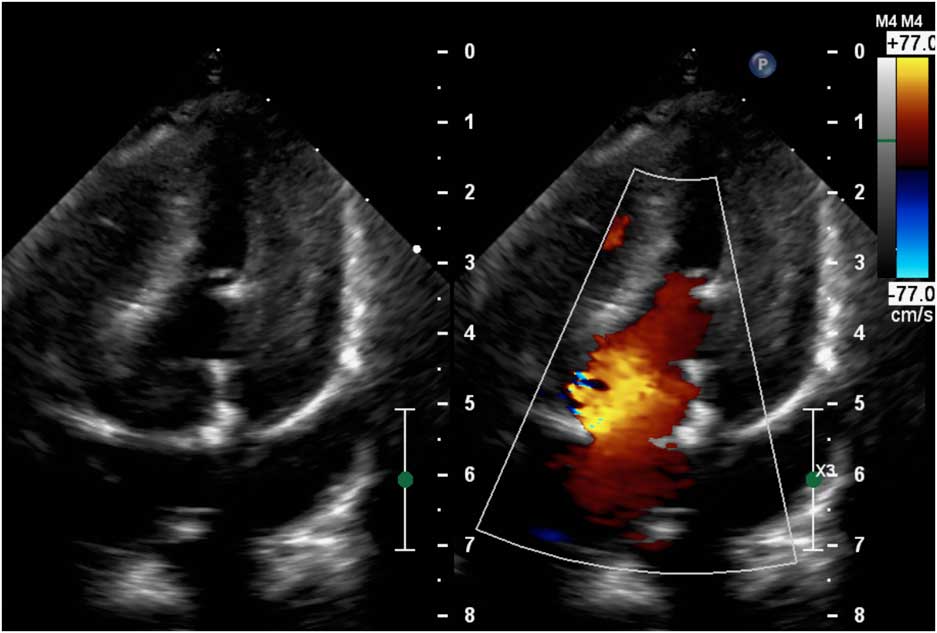
Our patient was an 11-month-old girl weighting 7.3 kg with corrected anomalous origin of the left coronary artery from the pulmonary artery (Bland–White–Garland syndrome) and severe dysfunction of mechanical mitral prosthesis, associated with blockade of one disc. After birth, the girl presented increasing heart insufficiency with mitral regurgitation and vomiting with failure to oral feeding. Anomalous origin of the left coronary artery from the pulmonary artery was first diagnosed in 1.5 months of life after general status deterioration. An emergency surgical correction was performed. The surgery was complicated with low cardiac output requiring extracorporeal membrane oxygenation support for 6 days. Severe mitral valve insufficiency was observed postoperatively without any improvement. The child was operated again in postoperative day 6 – after careful surgeon’s evaluation mitral valve plasty was impossible and the mitral valve replacement with St Jude® 17 mm mechanical valve was performed. The postoperative course was complicated with delayed sternal closure, renal insufficiency requiring peritoneal dialysis, diaphragm paralysis requiring plication, anti-reflux surgery, and the need of gastrostomy creation. Oral feeding was impossible because of unresolved problems with intestinal absorption – chronic intestinal pseudo-obstruction was finally diagnosed. Because oral coumarin was not tolerated, anticoagulation was maintained with low molecular weight heparins. But 7 months after mitral valve replacement the child’s general status deteriorated again. In echocardiography, it was observed that one disc of mechanical valve was blocked which was confirmed by angiographic examination (Fig 1; Supplementary Video 1). Neither heparin nor alteplase infusion resolved the problem. On continuous heparin infusion, the child was referred for another surgical mitral valve replacement. The use of biological prosthesis was considered because of persisted impossibility for oral anticoagulation. At the age of 11 months, the mechanical valve was removed. The new Edwards Inspiris Resilia® valve (19 mm) was implanted in reversed position. Postoperative echocardiography showed excellent function of the mitral prosthesis, with no pronounced regurgitation or stenosis as well as no left ventricular outflow tract obstruction (Fig 2; Supplementary Video 2). The postoperative course was complicated with temporary renal insufficiency requiring peritoneal dialysis and whole-body oedema because of capillary leakage syndrome. In postoperative day 9, the child was discharged from ICU in good general status, and after 2.5 months our patient was discharged home. Standard anti-aggregation therapy was prescribed until 3 months postoperatively. The follow-up time is now 6 months.
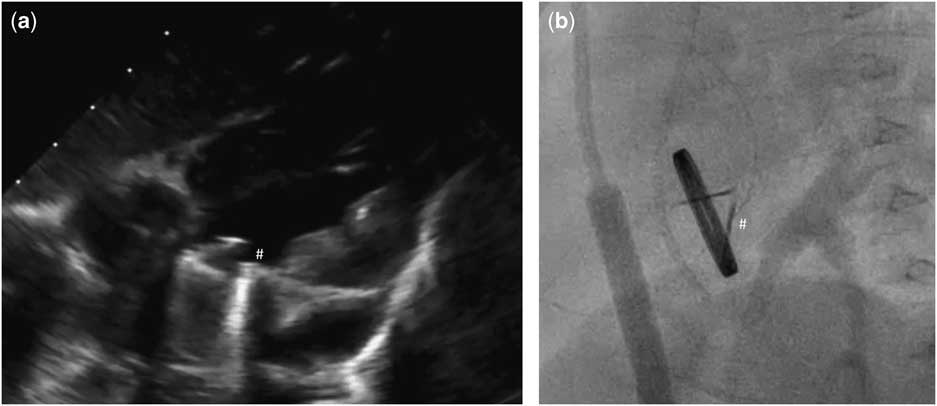
Figure 1 Echocardiographic (a) and angiographic (b) examination showing one disc blockade (#) of mechanical valve in mitral position.
