Introduction
Stem borers are one of the most important groups of rice (Oryza sativa L.: Poaceae) pests worldwide. Borers attack rice plants from seedling to maturity and are one of the reasons for low yields in the rice-growing countries of Africa and Asia (Akinsola, Reference Akinsola1984). Stem borer species attacking rice belong to two lepidopteran families, Crambidae and Noctuidae, and one dipteran family, Diopsidae (Pathak & Khan, Reference Pathak and Khan1994). The life cycles of and injury caused by boring lepidopterans are generally similar (Akinsola, Reference Akinsola1984). The damaging stages of stem borers, the larvae, are internal feeders. Eggs are laid on both sides of leaf blades in clusters of usually 2–100 eggs with individual eggs overlapping like fish scales. After hatching, the young larvae migrate to spaces between the leaf sheaths and stem where they feed inside the leaf sheath. Initial feeding by the larvae in the leaf sheath causes broad longitudinal reddish brown lesions at the feeding sites. Shortly thereafter, larvae bore into the stem and feed internally. At the vegetative stage of rice plant growth, feeding by stem borer larvae results in ‘deadhearts’, in which the young tillers and the leaves of the tillers die. During the reproductive stage, injury to tillers can destroy the panicles resulting in ‘whiteheads’. Extensive feeding can also lead to lodging of rice plants (falling over in rainy or windy conditions) (Holloway et al., 1928; Pathak, Reference Pathak1968; Castro et al., Reference Castro, Riley, Leonard and Baldwin2004). If injury occurs at an early stage, borer-injured plants can recover partially by producing new tillers (Bandong & Litsinger, Reference Bandong and Litsinger2005; Lv et al., Reference Lv, Wilson and Longnecker2008).
In 2011, rice was planted on about 1.18 million ha in USA with a value of approximately US$ 2.63 billion (USDA FAS, 2012). In Louisiana, rice was cultivated on 145,372 ha (LSU Agcenter, 2012) with an average yield of 7175 kg ha−1 (USDA NASS, 2012) and a production value of over US$ 360 million (USDA FAS, 2012). Stem borer species that have been reported to infest rice in Louisiana include the rice stalk borer; Chilo plejadellus Zincken, and sugarcane borer, Diatraea saccharalis (F.). The sugarcane borer is a major agronomic pest in the southeastern USA. Holloway et al. (Reference Holloway, Haley, Loftin and Heinrich1928) reported more than 20 host plants for the sugarcane borer and it is an economically important pest in sugarcane (Saccharum officinarum L.), corn (Zea mays L.), rice, and sweet sorghum (Sorghum bicolor L. Moench) (Roe et al., Reference Roe, Hammond, Reagan and Hensley1981). In recent years, rice farmers in the southern USA have experienced increased problems with D. saccharalis. In 2002, for example, approximately 3000 acres of rice in Concordia parish in central Louisiana were infested with D. saccharalis, damaging 70–95% of the rice crop on some farms (Castro et al., Reference Castro, Riley, Leonard and Baldwin2004). Moreover, another stem-boring species, the Mexican rice borer, has invaded Louisiana (Hummel et al., Reference Hummel, Hardy, Reagan, Pollet, Carlton, Stout, Beuzelin, Akbar and White2010) and has the potential for inflicting significant economic losses (Reay-Jones et al., Reference Reay-Jones, Wilson, Reagan, Legendre and Way2008).
With the increasing impact of stem borers on rice in the southeastern USA, there is an urgent need to develop management strategies for stem borers that incorporate all relevant tactics, including host plant resistance. Chemical control is the most widely used management tactic for suppression of stem borer populations (Browning et al., Reference Browning, Way and Drees1989; Reay-Jones et al., Reference Reay-Jones, Way and Reagan2007). General negative aspects of the use of insecticides include pest resurgence, hazards to users, environmental contamination, and costs associated with multiple applications (Chelliah & Bharathi, Reference Chelliah, Bharathi and Heinrichs1994). Moreover, the feeding habits of stem borers shelter them from non-systemic insecticides and thereby reduce their effectiveness (Litsinger et al., Reference Litsinger, Bandong, Canapi, Dela Cruz, Pantua, Alviola and Batay-An2005). Similarly, biological control has not been found feasible to control stem borers in rice in temperate climates such as USA (Lv et al., Reference Lv, Wilson, Beuzelin, White, Reagan and Way2011). Integrated pest management tactics that are more durable and easily applicable should be developed. Host plant resistance and cultural control are now the main tactics under development for stem borer management in China (Hao et al., Reference Hao, Han, Hou and Liao2008).
Rice is a typical silicon (Si)-accumulating graminaceous species (Takahashi et al., Reference Takahashi, Ma and Miyake1990; Ma et al., Reference Ma, Tamai, Yamaji, Mitani, Konishi, Katsuhara, Ishiguro, Murata and Yano2006; Zhao et al., Reference Zhao, Mitani, Yamaj, Shen and Ma2010). Although Si is not considered an essential element, Si-accumulating graminaceous plants grown without Si exhibit a range of abnormalities in growth, development, and reproduction (Yoshida, Reference Yoshida1975; Takahashi, Reference Takahashi, Matsuo, Kumazawa, Ishii, Ishihara and Hirata1995). Silicon uptake leads to formation of a thick silicate epidermal cell layer that can make the plants less susceptible to biotic and abiotic stresses (Ma, Reference Ma2004), including insect pests such as borers, hoppers, and mites (Djamin & Pathak, Reference Djamin and Pathak1967; Chandramani et al., Reference Chandramani, Rajendran, Muthiah and Chinniah2010). Silicon content in rice plants varies with plant age. Older plants and leaves typically have higher Si content than younger plants and leaves (Ishizuka, Reference Ishizuka and Santos1964).
Augmentation of soil using Si-based fertilizer is one crop management tactic that has proven beneficial for rice production, especially on soils low or limiting in this element. Beneficial effects include yield increases and improved disease and insect control (Savant et al., Reference Savant, Snyder and Datnoff1997; Alvarez & Datnoff, Reference Alvarez and Datnoff2001; Ma et al., Reference Ma, Miyake, Takahashi, Datnoff, Snyder and Korndorfer2001). A number of studies have shown positive correlations between increased Si content in plants and enhanced insect resistance (Djamin & Pathak, Reference Djamin and Pathak1967; Sharma & Chatterji, Reference Sharma and Chatterji1971; Moore, Reference Moore1984; Salim & Saxena, Reference Salim and Saxena1992). Elawad et al. (Reference Elawad, Allen and Gascho1985) observed increased resistance of sugarcane to sugarcane borer, D. saccharalis, with improved Si nutrition. Anderson and Sosa (Reference Anderson and Sosa2001) also observed that application of calcium silicate resulted in low sugarcane borer intensity on different sugarcane cultivars. Based on these previous studies suggesting a role for Si in resistance toward stem-boring species, Si amendments were expected to increase resistance of rice to D. saccharalis. Compared to the impact of other nutrients on rice production, the economic importance of Si is poorly understood in the southcentral USA (Kraska, Reference Kraska2009). We predict that soil Si amendments will result in decreased relative growth rates (RGRs) and boring success of D. saccharalis larvae in a susceptible and moderately resistant rice cultivar. This is the first study conducted on the effect of Si on D. saccharalis in rice.
