Introduction
Ventricular septal defects are the most common form of CHD after bicuspid aortic valve. Incidence of isolated ventricular septal defect is around 20%. Reference Minette and Sahn1 Surgical closure is the widely accepted gold standard of therapy for haemodynamically significant defects. Surgical closure was first performed by Lillehei in 1954, Reference Lillehei, Cohen, Warden, Ziegler and Varco2 and it remained as the only therapeutic option until 1988 when the first report of device closure of ventricular septal defect was published by Lock et al. Reference Lock, Block, McKay, Baim and Keane3
Since then, a wide range of devices have been designed and used, some of which have stood the test of time while some got withdrawn due to unacceptable complications. With the improvement in the expertise of the interventionist, device closure of ventricular septal defect has become a commonly performed procedure with very good results. Reference Liu, Wang, Gao, Tan, Zheng and Zhang4,Reference Arora, Trehan and Kumar5 Although a common procedure for older children and adolescents, the safety of the procedure is not quite adequately evaluated in young children weighing 10 kg or less with only very few operators reporting their experience in this weight category. Reference Narin, Pamukcu and Tuncay6–Reference Soriano and Villareal9 We report our initial experience with device closure of ventricular septal defects in less than 10 kg weight category with an immediate and short-term follow-up of outcome.
Materials and methods
The study was conducted at the Rabindranath Tagore International Institute of Cardiac Sciences, Kolkata. A total of 65 children of different ages were referred for ventricular septal defect closure between January, 2017 and September, 2019, out of which 16 were selected for transcatheter intervention based on our inclusion criteria. Retrospectively, the medical records of all the intervention patients were reviewed. Written, informed consent was obtained from all the parents prior to device closure. Being a retrospective study, the ethical committee clearance was waived.
Inclusion criteria: Device closure was attempted on patients with restrictive ventricular septal defects with features of growth failure or congestive heart failure, where echo demonstrated left heart volume overload. Overall, children with restrictive defects with haemodynamically significant left-to-right shunt as evidenced by left-sided chamber enlargement on echo were selected for device closure. Presence of at least 3 mm tissue rim separating the defect from the aortic or tricuspid valve and absence of significant aortic cusp prolapse and more than mild aortic regurgitation was necessary for patients to be selected for device closure. We retrospectively reviewed the records of children weighing less than 10 kg for the purpose of this study.
Exclusion criteria: Patients with malaligned defects, those associated with more than mild aortic regurgitation, associated mitral/tricuspid valve abnormalities requiring simultaneous valve repair, other lesions needing surgical correction, and non-consenting parents were referred for surgical closure.
Imaging: All patients underwent a thorough echocardiographic assessment of the defect prior to device closure. The echo parameters assessed included-location and size of the defect, its proximity to the aortic and tricuspid valves, involvement of these valve leaflets with the defect and presence of any aortic or tricuspid valve regurgitation. The defect was considered suitable for device closure if there was a aortic margin of 3 mm or more and aneurysm formation by tricuspid valve chordal tissue on the right ventricular side. Careful attention was also given to exclude mitral valve regurgitation by entrapment of chordal apparatus after device delivery from the left ventricular apex. Left heart volume overload was assessed by M-mode assessment of left atrium and ventricular size done from parasternal long-axis view. Left atrial enlargement was defined as left atrium/aorta ratio >1.5. Left ventricular enlargement was documented when the diastolic ventricular diameter z score was more than +2 for body surface area.
Chest X-ray was also performed on all patients to look for pulmonary overflow.
Pre-procedure preparation: All patients were admitted the night before the procedure. Blood investigations were done to rule out any active infection (complete blood count, C-reactive protein) to check the functional renal status (electrolytes, urea, creatinine), coagulation parameters (prothrombin time and activated partial thromboplastin time), and serology (HIV, Hepatitis B and C). A 12-lead electrocardiogram was also mandatory to check rhythm before embarking on the procedure. All patients received a dose of intravenous cefuroxime the night before and another dose immediately before the start of the procedure. Cardiac surgical back up was arranged before the procedures.
Procedure: A total of 16 patients with ventricular septal defect were under 10 kg and was considered for percutaneous device closure. Thirteen attempts were successful and three patients were referred for surgery. Ten out of 16 patients (62.5%) had perimembranous defects, and others had muscular defects (6 patients, 37.5%).
