Introduction
The European cherry fruit fly, Rhagoletis cerasi L. (Diptera: Tephritidae), is a pest of major agricultural importance. Females of this species oviposit on and larvae develop in fruits of sweet cherries (Prunus avium), sour cherries (P. cerasus) and honeysuckle (mainly Lonicera tartarica and L. xylosteum). The geographic distribution of R. cerasi includes almost all European and some Asian countries (Fimiani, Reference Fimiani, Robinson and Hopper1989; White & Elson-Harris, Reference White and Elson-Harris1992). R. cerasi has only one generation per year, since pupae undergo an obligatory diapause. Adults emerge in spring, and females oviposit usually one egg per ripening fruit.
Adults feed on nectar and honeydew. Larvae feed on mesocarp, destroying fruits and causing considerable economic loss (Fimiani, Reference Fimiani, Robinson and Hopper1989). Secondary infection of fruits by fungi and bacteria is facilitated by larvae activity, causing additional damage. The European cherry fruit fly received much attention as one of the targeted pests for the sterile insect technique (SIT) in the early 1970s.
In contrast to its economic importance, very little is known about the biology of this pest at the molecular, genetic and population level. A number of studies are focused on the biology of R. cerasi (Boller & Prokopy, Reference Boller and Prokopy1976; Boller et al., Reference Boller, Katsoyannos and Hippe1998; Kovanci & Kovanci, Reference Kovanci and Kovanci2006) and the development of control methods (Katsoyannos et al., Reference Katsoyannos, Papadopoulos and Stavridis2000); however, genetic and molecular data are still scarce. The cytogenetics (including the development of polytene chromosome maps) have been recently studied (Kounatidis et al., Reference Kounatidis, Papadopoulos, Bourtzis and Mavragani-Tsipidou2008). Polytene chromosome maps constitute a valuable tool for mapping of important traits. Cytoplasmic incompatibility in natural populations has recently been attributed to infection with different strains of the intracellular symbiont Wolbachia (Riegler & Stauffer, Reference Riegler and Stauffer2002; Arthofer et al., Reference Arthofer, Riegler, Schneider, Krammer, Miller and Stauffer2009b). These Wolbachia strains have been recently used for the development of an alternative and environment-friendly strategy to control a major agricultural pest, the Mediterranean fruit fly Ceratitis capitata (Zabalou et al., Reference Zabalou, Riegler, Theodorakopoulou, Stauffer, Savakis and Bourtzis2004, Reference Zabalou, Apostolaki, Livadaras, Franz, Robinson, Savakis and Bourtzis2009). This incompatible insect technique holds great potential for pest and disease control (Bourtzis, Reference Bourtzis2008; Saridaki & Bourtzis,Reference Saridaki and Bourtzisin press). A population analysis based on allozymes has been reported earlier (Schwartz et al., Reference Schwarz, McPheron, Hartl, Boller and Hoffmeister2003) and, more recently, the development of 13 microsatellite markers for this species (Arthofer et al., Reference Arthofer, Krumböck, Schuler, Rasool, Riegler, Köppler and Stauffer2009a). Both provide new tools for future population analysis.