Materials and methods
Plant growth and Si treatment
Plants for all experiments were grown in a greenhouse located on the campus of Louisiana State University, Baton Rouge. Two cultivars, ‘Cocodrie’ and ‘XL723,’ were used. Cocodrie is a widely grown, conventional long-grain cultivar and XL723 is a long-grain hybrid (2003 proprietary hybrid, Rice Tec, Alvin, TX). Prior experiments have shown Cocodrie to be susceptible to D. saccharalis while XL723 has been found to be moderately resistant (Sidhu & Stout, unpublished data). The soil mix used for planting consisted of two parts sterilized top soil (river sand, Entisol): one part peat moss and other part sand. Analysis of Si content of the soil mix was conducted by Soil Fertility laboratory, School of Plant, Environment and Soil Sciences, LSU Agricultural Center, using 0.5 M acetic acid extraction procedure (Narayanaswamy & Prakash, Reference Narayanaswamy and Prakash2010). The analysis showed the Si content to be approximately 20 ppm. Based on the soil Si levels in other soils (Histosols, Oxisols, and Ultisols), this soil Si level is considered low (Snyder, Reference Snyder, Datnoff, Snyder and Korndorfer2001). Seeds were planted in the soil mix in 15 cm diameter pots (3.8 liters) (Hummert International, Earth City, MO). Plants were maintained under greenhouse conditions with ambient lighting at approximately 29–33 °C. At the time of planting, approximately 1.2 g of 19:5:8 (N–P–K) controlled release fertilizer (Osmocote, Scotts Miracle-Gro, Marysville, OH) was added to the soil. Plants were thinned to a density of one plant per pot 5–7 days after planting. Plants were maintained in basins lined with pond liner and watered by flooding basins to a depth of ≈10 cm. The designation of rice plant stages followed the system outlined by Counce et al. (Reference Counce, Keisling and Mitchell2000). All experiments were conducted when plants were at the late tillering stage (50–55 days after planting).
At the two-leaf growth stage of rice plants, plants assigned to the Si augmentation treatment were treated by adding calcium silicate, Ca2SiO4 (slag) (Calcium Silicates Corporation, Columbia, TN) at 4 tons ha−1 (7.3 g per pot) directly on the soil surface in the pots and incorporated into the top layer by hands. This rate was chosen because it represents the highest field rate that could potentially be used economically in the field and would potentially have the maximum Si response (Datnoff et al., Reference Datnoff, Raid, Snyder and Jones1991).
Insects
D. saccharalis larvae used in experiments were obtained from a colony maintained continuously in the laboratory at Louisiana State University following the methods of Martinez et al. (Reference Martinez, Bard and Holler1988). The colony originated from larvae collected in rice fields near Crowley, LA, in 2005. Larvae were reared in 29.5 ml Solo soufflé cups (AceMart Restaurant Supply, San Antonio, TX) on sugarcane borer artificial diet (Southland Products, Lake Village, AR). Pupae were sexed according to Butt & Cantu (Reference Butt and Cantu1962) and equal numbers of males and females were placed into 3 liters plastic buckets with wax paper as a substrate for oviposition. Adults were provided with a 1:1 mixture of honey and beer (Milwaukee's Best Light, Miller Brewing Co., Milwaukee, WI) and distilled water. Eggs were put into eight cell trays for hatching. When the eggs hatched, neonates were placed on artificial diet and reared until use. The colony was maintained under controlled environmental conditions (14L;10D, 28 °C±2 °C, 38±2% R.H.). Insects collected from rice fields were added annually to the colony to maintain genetic variability.
Larval boring success
Greenhouse studies
Greenhouse studies using intact plants were conducted in 2011 and 2012 to assess the boring success of larvae on Si-treated and non-treated plants. In these studies, larvae were confined to either Si treated or untreated control plants (no choice-study). Boring success was defined as the proportion of second instar larvae entering the stems within 72 h of being placed on plants. Experiments were conducted as randomized block design (RBD) experiments with five replications. Blocks consisted of groups of four plants (one Si-treated and one non-treated plant of each of the two cultivars) spatially arranged on a greenhouse bench. At the late tillering stage, plants were infested using five second instar D. saccharalis larvae per plant. Small plastic tube cages (Icon Plastics, CA) were used to confine insects on the plants. These tubes were 15 cm long and 2.5 cm in diameter. Tubes were placed over the primary tiller of each plant and foam plugs (WVR International, Suwanee, GA) were used to seal the top and bottom of the tube cages enclosing the stem. Observations of numbers of larvae that remained outside the stems of the plants were taken 72 h after placing insects on plants. From this data, the percentage of larvae that bored into the stem was calculated. Frass coming out of the stem and visible entry holes were considered as confirmation of larval boring into the stem. Boring success was calculated using the formula:
Boring success in laboratory ‘cut stem’ assays
The effect of Si on boring success of D. saccharalis was also investigated in a laboratory experiment using cut stems in 2011. When greenhouse-grown plants reached the late tillering stage, they were brought back to the laboratory for experiment initiation. A 25 cm stem piece was cut from the base of the primary tiller near the soil line of each plant of each cultivar and placed in glass test tubes (Pyrex, Tewksbury, MA) measuring 20 cm length and 2.5 cm in diameter. The end of the stem placed in the tube was sealed using parafilm (Beemis Flexible Packaging, Neenah, WI). The other end was kept outside the test tube and the test tube was sealed using a foam plug. To keep the cut stems fresh, a wet cotton plug was placed on the stem end kept outside the test tube. The experiment was conducted as an RBD with five replications. A block consisted of a test tube rack containing randomly arranged test tubes. In each block, there were four test tubes with cut stems from plants of each cultivar, one Si-treated and one non-treated control. Infestations were achieved using five first instar D. saccharalis larvae per test tube. The larvae were released on the side of the test tube using a camel hair brush. Observations of numbers of larvae that remained outside the cut stems were taken 72 h after placing insects inside the glass test tube. From these data, the percentage of larvae that bored into the stem was determined as described above. Frass coming out of the stem and a visible entry hole were considered as confirmation of larval boring into the stem.