All the procedures were performed under conscious sedation. Right femoral artery and venous accesses were obtained. Patients were heparinised with 100 units/kg of heparin immediately after the introduction of the short sheath. Activated clotting time was checked at 1 hour into the procedure and repeated at that point again to maintain it above 200 seconds. Shunt assessment was performed immediately upon entry into the heart. For angiographic definition, we performed left ventricular pigtail injection on left anterior oblique projection with cranial tilting at 60o–20o and 30o–30o views for all the perimembranous defects (Fig 1a). Additionally, hepato-clavicular view was used for inlet muscular defects. We crossed the defect from the left ventricular side in six patients (46%) and from the right ventricular side in seven patients (53%). Crossing from the right ventricular side was initially attempted in all cases of perimembranous defects and if failed crossing from the left side was performed. After crossing the defect from the right side with a diagnostic Judkin’s right coronary catheter and 0.032 angled Terumo wire (GLIDEWIRE, Terumo Medical Corporation, Somerset, NJ, United States of America), the wire was parked in descending thoracic aorta. A long delivery sheath (AGA Medical Corporation, Golden Valley, MN, USA) was taken over this wire and its tip was kept inside the left ventricular cavity (Fig 1b). A device of predetermined size and shape was taken through the delivery sheath and its distal end was taken out inside the left ventricular cavity. Carefully the device was deployed across the defect by gradual unsheathing while checking its placement by transthoracic echo and fluoroscopy (Fig 1c). Particular care was taken to safeguard the mitral valve apparatus.
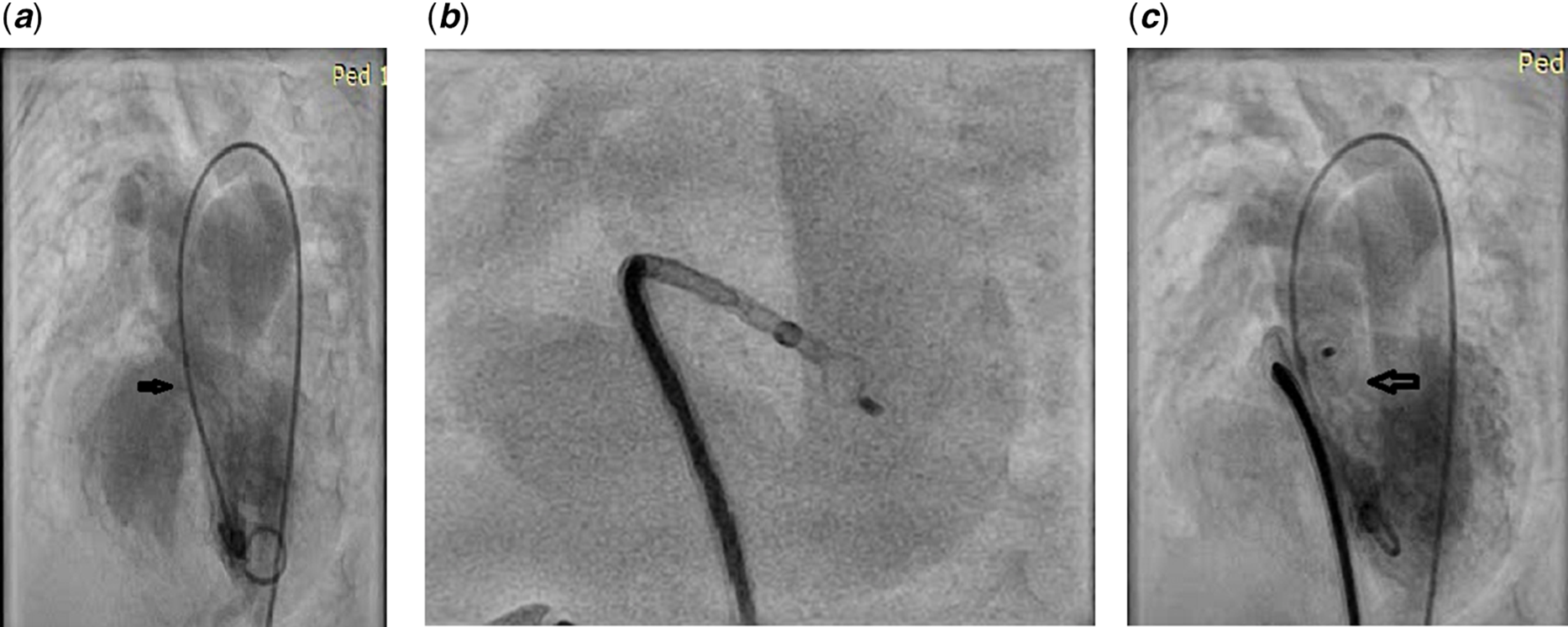
Figure 1. (a) Left ventriculogram in left anterior oblique projection to demonstrate perimembranous defect (patient 2, Table 2); (b) A 9/7 multifunctional occluder being delivered from the left ventricular apex (same patient as in Figure 1a); (c) Left ventricular angiogram after deploying the device across the defect.
