INTRODUCTION
Neospora caninum is a tissue cyst-forming coccidian closely related to Toxoplasma gondii that causes reproductive failure in cattle worldwide (Dubey et al. Reference Dubey, Schares and Ortega-Mora2007). Acute and chronic N. caninum infections in cattle are represented by 2 different phases of the disease, governed by tachyzoite and bradyzoite stages, respectively. The clinical consequences of these 2 stages differ significantly. For this reason, the study of the molecular mechanisms involved in tachyzoite-to-bradyzoite conversion and the subsequent employment of N. caninum stage-specific markers may contribute to our understanding of the pathogenesis of bovine neosporosis.
Until now, only 2 N. caninum bradyzoite-specific genes, the surface antigens NcSAG4 and NcBSR4, have been identified based on their homology with the T. gondii genome. The bradyzoite-specific expression of NcSAG4 and NcBSR4 has been shown by immunofluorescence and real-time reverse transcription polymerase chain reaction (RT-PCR) analysis of parasites from in vitro cultures (Fernández-García et al. Reference Fernández-García, Risco-Castillo, Zaballos, Álvarez-García and Ortega-Mora2006; Risco-Castillo et al. Reference Risco-Castillo, Fernández-García, Zaballos, Aguado-Martínez, Hemphill, Rodriguez-Bertos, Álvarez-García and Ortega-Mora2007), and by immunohistochemistry in mature bradyzoites from tissue cysts of a naturally infected calf (Risco-Castillo et al. Reference Risco-Castillo, Fernández-García, Zaballos, Aguado-Martínez, Hemphill, Rodriguez-Bertos, Álvarez-García and Ortega-Mora2007). Preliminary studies of NcBSR4 indicate that transcription of this gene occurs during the late bradyzoite stage, whereas transcription of NcSAG4 is thought to occur at an earlier stage (Risco-Castillo et al. Reference Risco-Castillo, Fernández-García, Zaballos, Aguado-Martínez, Hemphill, Rodriguez-Bertos, Álvarez-García and Ortega-Mora2007). These expression patterns are consistent with what has been suggested for T. gondii during the conversion of tachyzoites to bradyzoites (Cleary et al. Reference Cleary, Singh, Blader, Brewer and Boothroyd2002; Contini et al. Reference Contini, Giuliodori, Cultrera and Seraceni2006). Moreover, recent work has also shown that recombinant NcSAG4 protein could be a good marker for serodiagnosis of chronic infection in cattle (Aguado-Martínez et al. Reference Aguado-Martínez, Álvarez-Garcia, Fernández-García, Risco-Castillo, Arnaiz-Seco, Rebordosa-Trigueros, Navarro-Lozano and Ortega-Mora2008).
Previously, transcription levels of stage-specific antigens during experimental or natural N. caninum infection have been poorly investigated. The T. gondii orthologue to NcSAG4, TgSAG4, and some Plasmodium falciparum stage-specific genes have been successfully employed as molecular markers of the infection phase by RT-PCR or real-time RT-PCR in infected human samples (Niederwieser et al. Reference Niederwieser, Felger and Beck2000; Blair et al. Reference Blair, Witney, Haynes, Moch, Carucci and Adams2002; Cultrera et al. Reference Cultrera, Seraceni and Contini2002).
The kinetics of N. caninum infection in Balb/c mice have been previously described in detail, rendering a good model for evaluation of the expression patterns of stage-specific genes during infection. In these mouse models, N. caninum was found in the brain, where the parasite persists during chronic infection from the second to the third week post-infection (p.i.). Studies also showed significant differences in virulence between various isolates, which must be considered in the present work (Atkinson et al. Reference Atkinson, Harper, Ryce, Morrison and Ellis1999; Hemphill et al. Reference Hemphill, Vonlaufen and Naguleswaran2006). Infection with an Nc-Liv isolate induced greater morbidity and a higher parasite burden in the brain during chronic infection than infection with an Nc-1 isolate (Collantes-Fernandez et al. Reference Collantes-Fernández, López-Pérez, Álvarez-García and Ortega-Mora2006).