Relative growth rate
Greenhouse studies
No-choice greenhouse studies using intact plants were conducted in 2011 and 2012 to investigate the RGR of D. saccharalis larvae on Si-treated and non-treated plants of the two cultivars. Experiments were conducted as RBD experiments with five replications. The blocks consisted of groups of four plants spatially arranged on a greenhouse bench as described above. When the plants reached late tillering stage, infestations were achieved using one second instar D. saccharalis larva per plant. The larvae were taken off artificial diet, starved for 3 h and weighed prior to release on the stems to obtain an initial weight. Small plastic tubes identical to those used in the boring success experiment were used as cages to confine the insects to individual plants. The tube cages were placed over the primary tiller of each plant and foam plugs were used to seal the top and bottom of the tube cages enclosing the stem. Larvae were recovered after seven days, starved for 3 h and weighed (final weight). Weight gain and relative growth rates of the larvae were calculated using the formula:
 $${\rm RGR =} \displaystyle{{ {\rm Final}\,{\rm weight} - {\rm Initial}\,{\rm weight}} \over {\left\{ {\displaystyle{{ {\rm Final}\,{\rm weight + Initial}\,{\rm weight}} \over {\rm 2}}} \right\} {\rm \times Number}\,{\rm of}\,{\rm days}\,{\rm feeding}}}$$
$${\rm RGR =} \displaystyle{{ {\rm Final}\,{\rm weight} - {\rm Initial}\,{\rm weight}} \over {\left\{ {\displaystyle{{ {\rm Final}\,{\rm weight + Initial}\,{\rm weight}} \over {\rm 2}}} \right\} {\rm \times Number}\,{\rm of}\,{\rm days}\,{\rm feeding}}}$$(Waldbauer, Reference Waldbauer1968)
Laboratory growth rate studies
Laboratory experiments were conducted using cut stems in 2011 to further investigate the effect of Si on RGR of D. saccharalis. When plants in the greenhouse reached late tillering, they were brought back to the laboratory for setting up the experiment. From the central tiller of each plant two stem pieces were cut, each about 12 cm long. The two cut stems from each plant were placed in the center of a large Petri dish (14 cm diameter) (Corning™, NY) lined with wet filter paper to keep the stems fresh. The experiment was conducted as an RBD with five replications. A block was a rack with Petri dishes arranged randomly. In each block, there were four Petri dishes with cut stems from plants of each cultivar by Si treatment combinations. One second instar D. saccharalis larva was released into each Petri plate. The larvae had been taken off artificial diet, starved for 3 h and weighed (initial weight) prior to release on the stems. The Petri plates were then sealed with parafilm to prevent escape of the larvae. The larvae were recovered after 7 days. They were starved for 3 h and weighed again (final weight). Relative growth rates were calculated as described above.
Silicon content of plants