Knowledge of the genetic structure of natural populations of a pest can support the development of environmentally friendly control methods. Microsatellites, especially, have been extensively used in addressing important ecological issues, since they are highly polymorphic, abundant, dispersed, PCR-analyzable and co-dominantly inherited markers. More specifically, in Tephritidae, microsatellites have been used to answer important questions regarding recent invasions (Bonizzoni et al., Reference Bonizzoni, Zheng, Guglielmino, Haymer, Gasperi, Gomulski and Malacrida2001; Meixner et al., Reference Meixner, Mcpheron, Silva, Gasparich and Sheppard2002; Silva et al., Reference Silva, Meixner, McPheron, Steck and Sheppard2003; Bonizzoni et al., Reference Bonizzoni, Guglielmino, Smallridge, Gomulski, Malacrida and Gasperi2004; Zygouridis et al., Reference Zygouridis, Augustinos, Zalom and Mathiopoulos2009): the expansion routes of different species (Bonizzoni et al., Reference Bonizzoni, Malacrida, Guglielmino, Gomulski, Gasperi and Zheng2000; Augustinos et al., Reference Augustinos, Mamuris, Stratikopoulos, D'Amelio, Zacharopoulou and Mathiopoulos2005; Nardi et al., Reference Nardi, Carapelli, Dallai, Roderick and Frati2005; Aketarawong et al., Reference Aketarawong, Bonizzoni, Thanaphum, Gomulski, Gasperi, Malacrida and Gugliemino2007), the origin of annual outbreaks of local populations (Yu et al., Reference Yu, Frommer, Robson, Meats, Shearman and Sved2001; Gilchrist et al., Reference Gilchrist, Sved and Meats2004), the degree of genetic structuring in natural population that can be associated with incipient speciation (Michel et al., Reference Michel, Rull, Aluja and Feder2007; Cameron et al., Reference Cameron, Sved and Gilchrist2010) and patterns of mating in nature (Bonizzoni et al., Reference Bonizzoni, Katsoyannos, Marguerie, Guglielmino, Gasperi, Malacrida and Chapman2002; Kraaijeveld et al., Reference Kraaijeveld, Katsoyannos, Stavrinides, Kouloussis and Chapman2005; Bonizzoni et al., Reference Bonizzoni, Gomulski, Mossinson, Guglielmino, Malacrida, Yuval and Gasperi2006; Song et al., Reference Song, Drew and Hughes2007). Moreover, microsatellite markers have been used for the construction of cytogenetic maps (Stratikopoulos et al., Reference Stratikopoulos, Augustinos, Petalas, Vrahatis, Mintzas, Mathiopoulos and Zacharopoulou2008), which can facilitate the future mapping of important traits, such as resistance to insecticides (Ranson et al., Reference Ranson, Paton, Jensen, McCarroll, Vaughan, Hogan, Hemingway and Collins2004; Wondji et al., Reference Wondji, Morgan, Coetzee, Hunt, Steen, Black, Hemingway and Ranson2007).
In this study, we present the development of 13 microsatellite markers for the European cherry fruit fly through cross-species amplification, and we demonstrate their usefulness by performing a small-scale analysis of Greek populations of this species. This is the first demonstration of the possibility to transfer microsatellite markers from one Rhagoletis species to another, eliminating the time needed for de novo development of microsatellite markers for each species. More importantly, the analysis presented here includes a successful evaluation of these markers, managing to reveal the structuring of R. cerasi populations in Greece.
Materials and methods
Collection of fly samples and DNA extraction
Collection sites and the number of flies used in the study are shown in fig. 1 and table 1. Field-infested sweet cherries were collected from five different locations in Greece and kept in the laboratory until larval pupation. Adults emerging from pupae completing diapause were stored at −80°C in 95% ethanol. Genomic DNA was extracted as described in Ashburner (Reference Ashburner1989) .

Fig. 1. Sampling sites: 1, Kallipefki Larissa; 2, Agia Larissa; 3, Karditsa; 4, Kernitsa Achaia; 5, Hania Crete.
Table 1. Microsatellite marker cross-species amplification in Rhagoletis cerasi.

* , as revealed by agarose gel electrophoresis.