The aim of this work was to study the transcription profile of the bradyzoite-specific NcSAG4 gene during the course of N. caninum infection in mice. NcSAG1, which is specifically expressed in tachyzoites, was used as a control for the presence of tachyzoites during the course of the infection. For this purpose, transcription levels of both genes were measured by NcSAG1 and NcSAG4-based real time RT-PCR in brain tissue samples from mice experimentally infected with either the Nc-1 or Nc-Liv N. caninum isolate, at different days post-infection (p.i.). These transcription levels are discussed here and are related to parasite burden in the brain, as well as to the humoral immune response against soluble tachyzoite antigen and the N. caninum-recombinant proteins NcGRA7 and NcSAG4.
MATERIALS AND METHODS
Parasite culture and preparation of BALB/c mice inocula
Nc-1 and Nc-Liv isolates of N. caninum were routinely maintained by continuous passages in the cell line MARC-145, as previously described (Pérez-Zaballos et al. Reference Pérez-Zaballos, Ortega-Mora, Álvarez-García, Collantes-Fernández, Navarro-Lozano, García-Villada and Costas2005). For inoculum preparation, the tachyzoites cultures were scraped with a plastic cell scraper, passed through 21-gauge needles, harvested by centrifugation at 1350 g for 10 min, counted by the Trypan Blue vital dye and suspended at the required dose in a final volume of 200 μl of phosphate-buffered saline (PBS) per mouse. Afterwards, mice were immediately inoculated.
Experimental design and samples
Sixty-five 6-week-old female BALB/c mice (Harlan, Spain) were infected intraperitoneally (i.p.) with 2×106 tachyzoites from the Nc-1 (group 1) or Nc-Liv (group 2) isolate of N. caninum (Dubey et al. Reference Dubey, Hattel, Lindsay and Topper1988; Barber et al. Reference Barber, Trees, Owen and Tennant1993). A control group of 18 mice was inoculated i.p. with PBS (group 3). Mice were monitored daily for the presence of clinical signs of neosporosis. Five to eight random animals from each group were euthanized in a CO2 chamber on days 1 and 4 p.i. (acute infection), on days 16 and 32 p.i. (early chronic infection) and on days 64 and 155 p.i. (late chronic infection). Blood samples were collected in tubes with EDTA at necropsy. Peripheral blood cells, to be used for DNA extraction, and plasma to be used for enzyme-linked immunosorbent assays (ELISAs), were separated by centrifugation. Plasma was cryopreserved at −80°C until use. Brain tissue was also aseptically collected from each mouse. A brain hemisphere was longitudinally dissected into 2 different pieces for DNA and RNA extraction, respectively. Brain samples were immediately frozen in dry ice and cryopreserved at −80°C until analysis.
DNA extraction and PCR techniques
Genomic DNA from peripheral blood cells and brain tissue was extracted using commercial kits (Real Pure DNA Extracción ADN SSS or ADN genómico for blood or tissue samples, respectively, Durviz, Valencia, Spain). Genomic DNA from 106N. caninum tachyzoites was also extracted and used as a positive control. Neospora caninum DNA detection was carried out by nested-PCR based on the internal transcribed spacer 1 (ITS1) region of N. caninum (Buxton et al. Reference Buxton, Maley, Wright, Thomson, Rae and Innes1998). Real-time PCR of the N. caninum Nc-5 sequence was applied to quantify parasites in the brain tissues (Collantes-Fernandez et al. Reference Collantes-Fernández, Zaballos, Álvarez-García and Ortega-Mora2002).
RNA extraction and real-time RT-PCR
In order to determine transcription levels of the NcSAG4 and NcSAG1 genes in N. caninum PCR-positive brain tissues, levels of mRNA were analysed by real-time RT-PCR as previously described (Fernandez-Garcia et al. Reference Fernández-García, Risco-Castillo, Zaballos, Álvarez-García and Ortega-Mora2006) and standardized for tissue samples. Briefly, total RNA isolation was carried out by homogenizing 50 mg of brain tissue in 1 ml of Tri-Reagent™ (Sigma, Spain), according to manufacturer's instructions, using a Polytron® PT 1600E homogenizer (Kinematica AG). cDNA was synthesized from 5 μg of total RNA using Superscript II RNase H Minus Reverse Trancriptase (200 U, Invitrogen), random hexamers (2·5 μm, Applied biosystems) and RNase inhibitor (40 U, Ambion) in 20 μl reactions, according to the Superscript II RNase H Minus Reverse Trancriptase's instructions.