In 2012, an additional set of plants was grown in the greenhouse for plant Si analysis. These plants were treated and maintained under conditions identical to those described above. When the plants reached the late tillering stage, Si-treated and non-treated plants were cut at the soil line and entire plants were sent to the Department of Agronomy (School of Plant, Environment and Soil Sciences) for estimation of plant Si content. Plant tissue Si analysis was performed following a two-phase wet-digestion procedure and Molybdenum Blue Colorimetry method for determination of Si concentrations in digested plant samples as described by Kraska & Breitenbeck (Reference Kraska and Breitenbeck2010b).
Data analyses
Data from laboratory studies were analyzed as a factorial RBD experiment with block as a random effect and cultivar, treatment, and cultivar×treatment as fixed effects using a mixed model analysis of variance in PROC GLIMMIX (SAS, 2006). Data from greenhouse studies in 2011 and 2012 were analyzed together as replicated RBD factorial with year and block(year) as random effects and cultivar, treatment, and cultivar×treatment as fixed effects using a mixed model analysis of variance in PROC GLIMMIX (SAS, 2006).
Data for Si from Si analysis were analyzed as a factorial RBD experiment with block as a random effect and treatment, cultivar, and cultivar×treatment as fixed effects using a mixed model analysis of variance PROC GLIMMIX (SAS, 2006). Kenward–Rogers adjustment for degrees of freedom in mixed models was applied in all analyses (Littell et al., Reference Littell, Stroup and Freund2002).
Results
Boring success
Greenhouse studies
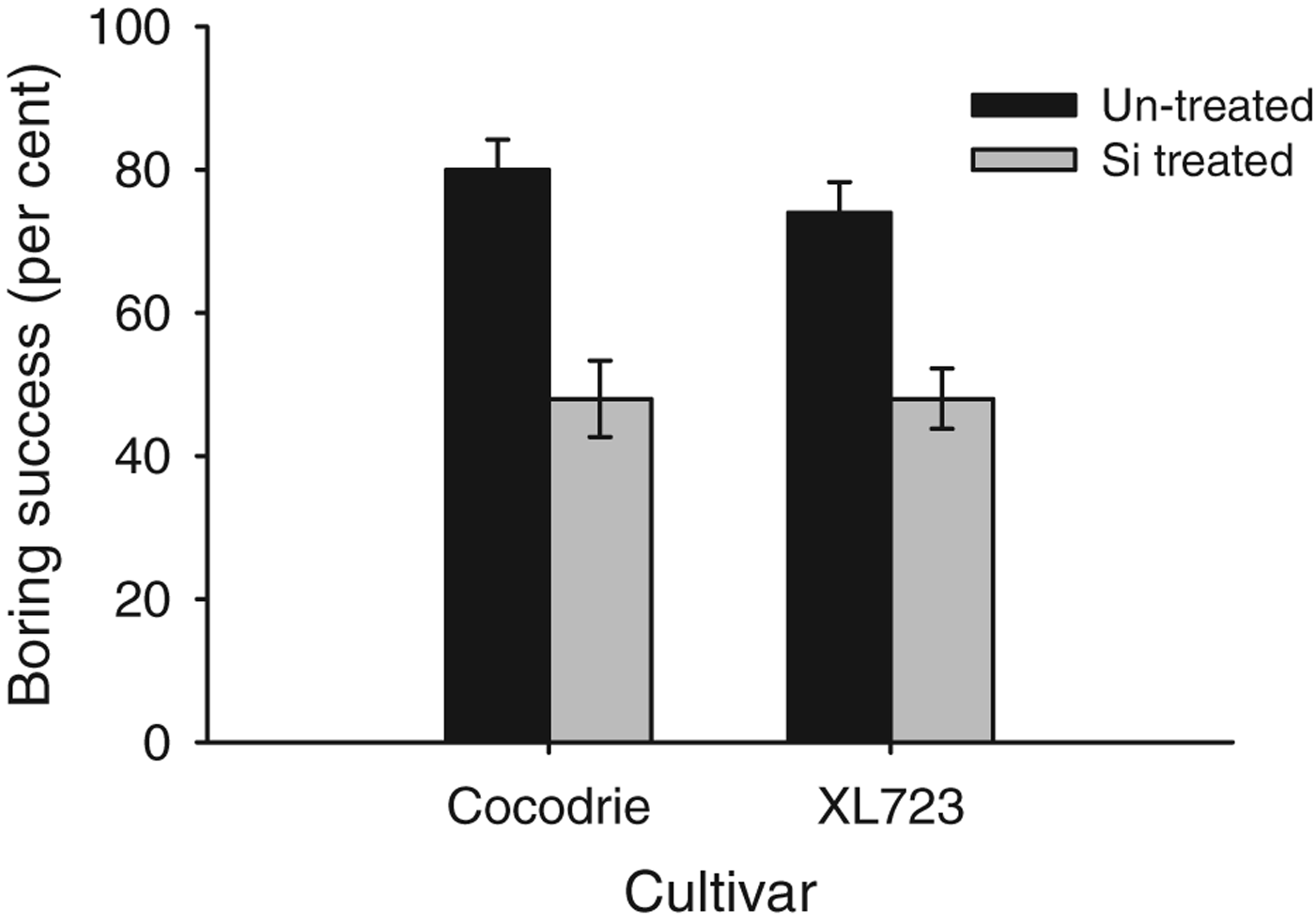
In the greenhouse, the percentage of second instar larvae that bored into rice stems within 72 h differed significantly by Si treatment (F 1,27=40.05, P<0.05) but not cultivar (F 1,27=0.43, P>0.05) (fig. 1). Overall, a higher percentage of larvae (78.00±4.50%) bored into the stems of non-treated plants compared to Si-treated plants (47.00±3.50%). The cultivar×Si treatment interaction was also not significant (F 1,27=0.43, P>0.05). The percentage of larvae boring into rice stems was reduced by approximately 40% on Si-treated plants of each cultivar.

Fig. 1. Mean larval boring success of D. saccharalis larvae on Si treated and un-treated plants of two rice cultivars in GH (2011, 2012). The bars represent standard error (SE).
Boring success in laboratory ‘cut stem’ assays
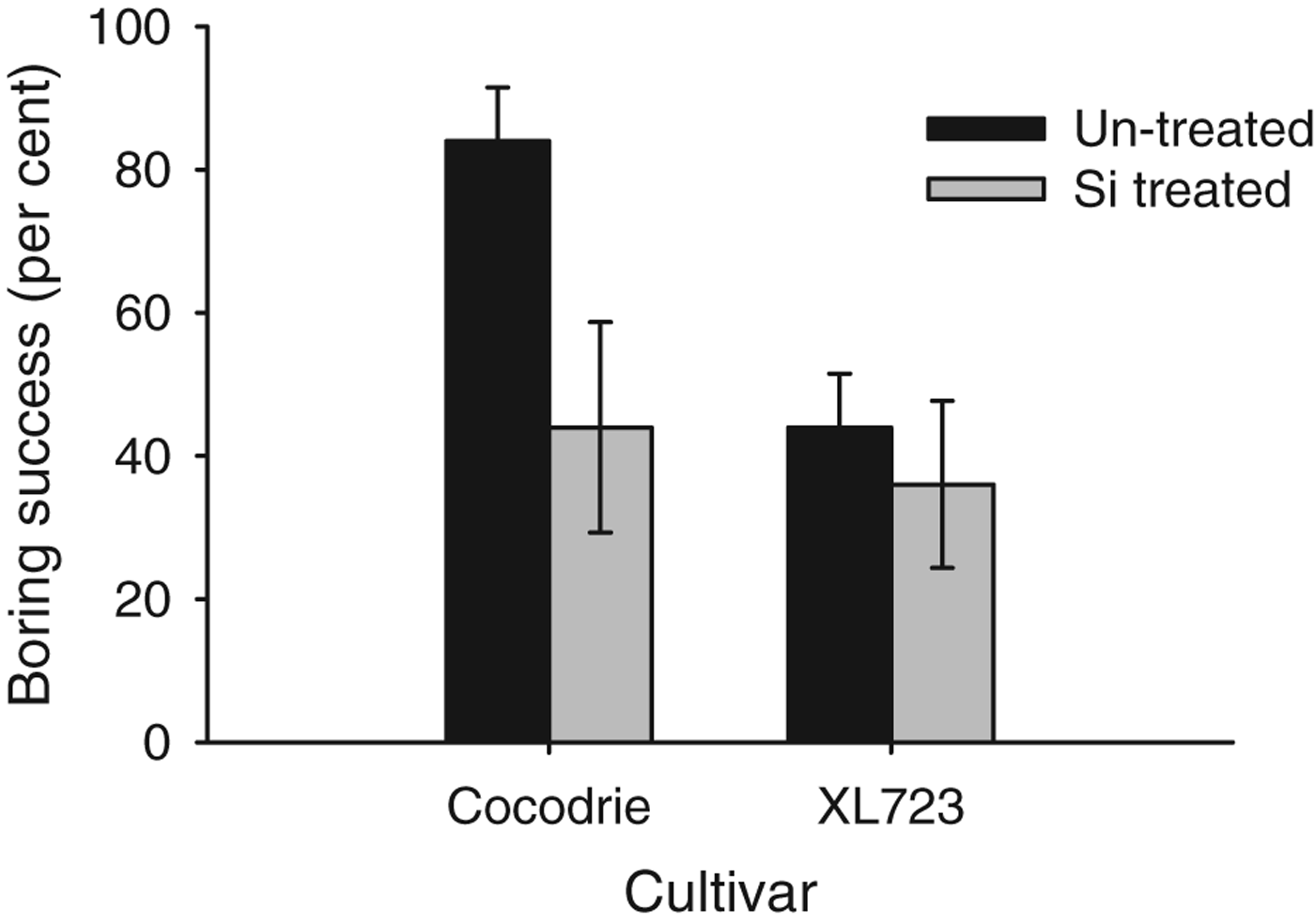
Cut stem assays revealed similar effects of Si on boring success of larvae (fig. 2). Significant differences among Si-treated and non-treated plants were observed (F 1,16=4.97, P<0.05) with only 40.00±6.20% of larvae boring into Si-treated plants compared to 64.00±8.10% in non-treated plants. Cultivar also affected boring success (F 1,16=4.97, P<0.05) as greater numbers of larvae bored into the stems of Cocodrie (64.00±10.24%) than XL723 (40.00±6.60%). The cultivar×Si-treatment interaction was not significant (F 1,16=2.21, P>0.05). For Si-treated Cocodrie plants, boring success was reduced by 47%, whereas for XL723 boring success was reduced by 18%.