** , as revealed by genotyping of 20 individuals by polyacrylamide gel electrophoresis.
Genotyping
Primer pairs designed for the amplification of microsatellite markers in different closely related species were tested for their efficacy to amplify specific amplicons in R. cerasi. PCRs were performed in 10 μl containing ~10 ng of DNA, using Kapa Taq DNA polymerase (KAPA Biosystems), (0.15 U Taq, 1×Buffer A, 0.25 mM of each dNTP and 0.5 μM of each primer). PCR products were analyzed on 1.3% agarose gels. Primer pairs that amplified a specific band were selected for the genotyping of multiple individuals. PCRs for genotyping were performed as above, with the only difference that one fifth of one of the primers of each pair was end-labeled with [γ32P]-ATP, using T4 polynucleotide kinase (MBI, Fermentas) (Zheng, Reference Zheng, Crampton, Beard and Louis1997). Amplifications were performed with an initial denaturation step (5 min at 95°C), followed by 30 cycles of: 15 s at 94°C, 30 s at 50–52°C and 30 s at 72°C, with a final elongation step of 5 min at 72°C. PCR products were electrophoresed on 5% denaturing polyacrylamide gels and visualized by autoradiography. Verification of the exact size of PCR products and of the presence of different alleles was performed through PCR of homozygotes, agarose gel electrophoresis of the products, gel extraction of the bands with the Jetquick gel extraction kit (Genomed) and direct sequencing with an ABI 310 DNA Sequencer.
Data analysis
Genetic variability was measured as the mean number of alleles per locus (n a), effective number of alleles (n e), observed (Ho) and expected heterozygosity (He), using POPGENE version 1.31 (Yeh et al., Reference Yeh, Boyle, Rongcai, Ye and Xiyan1999), and allelic diversity after correction for sample size, using FSTAT (Goudet, Reference Goudet2001). Deviations from Hardy-Weinberg Equilibrium (HWE) were tested with the G2 likelihood ratio test in POPGENE. Genotypic disequilibrium was tested with Genepop (Raymond & Rousset, Reference Raymond and Rousset1995), with Fisher's exact test, for all pairs of loci in all samples and across samples. Genetic distances were measured according to Nei (Reference Nei1972) using POPGENE (Yeh et al., Reference Yeh, Boyle, Rongcai, Ye and Xiyan1999). Population differentiation was estimated using the FSTAT software, at the significance level of 0.05. GENALEX 6.1 software (Peakall & Smouse, Reference Peakall and Smouse2006) was used for the analysis of molecular variance (AMOVA) and to estimate pairwise population PhiPT values. PhiPT matrix was used to perform principal components analysis (PCA). STRUCTURE software was used to determine the number of possible genetic clusters (Pritchard et al., Reference Pritchard, Stephens and Donnelly2000; Falush et al., Reference Falush, Stephens and Pritchard2003). We used all four different models, with a burn-in period of 50,000 and 50,000 MCMC repetitions after the initial burn-in. We tested for K=1 to K=5 (where K stands for the assumed number of populations). Isolation by distance was tested using Genepop (ISOLDE option), for plotting genetic distance matrix against geographic distance matrix; and Pearson's r coefficient of genetic and geographic distance was measured.
Results and discussion
Evaluation of microsatellite cross-species amplification in Rhagoletis
Microsatellites, although highly polymorphic, tend to be quite stable across related species. This is a great advantage since the development of microsatellite markers can be time- and money-consuming. Previous studies in Tephritidae have shown a high degree of microsatellite conservation, especially among species of the same genus (Baliraine et al., Reference Baliraine, Bonizzoni, Osir, Lux, Mulaa, Zheng, Gomulski, Gasperi and Malacrida2003; Augustinos et al., Reference Augustinos, Stratikopoulos, Drosopoulou, Kakani, Mavragani-Tsipidou, Zacharopoulou and Mathiopoulos2008; Stratikopoulos et al., Reference Stratikopoulos, Augustinos, Pavlopoulos, Economou, Mintzas, Mathiopoulos and Zacharopoulou2009). However, these studies did not perform a population analysis based on these markers (except Baliraine et al., Reference Baliraine, Bonizzoni, Guglielmino, Osir, Lux, Mulaa, Gomulski, Zheng, Quilici, Gasperi and Malacrida2004). Approximately 80% of C. capitata microsatellite markers could be transferred to other Ceratitis species (Baliraine et al., Reference Baliraine, Bonizzoni, Osir, Lux, Mulaa, Zheng, Gomulski, Gasperi and Malacrida2003). Likewise, microsatellite markers developed for Bactrocera papayae and Bactrocera dorsalis were cross-amplified in other Bactroceras at levels of >90% (Shearman et al., Reference Shearman, Gilchrist, Crisafulli, Graham, Lange and Frommer2006). However, the B. dorsalis case regards a complex species and not different species. Transferability was reduced to 40% for species outside the complex. In general, the transferability within genus ranges from 40–90% in Diptera. Between different species, percentages reduce dramatically (Augustinos et al., Reference Augustinos, Stratikopoulos, Drosopoulou, Kakani, Mavragani-Tsipidou, Zacharopoulou and Mathiopoulos2008; Stratikopoulos et al., Reference Stratikopoulos, Augustinos, Pavlopoulos, Economou, Mintzas, Mathiopoulos and Zacharopoulou2009).
