I. INTRODUCTION
Different investigations in the Ca1−xSrxAl2O4 system are based on the importance of these phases as cement phases and also as long term phosphorescence materials (and also when doped with foreign elements) (Carlson (Reference Carlson1955), Braniski (Reference Braniski1957,Reference Braniski1965,Reference Braniski1967), Knopp (Reference Knopp1962), Witzmann and Knopp (Reference Witzmann and Knopp1963), Rodehorst et al. (Reference Rodehorst, Carpenter, Marion and Henderson2003), Ye et al. (Reference Ye, Zhuang, Wang, Yuan and Qiao2006), Saines and Kennedy (Reference Saines and Kennedy2006), Chatterjee (Reference Chatterjee2009), Huang et al. (Reference Huang, Cui and Hao2009), Fu et al. (Reference Fu, Dong, Liu and Wang2010), Hwang et al. (Reference Hwang, Kang, Kim, Hwangbo and Kim2011), Mohr (Reference Mohr2011), Pöllmann (Reference Pöllmann2012)).
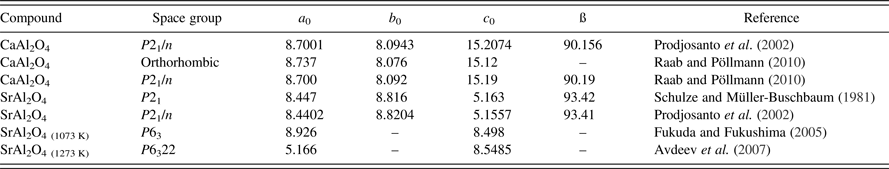
The components of the system and their structures are described by several authors (Table 1). Both components form different modifications. CaAl2O4 crystallizes in a monoclinic space group P21/n (Dougill, (Reference Dougill1957), Hörkner and Müller-Buschbaum (Reference Horkner and Muller-Buschbaum1975)), and confirmed by several authors. A metastable orthorhombic modification was synthesized by Raab and Pöllmann (Reference Raab and Pöllmann2010) by a low-temperature synthesis method. SrAl2O4 crystallizes in monoclinic P21 or P21/n at room temperature and transforms into a hexagonal high-temperature modification (P63) at about 650 °C. Avdeev et al. (Reference Avdeev, Yakovlev, Yaremchenko and Kharton2007) described a high-temperature phase in the space group P6322 at temperatures above 1273 K. Some selected crystallographic data for monocalcium aluminate and monostrontium aluminate are summarized in Table 1.
Table I. Crystallographic data of CaAl2O4 and SrAl2O4.

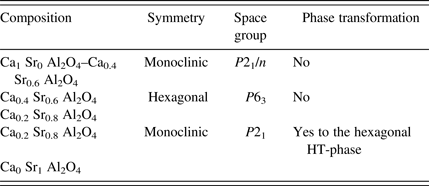
Table II. Symmetry within the solid-solution series of CaAl2O4 and SrAl2O4.

Solid-solution studies in the system CaAl2O4 and SrAl2O4 have been already investigated by several authors. A complete solution series was described by Massazza and Cannas (Reference Massazza and Cannas1959), but limited solid-solution series were described by Ito et al. (Reference Ito, Banno, Suzuki and Inagaki1977, Reference Ito, Banno, Suzuki and Inagaki1979), Kuroki et al. (Reference Kuroki, Saito, Matsui and Morita2009), and Prodjosanto et al. (Reference Prodjosantoso and Kennedy2002, Reference Prodjosantoso and Kennedy2003) using X-ray diffraction (XRD) and synchrotron powder data.
II. EXPERIMENTAL
All phases were synthesized via the Pechini method with final temperature steps between 700 and 1200 °C for 1 h. Powder X-ray diffraction (PXRD) was performed using a Panalytical ![]() PERT Pro diffractometer system equipped with
PERT Pro diffractometer system equipped with ![]() celerator RTMS detector in Bragg–Brentano-geometry using CuKα-radiation. Silicon was used as an internal standard. All phases were refined using the High Score Plus and Topas software. For temperature-dependent XRD an Anton Paar HTK-16 heating chamber with platinum strip was applied. SEM images were collected using scanning electron microscope JSM 6300 (JEOL). Structural images were obtained using ATOMS-program (DOWTY) and published atomic coordinates. Scanning electron microscope (SEM) images were collected using a scanning electron microscope JSM 6300 (JOEL). High-resolution images of the microstructure were recorded with a LEO 1525 Gemini field-emission SEM equipped with a high-efficiency InLens detector.
celerator RTMS detector in Bragg–Brentano-geometry using CuKα-radiation. Silicon was used as an internal standard. All phases were refined using the High Score Plus and Topas software. For temperature-dependent XRD an Anton Paar HTK-16 heating chamber with platinum strip was applied. SEM images were collected using scanning electron microscope JSM 6300 (JEOL). Structural images were obtained using ATOMS-program (DOWTY) and published atomic coordinates. Scanning electron microscope (SEM) images were collected using a scanning electron microscope JSM 6300 (JOEL). High-resolution images of the microstructure were recorded with a LEO 1525 Gemini field-emission SEM equipped with a high-efficiency InLens detector.
III. RESULTS
A. Synthesis of monostrontium aluminate
Pure monostrontium aluminate was synthesized via the Pechini method at different final temperature steps between 700 and 1200 °C. Whereas no significant crystallinity was observed at 700 °C, at increasing temperature the crystallinity improved as indicated by the sharpening of the reflections in the PXRD pattern (Figure 1).