Fig. 2. Mean larval boring success percentage of D. saccharalis larvae on Si treated and un-treated plants of two rice cultivars in lab 2011. The bars represent standard error (SE).
Relative growth rate
Greenhouse studies
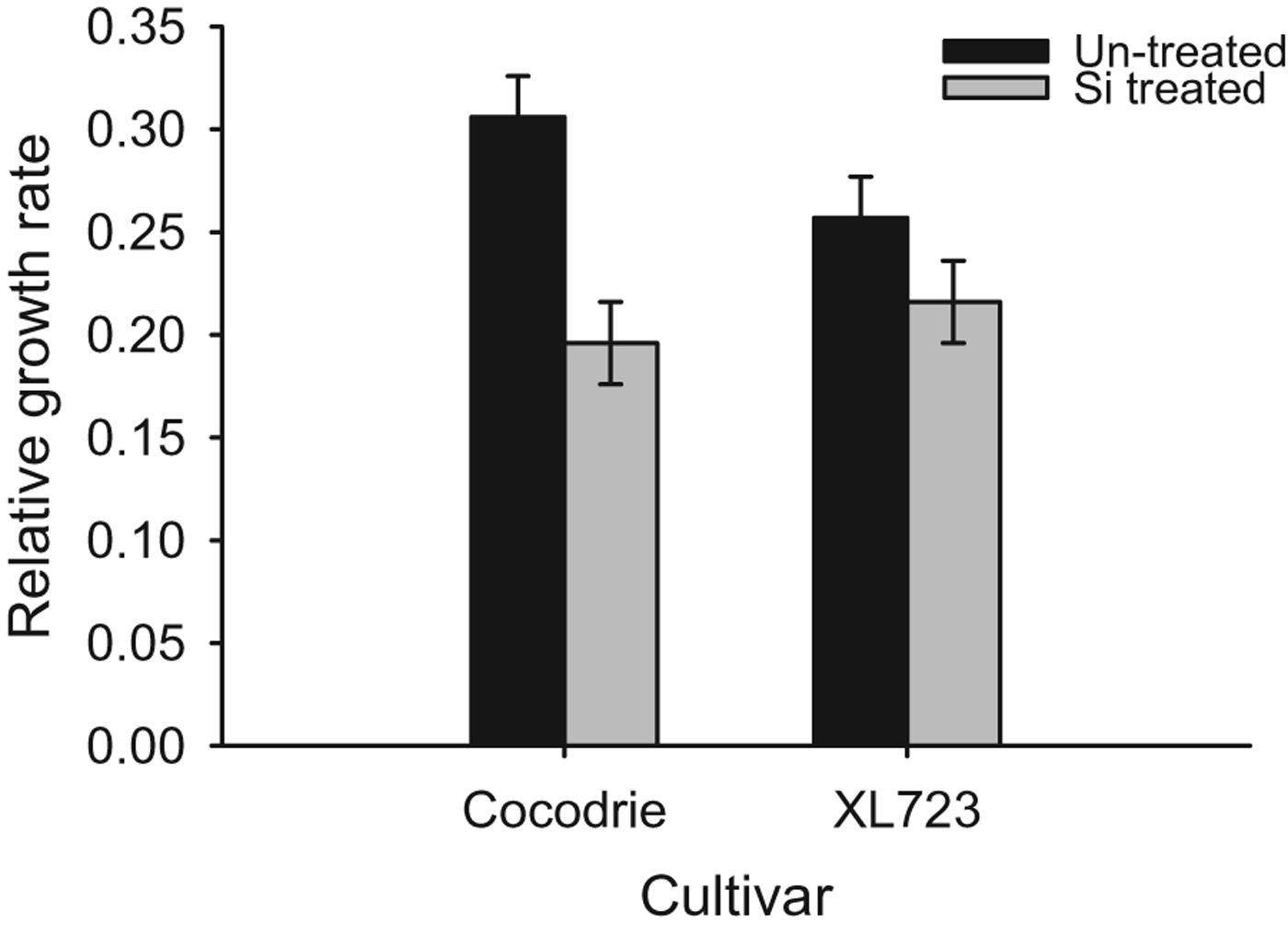
Relative growth rates of larvae recovered from Si-treated and non-treated plants after 7 days were significantly different (F 1,27=12.48, P<0.05) (fig. 3). RGRs were significantly lower (0.21±0.004) for Si-treated plants compared to the non-treated plants (0.28±0.01). RGRs did not differ significantly among cultivars (F 1,27=0.44, P>0.05). The Cultivar×Si treatment interaction was also not statistically significant (F 1,27=2.62, P>0.05) although there was a trend toward greater reduction in RGR on Si-treated Cocodrie plants. RGRs were reduced by 36% for Si-treated Cocodrie plants and approximately 16% for the hybrid XL723.

Fig. 3. Mean relative growth rate of D. saccharalis larvae on Si treated and un-treated plants of two rice cultivars in GH (2011, 2012). The bars represent standard error (SE).