Based on previous studies, primer pairs developed for the amplification of microsatellite markers in other Tephritidae species were tested for their ability to produce specific amplicons in the European cherry fly. We tested 19 primer pairs developed for the amplification of Rhagoletis pomonella microsatellite loci (in some cases new primers were designed and tested) (Velez et al., Reference Velez, Taylor, Noor, Lobo and Feder2006) and 16 that were designed for Rhagoletis indifferens (Maxwell et al., Reference Maxwell, Rasic and Keyghobadi2009). We also designed two primer pairs for microsatellites obtained from Ceratitis capitata (Stratikopoulos et al., Reference Stratikopoulos, Augustinos, Pavlopoulos, Economou, Mintzas, Mathiopoulos and Zacharopoulou2009) and Bactrocera oleae (Augustinos et al., Reference Augustinos, Stratikopoulos, Drosopoulou, Kakani, Mavragani-Tsipidou, Zacharopoulou and Mathiopoulos2008). Fourteen of the R. pomonella primer pairs and ten of R. indifferens amplified distinct products, as revealed by agarose gel electrophoresis. These primers were used for the genotyping of a sample consisting of 20 individuals (data not shown). Only six and five markers, respectively, were polymorphic, amplifying clear and easily scorable bands and reproducible results. The rest were monomorphic or poorly defined on polyacrylamide gel electrophoresis (multiple bands, faint signal) or failed to generate PCR products (i.e. harboured null alleles). These results are summarized in table 1. In total, 11 of the 35 markers tested were successfully transferred to R. cerasi. This does not include the two markers derived from C. capitata [RcMic(M101)] and B. oleae [RcMic(B3b)], since, in these cases, we had already sequenced cross-amplification PCR products for R. cerasi and designed new primer pairs, specific for R. cerasi (see Augustinos et al., Reference Augustinos, Stratikopoulos, Drosopoulou, Kakani, Mavragani-Tsipidou, Zacharopoulou and Mathiopoulos2008; Stratikopoulos et al., Reference Stratikopoulos, Augustinos, Pavlopoulos, Economou, Mintzas, Mathiopoulos and Zacharopoulou2009).
Genetic diversity
We used these markers to genotype 130 individual flies collected from five different locations in Greece (fig. 1). These proved medium polymorphic, harboring 2–8 alleles (mean 4.69 alleles per locus). Expected heterozygosity values were relatively high, ranging from 0.13 to 0.78 (mean 0.46); however, observed heterozygosity values were substantially lower (mean 0.36). Observed heterozygosity deficiency can generally be attributed either to null alleles (non-amplifying alleles due to changes in the primer binding regions), small sample size or extensive inbreeding. We have no indications for extensive null allele presence (for example, a number of individuals repeatedly failing to amplify at specific loci – all such loci were excluded prior to this analysis). Seven of the loci showed departure from HWE, according to the G2 criterion, at the significance level of 0.05. These deviations were attributed either to homozygote excess or to the presence of few unexpected genotypes. Since no indication of null alleles exist and according to the analyses presented below, we can assume that these deviations are attributed to the structuring of R. cerasi natural populations and the small sample size analyzed in some cases. However, as far as marker RcMic(Rp18) is concerned, there is a clear homozygosity excess. All above results are summarized in table 2
Table 2. Genetic variability of microsatellite markers.

N, sample size; na, observed number of alleles; ne, effective number of alleles (Kimura & Crow, 1964); AR, allelic richness; He, expected heterozygosity; Ho, observed heterozygosity (computed using Levene (1949)); HWE (+), in equilibrium at the significance level of 0.05.