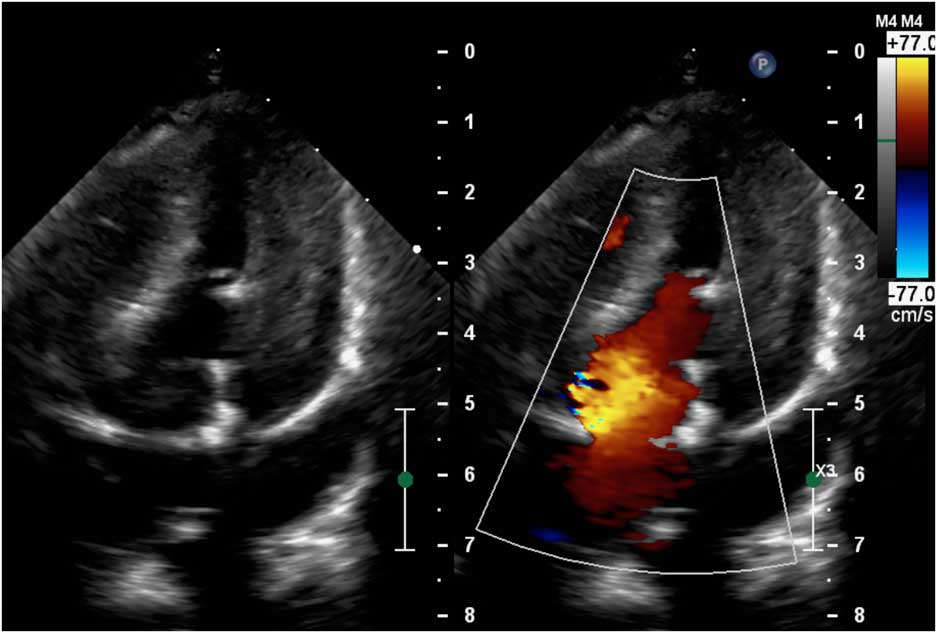
Figure 2 Postoperative echocardiography showing excellent function of the Edwards Inspiris Resilia® valve in mitral position.
Comment
Mitral valve surgery in children with anomalous origin of the left coronary artery from the pulmonary artery remains a controversial issue. There is no consensus whether it should be performed at the initial operation at all. Theoretically, the coronary replacement allows improvement in left ventricle function with mitral insufficiency reduction in consecutive days after surgery.Reference Alexi-Meskishvili, Nasseri and Nordmeyer 3 But in our patient even 6 days after anomalous origin of the left coronary artery from the pulmonary artery correction despite extracorporeal membrane oxygenation, the mitral valve insufficiency did not improve. At the second operation, the mitral valvuloplasty was impossible and mitral valve replacement was performed. Mitral valve replacement is not a common procedure in children and reported case series underline poor outcomes – perioperative mortality remains high in infants and reaches even 42%.Reference Sawan, Brink and Soquet 1 , Reference Beierlein, Becker and Yates 4 Late observations in this specific group of patients are also unsatisfactory with reoperation rate as high as 50% at 10 years of follow-up and late mortality of even 30%.Reference Sawan, Brink and Soquet 1
The use of bio-prosthesis in the mitral position is not new, but to the best of our knowledge, this is the first report where biological valve has been implanted in a so small child after mechanical mitral valve failure with impossible oral drugs intake. There are attempts to find the best option for children with the strong indication for mitral valve replacement with biological valve. One of the possibilities is autotransplant with own aortic valve (Ross II operation). It is a valuable alternative in low-risk patients, but its limitations are known.Reference Abdullah, Ramirez, McElhinney, Lock, del Nido and Emani 5 Another possibility is the off-label implantation of Melody® valve in the mitral position, which was primarily approved for transcatheter replacement of the pulmonary valve. It was first performed in 2010 and has been followed worldwide.Reference Athanasiou, Cherian and Ross 6 – Reference Quiñonez, Breitbart, Tworetsky, Lock, Marshall and Emani 8 There are also reports of implantation of the Edwards Sapien 3® valve, which is thought by some authors to have potential advantages relative to the Melody® valve.Reference Trezzi, Cetrano, Iacobelli and Carotti 2
In our opinion, the Edwards Inspiris Resilia® may be a reasonable option as biological valve for mitral valve replacement. The smallest diameter of 19 mm makes it one of the smallest biological valves suitable for children. According to the manufacturer information the valve is made of bovine pericardial tissue transformed by integrity preservation technology with stable-capping and glycerolisation which should improve anti-calcification properties. 9 The valve incorporates also two features designed for potential future valve-in-valve procedures – it has fluoroscopically visible size markers at each commissure and expansion zone. Finally, it does not require oral anticoagulation, which was impossible to maintain in our patient. One of the limitations of implantation of the Edwards Inspiris Resilia® valve in mitral position is the possibility of left ventricle outflow tract obstruction as well as pulmonary vein obstruction because of the relative high profile of the valve designed for aortic position. This fortunately has not occurred in our patient.
Our report highlights the possibility of the use of Edwards Inspiris Resilia® valve for the treatment of mitral valve disease in children with a valve annulus of appropriate size and may be a therapeutic option with off-label indications for selected patients. However, long-term outcomes remain unknown and require further observation.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951118001816
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency, or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human care and with the Helsinki Declaration of 1975, as revised in 2008.