Laboratory growth rate studies
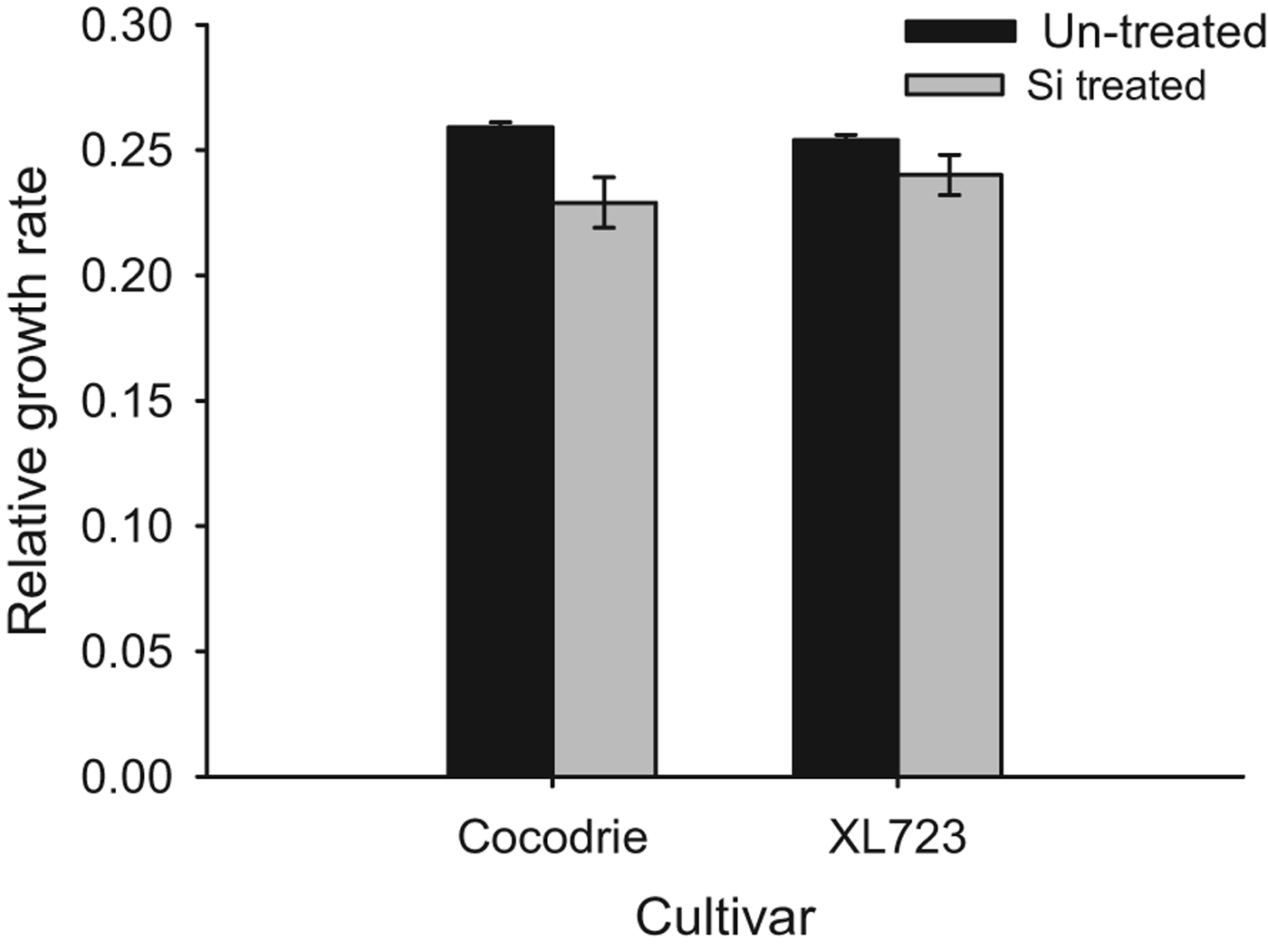
Results from the RGR assays conducted in the labrotary were similar to those from the greenhouse studies. RGRs of larvae were significantly lower (F 1,16=9.47, P<0.05) on Si-treated plants (0.23±0.01) than on non-treated plants (0.26±0.001). In Si-treated plants, RGRs of the larvae recovered after 7 days were approximately 12% lower on Si-treated Cocodrie plants and 4% lower on Si-treated XL723 (fig. 4). There was no significant effect of cultivar (F 1,16=0.35, P>0.05), and cultivar×Si treatment interaction was also not significant (F 1,16=1.73, P>0.05).

Fig. 4. Mean relative growth rate of D. saccharalis larvae on Si treated and un-treated plants of two rice cultivars in Lab 2011. The bars represent standard error (SE).
Silicon content in rice stalks
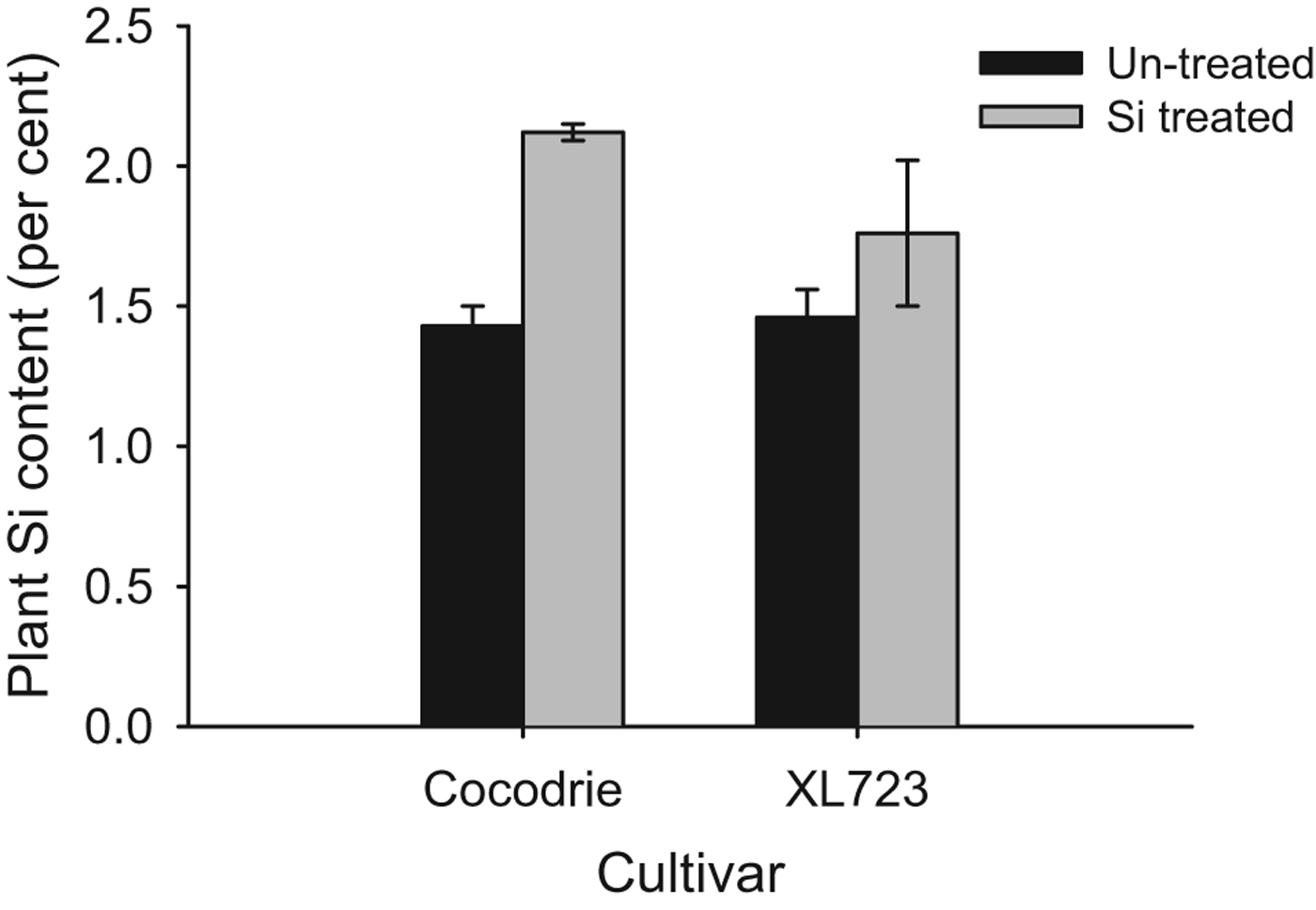
Amendment of soils with calcium silicate in the greenhouse increased Si content in rice plants (fig. 5). Silicon content of treated plants was significantly higher (1.94±0.07%) than non-treated plants (1.44±0.11%) (F 1,6=13.70, P<0.05). There was no significant effect of cultivar (F 1,6=1.52, P>0.05) and the cultivar×Si treatment interaction was also not statistically significant. Treated plants had 32 and 17% more Si in Cocodrie and XL723, respectively.