* derived from Ceratitis capitata Medflymic101, through cross-species amplification, sequencing of PCR products and new primer design, specific for R. cerasi (see Stratikopoulos et al., Reference Stratikopoulos, Augustinos, Pavlopoulos, Economou, Mintzas, Mathiopoulos and Zacharopoulou2009).
** derived from B. oleae Boms3b, through cross-species amplification, sequencing of PCR products and new primer design, specific for R. cerasi (see Augustinos et al., Reference Augustinos, Stratikopoulos, Drosopoulou, Kakani, Mavragani-Tsipidou, Zacharopoulou and Mathiopoulos2008).
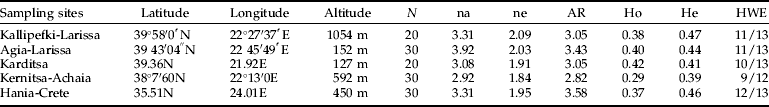
Twenty to 30 individuals per site were analyzed (table 3). According to all measures, the sample from Kernitsa was the least polymorphic and also presented the greatest deficiency of heterozygotes. The remaining samples were comparably polymorphic to each other. HWE was tested for all locus – population pairs, at the significance level of 0.05, and very few deviations occurred (13/64 tests). These deviations were not restricted to specific loci [except RcMic(Rp18), which was out of equilibrium in all samples], showing that the deviations observed before must be attributed to population structuring. Genotypic disequilibrium was tested for all pairs of loci and populations and none was observed.
Table 3. Samples' description and genetic variability.
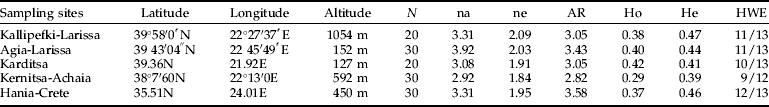
N, sample size; na, observed number of alleles; ne, effective number of alleles (Kimura & Crow, 1964); AR, allelic richness; He, expected heterozygosity; Ho, observed heterozygosity (computed using Levene (1949); HWE, number of loci in equilibrium at the significance level of 0.05.
The microsatellite markers presented here are medium polymorphic in R. cerasi and seem to be less polymorphic than in the species of origin. This does not necessarily reflect the degree of polymorphism of natural populations of the European cherry fly. It is known that microsatellites generally have more repeats and bigger uninterrupted motifs in those species from which they were initially isolated (Fitzsimmons et al., Reference Fitzsimmons, Moritz and Moore1995; Forbes et al., Reference Forbes, Hogg, Buchanan, Crawford and Allendorf1995; Rubinsztein et al., Reference Rubinsztein, Amos, Leggo, Goodburn, Jain, Li, Margolis, Ross and Ferguson-Smith1995), being therefore more polymorphic. On the other hand, the average degree of polymorphism is high enough to reveal the structure of natural populations of the cherry fly.
Structuring of R. cerasi populations in Greece
Genetic distances between the five samples were measured according to Nei (Reference Nei1972) and were high (table 4), ranging from 0.029 (Agia Larissa–Kallipefki Larissa) to 0.1267 (Agia Larissa–Hania Crete). Statistical analysis of population differentiation showed that samples from Larissa (Agia and Kallipefki) and Karditsa (all from Thessaly, central Greece) are not differentiated from each other. However, this group is differentiated from both Kernitsa (south/central Greece) and Hania (south Greece) (table 4).
Table 4. Genetic distance and Fst values.

Nei's (Reference Nei1972) genetic distance (below diagonal); population differentiation (above diagonal), based on Fst values;
*, significant population differentiation at 0.05 level; NS, not significant at 0.05 level.