Figure 1. (Color online) Improved crystallinity of SrAl2O4 synthesized via Pechini Method at different final temperature steps.
Depending on the temperature the modification of SrAl2O4 changes from P21 to P63 and for temperatures above 1000 °C into P6322 as reported by Avdeev et al. (Reference Avdeev, Yakovlev, Yaremchenko and Kharton2007). In PXRD, both polymorphs can be clearly distinguished owing to their specific reflection pattern, as demonstrated in Figure 2.

Figure 2. (Color online) Comparison of the simulated PXRD pattern of the different SrAl2O4 modifications: P21 (bottom), P63 (middle), and P6322 (top).
However, irrespective of the final temperature, the synthesized SrAl2O4 always possessed the monoclinic low-temperature modification after rapid cooling. Even in the PXRD pattern of the sample produced at 1200 °C, no reflections indicating a hexagonal high temperature form are present (Figure 3). The crystallized material shows a thin platy morphology, as presented in Figure 4.

Figure 3. (Color online) PXRD pattern of monoclinic low-temperature modification of SrAl2O4 synthesized via the Pechini method with final temperature step at 1200 °C (red: measurement, blue: LeBail fit, green: reflections).

Figure 4. SEM micrograph of pseudohexagonal platelets of SrAl2O4.
B. Synthesis of solid-solution series between CaAl2O4 and SrAl2O4
By varying the stoichiometric Sr/Ca ratio, different compositions of Ca1−xSrxAl2O4 were synthesized via the Pechini method with a final temperature step at 1200 °C. The ionic radius of strontium being significantly larger than that of calcium, the solid-solution series is subdivided into three fields of different modifications that can be clearly distinguished by PXRD (Figure 5). A substitution of Ca by Sr in the monoclinic CaAl2O4 (CA, SG P21/n) is possible up to 60 mole%, i.e. Ca0.4Sr0.6Al2O4 without a change of the modification. Between 60 and 75 mole% Ca substituted by Sr the modification of the synthesized compounds corresponds to the hexagonal high-temperature polymorph of monostrontium aluminate (SG P63). Compositions with larger strontium contents, i.e. Ca0.2Sr0.8Al2O4 towards pure SrAl2O4 (SrA), crystallize in the monoclinic low-temperature modification of monostrontium aluminate with SG P21.

Figure 5. (Color online) PXRD pattern of samples with different Ca/Sr ratios within the investigated solid-solution series Ca1−xSrxAl2O4 synthesized via the Pechini method with final temperature step at 1200 °C. The modifications dependent on the composition are given in the right column.
The substitution of Ca by Sr in general leads to increased unit cell dimensions of the calcium–strontium–aluminate compositions, shifting the strongest lines in the PXRD pattern towards the lower diffraction angles, as can be seen in Figure 5. According to Avdeev et al. (Reference Avdeev, Yakovlev, Yaremchenko and Kharton2007), the phase transitions between the observed modifications are displacive, and thus the principal structural features are similar, except for the distortions of coordination polyhedron, deviation of certain atom positions, and preferred occupations of the calcium positions. However, the unit-cell setups are differently related to the principal structural features, as given in Figure 6.