Fig. 5. Mean silicon content of treated and un-treated plants of two rice cultivars. The bars represent standard error (SE).
Discussion
The stem borers D. saccharalis and C. plejadellus have historically been considered important insect pests in Louisiana rice (Douglas & Ingram, Reference Douglas and Ingram1942; Oliver et al., Reference Oliver, Gifford and Trahan1972), and serious infestations of these insects have been reported over the last decade in Louisiana (Castro et al., Reference Castro, Riley, Leonard and Baldwin2004; MJS personal observation). Moreover, another invasive stem borer species, Eoreuma loftini (Mexican rice borer) has now moved through the Texas rice belt into Louisiana as predicted by Reay-Jones et al. (Reference Reay-Jones, Wilson, Reagan, Legendre and Way2008). This species was first found in Louisiana in 2008 from two pheromone traps approximately 8 km from a rice field near the Texas border (Hummel et al., Reference Hummel, Hardy, Reagan, Pollet, Carlton, Stout, Beuzelin, Akbar and White2010). Reay-Jones et al. (Reference Reay-Jones, Wilson, Reagan, Legendre and Way2008) predicted an annual loss of up to US$45 million by E. loftini, assuming the entire rice industry is infested by this pest by 2035. Despite the importance of stem borers in the past and in the future, there is currently no sound management program for stem borers in Louisiana. This study was conducted to investigate the potential of Si soil amendments to increase rice resistance to D. saccharalis. Results from the present study showed that Si incorporation into soil led to an increase in levels of Si in plant tissues and reduced performance of D. saccharalis larvae as manifested by reduced boring success of larvae into the stems of rice plants and reduced relative growth rates of larvae feeding in rice stems.
Incorporation of Si into the soil led to an uptake of Si and an increase in tissue silica concentrations in both rice cultivars used in this study. Soil Si augmentation increased Si content in plant tissues by approximately 32 and 17% in Cocodrie and XL723, respectively. These increases in levels of Si in leaves and stems are comparable to the increases reported by Hou & Han (Reference Hou and Han2010) in Chinese cultivars. In their study, plant Si content increased by approximately 15–20% in a susceptible cultivar and 15–24% in a resistant cultivar following soil augmentation. Plant Si contents in the present study were also comparable to those reported in other studies. Djamin & Pathak (Reference Djamin and Pathak1967) investigated varietal differences in Si content and borer susceptibility of 20 cultivars. They found that Si content of these cultivars ranged from 4.53% in a susceptible cultivar to 6.49% in a resistant cultivar. Datnoff et al. (Reference Datnoff, Deren and Snyder1997) evaluated ten different genotypes for Si accumulation and brown spot development on a low Si soil fertilized with 0 and 2.2 tons ha−1 of Si (calcium silicate). Silicon content in different genotypes varied from 3.4 to 4.9%. Datnoff et al. (Reference Datnoff, Deren and Snyder1997) reported that Si augmentation resulted in approximately 38–60% increase in the mean percent Si concentration of different rice cultivars in their study, a somewhat larger increase than that observed in the current study. Silicon content in rice tissues is influenced by a number of factors including differential uptake in different cultivars, methods of application, type of Si source used and methods used for analysis of plant Si content (Datnoff et al., Reference Datnoff, Deren and Snyder1997; Deren, Reference Deren, Datnoff, Snyder and Korndorfer2001; Moraes et al., Reference Moraes, Goussain, Carvalho and Costa2005; Ma et al., Reference Ma, Yamaji, Tamai and Mitani2007; Chandramani et al., Reference Chandramani, Rajendran, Muthiah and Chinniah2010; Kraska & Breitenbeck, Reference Kraska and Breitenbeck2010b).
There is a long history of studies that support a role for Si in rice resistance to stem-boring lepidopterans. The first study on the role of Si in plant resistance to insects was conducted by Sasamoto (Reference Sasamoto1953) on rice stem borer, Chilo simplex (Reynolds et al., Reference Reynolds, Keeping and Meyer2009). Ukwungwu & Odebiyi (Reference Ukwungwu and Odebiyi1985) recorded a negative correlation between percent Si content in different rice cultivars and the percentage of stems bored by the African striped borer, Chilo zacconius Bleszynski (Lepidoptera: Pyralidae), and the number of living larvae per plant. Panda et al. (Reference Panda, Pradhan, Samalo and Rao1975) reported that larvae of yellow rice borer, Scirpophaga incertullas Walker (Lepidoptera: Crambidae), were unable to attack rice plants because of the high Si content of their stems. Sasamoto (Reference Sasamoto1958, Reference Sasamoto1961) reported that Chilo suppressalis larvae preferred to feed in rice plants with low Si content as compared to plants with high Si content. Nakano et al. (Reference Nakano, Abe, Taketa and Hirano1961) found severe rice stem borer infestations in some rice fields where plant available Si in soil was low. Application of calcium silicate decreased both the insect damage and populations in those fields. Ma & Takahashi (Reference Ma and Takahashi2002) conducted Petri dish trials and observed a negative correlation between Si content of rice plants and the number of larvae that bored into the stems and the amount of feces produced by larvae. Similarly, Hou & Han (Reference Hou and Han2010) observed a significant reduction in weight gain by Asiatic rice borer, C. suppressalis Walker (Lepidoptera:Crambidae) on Si treated rice plants compared to un-treated plants.
Consistent with these previous studies on other stem-boring species, the experiments reported here demonstrate, for the first time, increases in rice resistance to D. saccharalis in the US rice cultivars as a result of soil Si amendment. The positive effect of Si on rice resistance was observed in both greenhouse and laboratory studies using two measures of resistance, larval boring success and relative growth rate in two cultivars. Although the increases in plant Si content did not significantly differ between the two cultivars, a stronger increase in resistance was observed in the more susceptible cultivar, Cocodrie, compared to the moderately resistant XL723. Thus, this study was a robust demonstration of the potential for Si to increase resistance to stem borers in the US rice.