Analysis of molecular variance (AMOVA) indicates an extensive structuring of R. cerasi natural populations, since 10% of the variance observed is attributed to inter sample differentiation. Principal components analysis (PCA) of the PhiPT distance matrix (fig. 2) indicates clustering in three well-defined groups, very distinct from each other, in accordance with their geographic origin: samples from central Greece (Agia Larissa, Kallipefki Larissa and Karditsa) constitute one group, and the other two samples (Kernitsa and Hania) are distinct not only from the Thessaly group, but from each other as well.

Fig. 2. Principal components analysis of the PhiPT distance matrix.
We also performed a Bayesian analysis to confirm the true number of genetic clusters of the samples presented here, using the STRUCTURE software. Applying a number of population parameters ranging between K=1 and K=5 and testing all available models, the hypothesis of three well-defined and distinct genetic clusters was always confirmed (fig. 3).

Fig. 3. Structure analysis assuming three genetic clusters (K=3): 1, Kallipefki Larissa; 2, Agia Larissa; 3, Karditsa; 4, Kernitsa Achaia; 5, Hania Crete. Analysis is based on the admixture model with correlated frequencies, after 50,000 burn-in period and 50,000 MCMC repeats.
Isolation by distance
One of the major factors that can lead to genetic differentiation is the geographic distance. To test this hypothesis for our data, we plotted the genetic distance matrix against the geographic distance matrix, and we estimated the degree of their correlation using Pearson's r. We found a highly significant positive correlation (r=0.92, P≪0.001), indicating that geographic distance among samples has greatly contributed to the creation of the genetic distance observed.
Can life history of R. cerasi explain the extended differentiation?
R. cerasi is an oligophagous pest, since adults oviposit on and larvae develop in specific fruits (mainly sweet cherries). Especially in Greece, cherry orchards are patchily distributed; they are rather isolated from each other and located mainly at high altitudes. Furthermore, R. cerasi produces only one generation per year and overwinters as pupae undergoing obligatory pupal diapauses. These life history traits constrain the capacity of the European cherry fly to disperse over larger distances and may contribute to allopatric differentiation. The presence of Wolbachia, a symbiotic bacterium that has been reported to cause incompatibility between populations with different infection status, may also contribute to reproductive isolation. A characterization of the different Wolbachia strains present in Greek populations of R. cerasi will be required to determine the extent of this contribution.
Conclusions
In the present study, we developed and evaluated a set of 13 microsatellite markers for the European cherry fly through cross-species amplification. The first important observation is that cross-species amplification can be used for the development of informative microsatellite markers in Rhagoletis, bypassing the time needed for de novo development of markers. Second, the markers presented here, although only medium polymorphic, seem to be adequate to reveal the genetic structure of the natural populations of R. cerasi and will probably be even more powerful in combination with other available markers (Arthofer et al., Reference Arthofer, Krumböck, Schuler, Rasool, Riegler, Köppler and Stauffer2009a). Finally, this study reveals a high structuring of natural R. cerasi populations in Greece, that has not been observed for the other Tephritidae species of Greece, such as B. oleae (Augustinos et al., Reference Augustinos, Mamuris, Stratikopoulos, D'Amelio, Zacharopoulou and Mathiopoulos2005) and C. capitata (Economou et al., in prep.).
Acknowledgements
This research was supported in part by grants from the European Community's Seventh Framework Programme CSA-SA_REGPROT-2007-1 under grant agreement no. 203590 and intramural funding from University of Ioannina to KB and by the European Social and National Resources – EPEAEK II – Pythagoras, and the Greek ministry of education and intramural funding from University of Thesssaly to NTP.
The authors would like to thank Prof. Zacharopoulou, Antigone (Department of Biology, University of Patras, Greece) for her generous support and encouragement throughout this study. Also, Dr Tsiamis, G. for assistance in sequencing analysis, Dr Velez, S. for providing R. pomonella raw microsatellite sequences and primer pairs sequences and Dr Stefan Oehler for comments on the manuscript. Finally, the authors would also like to thank Mavragani–Tsipidou, P., Kounatidis, I. (Aristotle Univeristy of Thessaloniki), Moraiti, K., Papanastasiou, S. and Diamantidis, A. (Univeristy of Thessaly) for their help in the collection of R. cerasi natural populations.