For procedures that were performed from the left ventricular side, the defect was crossed with the same catheter and wire assembly and the wire was snared out from the femoral vein or right internal jugular vein (for muscular defects). After the creation of this stable arteriovenous loop, a long delivery sheath was passed from the venous side up to the ascending aorta and the device was taken through the sheath and deployed under echo and fluoroscopic guidance. We deployed 12 devices (75%) via antegrade approach, i.e., from venous side, through which we deployed Multifunctional Occluder (KONAR-MFO, Lifetech, Shenzhen, China) or Amplatzer duct occluder I (ADO I, AGA Medical Corporation, Golden Valley, MN, USA). In four patients (25%), Amplatzer Duct Occluder II (ADO II, AGA Medical Corporation) was used for retrograde device delivery from the aorta after crossing the defect from the left ventricular side where no arteriovenous loop was formed. In three patients with muscular defects (two anterior muscular and one mid-muscular), right internal jugular vein was used for device delivery.
Crossing the defect from the right ventricular side was particularly advantageous because it could avoid arteriovenous looping, potentially destabilising the cardiovascular haemodynamics in small children.
Choice of the device was carefully made based on both echocardiographic and angiographic assessment of the defect. On echo, the defect was measured both on the left and right ventricular side and the device size was decided based on the largest measured dimension (Fig 2a). Generally, the device chosen was 1–2 mm larger than the largest defect diameter measured on the right ventricular side (Fig 2b).

Figure 2. (a) Modified four chamber view showing a muscular defect measuring 6 mm (patient 9, Table 2); (b) Sub costal view showing closure of the same defect using a Cera muscular device.
Post–procedure, the patients were kept in ICU for observation. Cardiac rhythm was monitored for the next 24 hours. Echocardiography and 12-lead ECG were done on all patients on next 2 consecutive days. Patients were discharged from intensive care on the following day and subsequently to home the next day. All the patients were started on oral aspirin (5 mg/kg/day – single dose after food) and advised to continue that for a total of 6 months.
All the procedures in this study were approved by the Institutional Ethics Committee and were in accordance with guidelines provided by the World Medical Association Declaration of Helsinki on Ethical Principles for Medical research involving human beings. All procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Follow-up: All the patients were called for follow-up after 7 days of discharge from the hospital and subsequently at 1 month, 3 months, 6 months, and at 1-year interval. On each follow-up visit, the patients again had 12-lead ECG and echocardiography for the assessment of rhythm and device position.
Statistical analysis
Data are expressed as percentage for categorical variables and mean ±standard deviation/median (range) for continuous variables.
Results
Out of a total of 65 patients who were referred to us for treatment of ventricular septal defect, 16 (24%) patients weighing less than 10 kg were found suitable for device closure. Out of these, 13 attempts were successful (81%), while 3 patients were sent for surgical closure (Table 3) due to various reasons. Demographic data of all 16 patients are shown in Table 1. Mean age of the patients was 17.3 ± 12.7 months. Male:Female ratio was 1.1:1. Mean weight was 6.8 ± 3.2 kg. Right heart catheterisation was done in all prior to device closure. Mean pulmonary: systemic flow (Qp/Qs) ratio was 2.3 ± 0.5. Mean defect diameter was 6 mm (range 3–10). Mean fluoroscopy time and mean procedure time was 24.6 ± 8.4 min and 42 ± 9.5 min, respectively. Mean radiation dose was 1582 ± 525 cGy/min. Various devices that were used in our series ranged from Multifunctional occluder (Lifetech Scientific Co. Ltd, Shenzhen, China), Cera muscular ventricular septal defect occluder (Lifetech Scientific Co. Ltd, Shenzhen, China), and Amplatzer duct occluders 1 and 2 (St Jude Medical Inc., United States of America). Devices were delivered through long delivery sheaths, which in most cases were Amplatzer ductal device delivery system and guiding catheter (Cook) of various sizes.