Real-time PCR was performed with 6 μl of cDNA using Platinum SYBR Green qPCR SuperMix UDG (Invitrogen) and specific forward and reverse primers for NcSAG4, NcSAG1 or N. caninum 18S ribosomal RNA (Nc18sR) genes (Fernandez-Garcia et al. Reference Fernández-García, Risco-Castillo, Zaballos, Álvarez-García and Ortega-Mora2006) in the ABI Prism 7000 Sequence Detector (Applied Biosystems). Amplification of each gene was performed according to the protocol supplied by Applied Biosystems, with an initial incubation of 50°C for 2 min, followed by 95°C for 10 min. Forty amplification cycles of 95°C for 15 sec and 60°C for 1 min were then carried out. After amplification, melting curves of PCR products were acquired by adding a dissociation step consisting of 95°C for 15 sec, 60°C for 30 sec and 95°C for 15 sec. All samples were processed in triplicate and the standard deviation (s.d.) of the threshold cycle (Ct) values for each gene did not exceed 0·5. The absence of DNA in RNA samples was determined by the real-time PCR amplification of Nc18sR, using 1·5 μg of total RNA (proportional to the amount of cDNA employed in the real-time PCR) from each sample. Results of each reaction are expressed as the relative transcription levels of NcSAG4 or NcSAG1, after conversion of the raw mean Ct values to the linear form 2−ΔCt (Livak and Schmittgen, Reference Livak and Schmittgen2001). The ΔC t was calculated as the difference between mean C t values of NcSAG4 or NcSAG1 (targets) and Nc18sR (normaliser) amplicons for each sample.
Serological techniques
Detection of N. caninum-specific IgG1 and IgG2a and specific-IgGs against NcGRA7 and NcSAG4 recombinant proteins (rNcGRA7 and rNcSAG4) in plasma samples was performed by ELISA using soluble N. caninum tachyzoite antigen, rNcGRA7 or rNcSAG4 according to previously described methods (Collantes-Fernández et al. Reference Collantes-Fernández, López-Pérez, Álvarez-García and Ortega-Mora2006; Aguado-Martínez et al. Reference Aguado-Martínez, Álvarez-Garcia, Fernández-García, Risco-Castillo, Arnaiz-Seco, Rebordosa-Trigueros, Navarro-Lozano and Ortega-Mora2008).
Statistical analysis
Comparisons of the parasite burdens and relative transcription levels of each gene between groups 1 and 2 were performed by the Mann-Whitney U test. The same variables over time were compared using the Kruskal-Wallis H test followed by a nonparametric multiple-comparison test whenever significant differences were found. Regarding specific immunoglobulin levels, a one-way ANOVA test was applied to compare levels of the 4 measured IgGs at each time-point and over time (N. caninum-specific IgG1 and IgG2a and rNcGRA7 and rNcSAG4-specific IgGs). The Tukey's multiple comparison test was applied whenever significant differences were found. The same variables between both N. caninum-inoculated groups were compared by the two-way ANOVA test.
RESULTS
Neospora caninum-associated clinical signs
None of the mice died spontaneously during the follow-up period. Slight N. caninum-associated clinical signs (mainly rough hair coat, inactivity and anorexia) were observed in 3 Nc-1-infected mice which were sacrified on days 32, 64 and 155 p.i., respectively. Regarding Nc-Liv-infected mice, all animals sacrified on days 32, 64 and 155 p.i. showed rough hair coat and 2 of them neurological signs (ataxia and pelvis limb weakness).
Neospora caninum DNA detection by nested-PCR
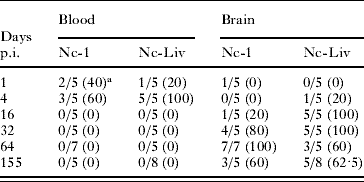
Parasitaemia was only detected on days 1 and 4 p.i. (Table 1). On day 4 p.i., 60% and 100% of blood samples from Nc-1- and Nc-Liv-inoculated mice, respectively, were found to be positive. Parasite DNA was detected in the brain from days 32 and 16 p.i. until the end of the experiment in most Nc-1- and Nc-Liv-inoculated mice, respectively. On day 64 p.i., 100% of brains from Nc-1-inoculated mice were PCR-positive and this number decreased to 60% on day 155 p.i. In the group inoculated with the Nc-Liv isolate, 100% of the brains were PCR-positive on days 16 and 32 p.i. and this number decreased to 60% on days 64 and 155 p.i. (Table 1).
Table 1. Neospora caninum DNA detection in blood and brain tissues
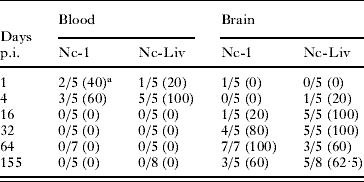
a No. of positive samples/number of total samples (%).