The mechanisms by which Si soil amendments increase the resistance of plants to insects are not fully understood (Kvedaras & Keeping, Reference Kvedaras and Keeping2007). The most widely cited potential mechanism is a reduction in insect growth and reproduction due to reduced feeding and tissue digestibility resulting from increased hardness and abrasiveness of plant tissues (Kaufman et al., Reference Kaufman, Dayanandan, Franklin and Takeoka1985; Ma et al., Reference Ma, Miyake, Takahashi, Datnoff, Snyder and Korndorfer2001; Massey et al., 2006; Massey & Hartley, 2009). Silicon is deposited as a cuticle–silica double layer in the epidermal layer of leaf sheath, leaf blades and vascular tissue (Yoshida et al., Reference Yoshida, Onishi and Kitagishi1962; Sangster et al., Reference Sangster, Hodson, Tubb, Datnoff, Snyder and Korndorfer2001; Ma & Takahashi, Reference Ma and Takahashi2002). Hou & Han (Reference Hou and Han2010) proposed that lower feeding damage on Si-treated plants may result from improper digestion of Si -treated rice tissue. The presence of Si in plants can also increase the bulk density of diet such that the insects are unable to ingest sufficient quantities of nutrients and water (Panda & Khush, Reference Panda and Khush1995). Pathak (Reference Pathak1971) observed that high plant Si content in rice plants interfered with larval feeding and larvae feeding on a resistant rice cultivar (high Si content) had worn mandibles and exhibited low feeding efficiency. Larvae were unable to bore into the stems and suffered higher mortality on cultivars with higher Si compared to cultivars with low Si content.
In addition to interfering with the feeding and growth of stem-boring larvae, silica amendments may alter expression of defense-related genes and proteins in plants (Datnoff et al., Reference Datnoff, Rodrigues, Seebold, Datnoff, Elmer and Huber2007). Most studies of plant gene expression following Si treatment show only a limited number of genes affected (Watanabe et al., Reference Watanabe, Shimoi, Ohkama, Hayashi, Yoneyama, Yazaki, Fujii, Shinbo, Yamamoto, Sakata, Sasaki, Kishimoto, Kikuchi and Fujiwara2004; Fauteux et al., Reference Fauteux, Chain, Belzile, Menzies and Bélanger2006; Chain et al., Reference Chain, Cote-Beaulieu, Belzile, Menzies and Belanger2009; Ghareeb et al., Reference Ghareeb, Zoltan, Ott, Repenning, Stahl and Wydra2011) although Brunings et al., (Reference Brunings, Datnoff, Ma, Mitani, Nagamura, Rathinasabapathi and Kirst2009) reported that silicon amendment resulted in differential regulation of 221 genes in rice without being challenged with the pathogen. Several studies have shown lower disease severity in Si-treated plants due to increased activity of defensive enzymes upon being challenged with a pathogen (Yang et al., Reference Yang, Liang, Lou and Sun2003; Rodrigues et al., Reference Rodrigues, Jurick, Datnoff, Jones and Rollins2005; Cai et al., Reference Cai, Gao, Luo, Zeng, Yang and Zhu2008). Microarray analysis of rice infected with Magnaporthe oryzae showed that Si triggers activation of the ethylene signaling pathway, the role of which in resistance against blast is well known (Iwai et al., Reference Iwai, Miyasaka, Seo and Ohashi2006; De Vleesschauwer et al., Reference De Vleesschauwer, Djavaheri, Bakker and Höfte2008; Brunings et al., Reference Brunings, Datnoff, Ma, Mitani, Nagamura, Rathinasabapathi and Kirst2009). Thus, Si acts as an elicitor of plant defenses via priming of defensive compounds in stressed plants (Chérif et al., Reference Chérif, Asselin and Bélanger1994; Fawe et al., Reference Fawe, Abou-Zaid, Menzies and Bélanger1998; Rodrigues et al., Reference Rodrigues, McNally, Datnoff, Jones, Labbé, Benhamou, Menzies and Bélanger2004).
In addition to directly affecting performance of insect herbivores on plants, Si amendments may also aid in pest management indirectly by facilitating the activity of natural enemies and other mortality factors. Silicon may lead to a change in profiles of herbivore-induced plant volatiles, as increases in plant attractiveness to natural enemies has been shown following Si treatment in cucumber (Kvedaras et al., Reference Kvedaras, An, Choi and Gurr2010). Increase in Si content of plants may delay penetration by larval stem borers into the stem, thereby increasing time spent outside the stem and increasing exposure to natural enemies, adverse climatic conditions, and insecticides (Kvedaras & Keeping, Reference Kvedaras and Keeping2007). Thus, changes in stem borer behavior on Si amended plants may lead to greater reduction of stem borer population by natural mortality or by properly timed chemical control. The plant Si levels attained in this study after soil augmentation were not particularly high for a typical Si-accumulating crop such as rice (Takahashi et al., Reference Takahashi, Ma and Miyake1990), yet significant effects were still observed. Higher levels of the order of 5% are likely to produce a more pronounced suppressive effect on borers.
The greater responsiveness of the susceptible cultivar to Si amendment may provide rice growers with an option for cultivation of high yielding, borer susceptible cultivars in Louisiana when no other host plant resistance and chemical control options are viable or cost effective. Although it is generally assumed in USA that soils containing appreciable amounts of silicate clays supply adequate Si to meet crop demands, there is little evidence to support this assumption (Kraska & Breitenbeck, Reference Kraska and Breitenbeck2010a). Field studies by Bollich et al. (Reference Bollich, Robichaux, Groth, Oard, Bell, Datnoff, Snyder and Korndorfer2001) demonstrated that the use of Si soil amendments in Louisiana had the potential to reduce disease incidence and increase grain yield. As soil Si amendments are easily applicable, they may be applied on an areawide basis for management of the borer population, potentially reducing the need for insecticides. Silicon amendments are beneficial for plant and soil health besides having no adverse effects on the environment (Alvarez & Datnoff, Reference Alvarez and Datnoff2001). With the increasing need for environmentally safe strategies for insect pest management, Si could provide a valuable tool for use in agriculture. Future studies will focus on understanding the role of Si amendments as a component of IPM programs that incorporate insecticides, natural enemies, and genotypes with varying levels of resistance against stem-boring pests.
Acknowledgements
We thank Brenda Tubana and Narayanaswamy Chowdappa for their valuable contribution in plant and soil Si analysis. We thank Srinivas Lanka and other laboratory members for assistance with greenhouse and laboratory experiments. This manuscript is published with approval of the Director of the Louisiana Agricultural Experiment Station, as manuscript number 2013-234-7879.