Table 1. Demographic data

PM = perimembranous; IM = inlet muscular; UM = upper muscular; AR = aortic regurgitation; MR = mitral regurgitation; PS = pulmonary stenosis; LM = lower muscular.
All the patients had a single defect except in one, where there was a small additional lower muscular defect (patient 15, Table 1), which was left alone. There were two patients who had Trisomy 21. Moderate mitral regurgitation was present in one patient (patient 10, Table 1) while two others had severe mitral regurgitation (patients 3 and 12, Table 1) due to mitral annular dilation. Pre-existing minimal aortic regurgitation was noted in two patients (patients 1 and 8, Table 1), which were thought to be due to cusp distortion due to venturi effect caused by high-velocity blood flow across ventricular septum, hence we proceeded with device closure. One patient (No. 13, Table 1) had severe valvar pulmonary stenosis associated with a moderate-sized septal defect. Here, we opened the pulmonary valve first with a Tyshak balloon and did the device closure afterwards.
Device was successfully positioned in 13 patients (81%). Immediate complete closure of left-to-right shunt was achieved in eight (61.5%) patients. Five others had intra-device residual shunts (38%), which were judged to be acceptable and not interfering with the stability of device position. In two patients, the device position was judged to be suboptimal with one of them showing significant residual shunt (patient 14, Table 1) and another having insufficient rim of tissue to hold the device adequately (patient 15, Table 1). Device was not released in both the patients and they were referred for surgical closure. There was accidental perforation of anterior wall of right ventricle in one patient (patient 16, Table 1) while trying to cross the defect from RV side. The defect was temporarily sealed with an 8 mm muscular defect occluder to prevent tamponade. We immediately shifted the baby to the operating room after quick aspiration of pericardial effusion, and the defect was surgically closed following repair of the right ventricular perforation. Patient was discharged home on day 6 after surgery Procedural data have been summarised in Table 2.
Table 2. Catheterisation data

ADO1 = Amplatzer duct occluder 1; ADO2 = Amplatzer duct occlude 2; F = French; FA = femoral artery; FV = femoral vein; I-M = inlet muscular; LV = left ventricle; MFO = multifunctional occluder; MM = mid muscular; MVO = muscular VSD occluder; PM = perimembranous; RIJV = right internal jugular vein, RV = right ventricle; UM = upper muscular.
Complications were classified into minor and major (Table 4). Minor complications observed in our series were transient loss of pulse (3/16, 18.7%), trivial new-onset aortic regurgitation (2/16, 12.5%), new-onset right bundle branch block (3/16, 18.7%). There was one major complication of right ventricular free-wall perforation, and this patient was salvaged by surgery as detailed earlier. There were no other major complications encountered in our series. Specifically, we had zero incidence of severe tricuspid/mitral valve injury, complete heart block, or left bundle branch block in our series. There was no incidence of death, device embolisation, haemolysis, or infective endocarditis.
Table 3. Patients referred for surgery

M = male; F = female; PM = perimembranous; IM = inlet muscular; AM = anterior muscular; HR-LT = Heart R by Lifetech Inc.; Cera LT = Cera muscular device by Lifetech Inc.; FV = femoral vein; RIJV = right internal jugular vein.
Table 4. Complications

AR = aortic regurgitation; LBBB = left bundle branch block; RBBB = right bundle branch block.
Follow -up was complete in all patients. Median follow-up duration was 6 months (range 1–24 months). Anti-failure medications were stopped within 1 month of the procedure. Complete closure was observed in all five patients within 3–6 months follow-up. There was no progression of new-onset aortic valve regurgitation that was noted earlier after device release and the pre-existing regurgitation disappeared on follow-up possibly due to correction of the haemodynamic condition underneath. All 13 patients remained in sinus rhythm during follow-up.