Figure 6. (Color online) Unit-cell parameters a 0, b 0, c 0 of different compositions Ca1−xSrxAl2O4 corresponding to the observed modification (inset in the three different fields). Owing to the different unit-cell setups the dimension of the parameters are adapted to comparable scales.
Within the CA-type solid solution (Ca1−xSrxAl2O4, 0 ≤ x ≤ 0.6, SG P21/n) the unit-cell parameters systematically increase (Figure 6). Increasing x(Sr) to 0.65 and changing the modification from CA-type (SG P21/n) into the high-temperature SrA-type (SG P63), a subsequent strong increase is observed for c 0(P63), which structurally corresponds to b 0(P21/n). The a 0 parameter, commonly designated for both modifications, is slightly lowered and is barely increased towards higher incorporation of Sr. A similar behaviour is found comparing ![]() $a_0 /\sqrt 3 \lpar P6_3 \rpar $ to c 0(P21/n), bottom of Figure 6. This value strongly increases when the modification is changed into the monoclinic low-temperature form of SrA-type (SG P21) as the Sr concentration increases. The parameter a 0(P21) follows the increasing tendency of c 0(P63), whereas b 0(P21) remains relatively constant.
$a_0 /\sqrt 3 \lpar P6_3 \rpar $ to c 0(P21/n), bottom of Figure 6. This value strongly increases when the modification is changed into the monoclinic low-temperature form of SrA-type (SG P21) as the Sr concentration increases. The parameter a 0(P21) follows the increasing tendency of c 0(P63), whereas b 0(P21) remains relatively constant.
As a consequence of the increasing unit-cell dimensions as the Sr concentration increases the unit-cell volumes are enlarged, too. To enable comparability between the different unit-cell setups of the observed modifications the specific volume per formula unit is (V unit-cell/Z). As presented in Figure 7, the specific volume depends on a distinct polynomial function on the stoichiometry: V spec = f(x). Whereas, V spec increasingly rises within the range of the CA-type solid solution, over the ranges of the hexagonal and the monoclinic SrA-type solid solutions the increase is diminished towards the pure SrAl2O4 (Figure 7). Exceptionally the specific volume for the composition Ca0.35Sr0.65Al2O4 is lower than the functionally expected value and corresponds well with the turning point of the overall function at x = 0.67. Taking into account the unit-cell parameters: a 0(P63) < a 0(P21/n) and c 0(P63, x = 0.65) are significantly lower than c 0(P63, x = 0.7), this composition obviously marks the stoichiometry-related phase-transition point. The result also corresponds to the finding of Prodjosantoso and Kennedy (Reference Prodjosantoso and Kennedy2002) on the preferred site occupation of strontium on the calcium positions. However, in contrary to the phase mixtures at these Ca/Sr ratio observed by Prodjosantoso and Kennedy (Reference Prodjosantoso and Kennedy2002, Reference Prodjosantoso and Kennedy2003), whose used the solid-state reaction synthesis, applying the Pechini method pure single phases could be obtained.

Figure 7. (Color online) Specific volume (V unit-cell/Z) of solid solutions in the system CaAl2O4–SrAl2O4.
As reported by Avdeev et al. (Reference Avdeev, Yakovlev, Yaremchenko and Kharton2007) the pure monoclinic SrAl2O4 undergoes polymorphic phase transitions when the temperature is increased over 1000 °C: monoclinic P21 → hexagonal P63 at about 680 °C → P6322 at about 860 °C. In order to prove the influence of the substitution of Sr by Ca in the monoclinic SrA-modification P21 high-temperature XRD (HT-XRD) was carried out for the composition Ca0.2Sr0.8Al2O4 (Figure 8). Therefore, the phase transition P21 → P63 was found to be shifted towards an elevated temperature and takes place in a range between 800 and 850 °C (Figure 8).

Figure 8. (Color online) HT-XRD measurement of Ca0.2Sr0.8 Al2O4 between 25 and 1000 °C.
IV. SUMMARY AND CONCLUSION
Within the system monostrontium- and monocalcium aluminate a replacement of calcium by strontium is possible resulting in a solid-solution series. The exchange can be given as:
Three different regions of solid solutions can be distinguished. In the calcium-rich part the solid solutions are based on the monoclinic structure of monocalcium aluminate (SG P21/n), in the strontium-rich part the solid solutions are based on the monoclinic monostrontium-aluminate (SG P21). A region in between strontium-rich (0.8–0.6 mole%) and calcium-rich (0.4–0.2 mole%) is based on the hexagonal high-temperature modification of mono-strontium aluminate (SG P63). The monoclinic strontium-based solid solutions also undergo a transformation to a higher hexagonal symmetry at increasing temperature.
ACKNOWLEDGEMENTS
The authors acknowledge ZWL/Lauf for the high-resolution scanning electron microscopy, especially Dipl.-Ing. S Winter and Dr. J. Göske.