Discussions
Surgery is the established gold standard of treatment for haemodynamically significant ventricular septal defects. With improvement in the available devices and the skill of the operator, transcatheter treatment for these defects has gained popularity. Furthermore, many patients and parents nowadays prefer transcatheter treatment due to less pain, absence of chest wall scar, and comparable success rate in appropriately selected patients.
Due to higher risk of complications, reports of transcatheter closure of ventricular septal defects in less than 10 kg weight category are rare. Only five other case series have been published with a good outcome on short and medium-term follow-up Reference Narin, Pamukcu and Tuncay6–Reference Ghosh, Mukherji and Chattopadhyay10 with authors concluding that it is feasible to close ventricular septal defects in catheterisation laboratory in early infancy. To the best of our knowledge, ours is the sixth series and only third from India to report the experience of percutaneous ventricular septal defect closure in less than 10 kg weight category.
Inspired by our results of success in closing ventricular septal defects in older children (unpublished data), we went ahead with the closure of such defects in children weighing less than 10 kg and aged less than 1 year.
All the procedures were performed under conscious sedation using an adequate dose of injection ketamine, fentanyl, and midazolam. This is in contrast to the other case series, where general anaesthesia was routinely used. Reference Narin, Pamukcu and Tuncay6–Reference Soriano and Villareal9 We intubated only one patient who had accidental right ventricular perforation before shifting to operating room.
We used a multitude of devices in our series depending on the shape of the defect. We chose to implant duct occluders (Amplatzer duct occluders 1 and 2, Abbott Scientific) for perimembranous defects when the defect appeared funnel shaped on echo due to crowding on the right ventricular side by tricuspid valve leaflet tissue. Specifically, type 2 duct occluder was used when the defect size was 4–5 mm or less on the right ventricular side. The choice was influenced chiefly due to the low-profile nature of the device. However, this device cannot be used for defects sizes larger than 5 mm because of the limitations of sizes available. Double-disc devices (muscular ventricular septal defect occluder, Abbott Scientific or Multifunctional occluder, Lifetech Scientific Co. Ltd) were used for muscular defects and also in perimembranous location when there was aneurysmal tissue, but without much anatomical narrowing on the right side. Multifunctional occluder has a specific advantage over muscular device in being medium profile, and it has the ability to be delivered through a smaller delivery sheath, which is an advantage when dealing with small children as in our series. Selection of device was made after careful analysis of echo and angiogram. Device size for perimembranous defects was chosen by adding 1–2 mm to the largest diameter of the defect on the right ventricular side. In presence of aneurysmal tissue with restriction on the right ventricular side, we tried to deploy the device within the aneurysm sac to minimise friction of the device with aortic valve. For muscular defects similarly, the defect was 1–2 mm larger than the largest diameter.
Overall successful device implantation rate and immediate closure rate in our series were 81.25% and 61.5%, respectively. These rates are comparable with Narin et al Reference Narin, Pamukcu and Tuncay6 who reported an immediate closure rate of 66.6% and 83.3% after 1 month and Pillai et al., Reference Pillai, Rangasamy and Balasubramonian7 overall success rate of 85.7% after 1 month of follow-up. On 1-month follow-up, all of our patients with residual shunt had shown complete disappearance of all residual left-to-right flow taking our success rate to 81.25% at the end of 1 month. The mean defect size in our series was 6 mm. The mean defect size in the other three series also varied from 4 to 6 mm. Reference Narin, Pamukcu and Tuncay6-Reference Soriano and Villareal9
In our series, the mean fluoroscopy time and procedure time were 24.6 ± 8.4 minutes and 42 ± 9.5 min, respectively. Mean radiation dose was 1582 ± 525 cGy/min. The fluoroscopic time and total radiation dose are comparable to that reported by Narin et al. Reference Narin, Pamukcu and Tuncay6 In another recent series reported from India, Reference Ghosh, Mukherji and Chattopadhyay10 consisting of 35 children under 10 kg, the authors could achieve a remarkably low fluoroscopic time of 9.2 ± 2.9 min, and consequently a total mean procedure time of 34.1 ± 13.1 min.
Crossing of the defect from the right ventricular side has been demonstrated to have a definite benefit of being able to avoid arteriovenous looping, which in small children may cause cardiovascular compromise by splinting of the heart. Reference Ghosh, Mukherji and Chattopadhyay10 Although attempted in all cases, we were only able to cross the defect from right side in seven patients. Crossing by this technique requires good synchrony between the operators.
Careful assessment of defect size by means of echo and left ventricular angiogram is essential to choose an appropriate sized device and to strike a balance between the risk of touching of aortic valve and complete heart block versus risk of residual shunt and device embolisation.
Key factors associated with the successful outcome in our series were the selection of patients with some aneurysm formation in the perimembranous location, presence of at least 3 mm distance from the aortic valve, avoiding oversizing, and implantation of the device within the aneurysm sac. Two patients in whom devices were deployed, but not released had significant residual in one and inadequate tissue rim in another for which they were referred for surgery.
In the earlier era, a higher rate of early and late complete heart block, up to 6% was reported with the use of devices such as Amplatzer eccentric and muscular devices in the perimembranous location. Reference Ghosh, Mukherji and Chattopadhyay10,Reference Carminati, Butera and Chessa11 Statistical analysis had shown a significant association of complete heart block after ventricular septal defect device closure with lower age and weight of the patients. However, in recent times, off label use of ductal occluders in the perimembranous location has given very good results, with complete heart block rate documented to be less than 1%. Reference Narin, Pamukcu and Tuncay6,Reference Pillai, Rangasamy and Balasubramonian7,Reference Ghosh, Mukherji and Chattopadhyay10–Reference Nguyen, Phan and Doan13
Risk of heart block or aortic regurgitation is reportedly less when the device is placed inside the aneurysm sac. Reference Lin, Chen and Hsu14 In our series, we had zero incidence of complete heart block. There were two patients with pre-existing mild aortic regurgitations and two others with mild post-procedure aortic regurgitation. On follow–up, we noted the disappearance of pre-existing regurgitation and the post-procedure regurgitation remained the same on 3 months follow-up. In their large series of 105 perimembranous defects patients, Lin et al. have also described 1% incidence of new-onset aortic regurgitation and non-progression of previous mild ones. Reference Lin, Chen and Hsu14
Experience with multifunctional occluder device is still very limited. Few studies have reported successful use of this device in perimembranous and muscular positions. Results have been good with a very high immediate shunt closure rate and minimal complications. Reported major complications are complete heart block, tricuspid, and aortic regurgitations due to the device interference with the valve leaflets. The advantages of the device are its medium profile, dual screw hubs making both antegrade and retrograde delivery possible, and specially designed low-profile delivery system, which enables it to be used in smaller children. Reference Ghosh, Mukherji and Chattopadhyay10,Reference Damsky Barbosa, Alonso and Ferrín15–Reference Haddad, Daou and Saliba18 In our series, we successfully used multifunctional occluder in five patients to close quite large-sized defects (Table 2). All the cases were done antegradely by which we wanted to ensure device placement away from the aortic valve (Table 2). Specifically, in one patient, (No. 12, Table 2) the defect was actually an intermediate Gerbode defect, which was closed by size 12/10 antegradely. Post–procedure, there was moderate tricuspid regurgitation, which remained the same on follow-up after 6 months.
To conclude, percutaneous closure of ventricular septal defect in small children with lower body weight is feasible with good short and medium-term results. Careful sizing of the defect for appropriate device selection is essential and crucial to avoid complications. Because patients in this category are particularly prone to complications, only experienced operators should venture into this, by keeping necessary surgical backup ready for unprecedented emergencies. Small number of patients, short follow-up duration, and retrospective data collection and analysis are the limitations of this study. Further multicentric studies are required to assess the safety and feasibility of device closure in this patient category.
Acknowledgement
The authors acknowledge the contribution by the cardiac anesthesia team, cathlab technical staff members and the nursing team for their unwavering encouragement and support. We also acknowledge the patients and their parents for putting their faith on us and for kindly agreeing to let us publish our data.
Disclosures
None.
Conflict of interest
None.
Ethical standards
The study being retrospective in design, ethical committee clearance was waived.