Parasite burden in brains
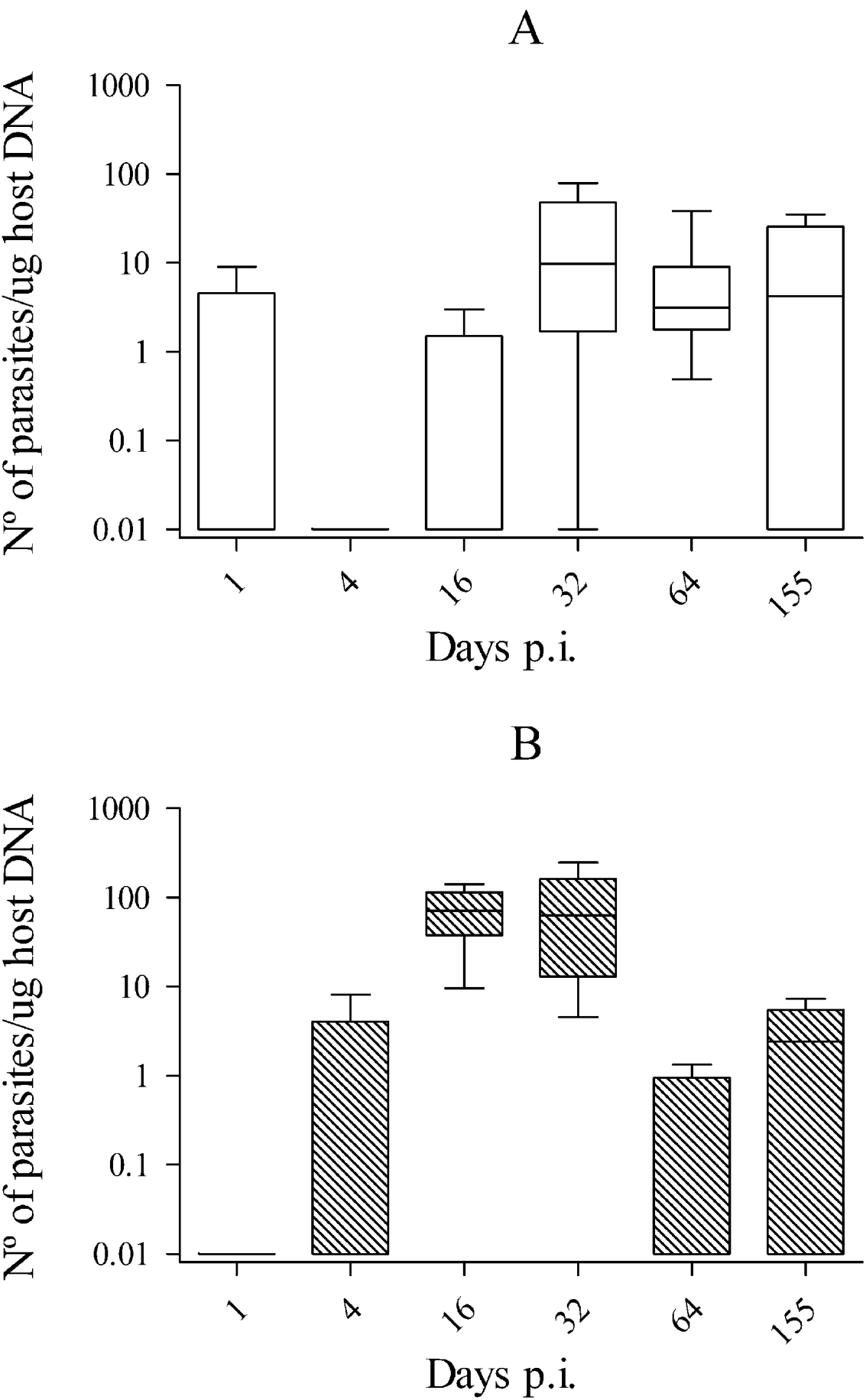
Parasite burdens in brains from Nc-1-inoculated mice were low, peaking on day 32 p.i., with more than 5 tachyzoites/μg of tissue in most of the samples analysed. Parasite burden slightly decreased on day 64 p.i. and then remained consistent until day 155 p.i., although no statistically significant differences were found in the parasite burden from day 16 p.i. to day 155 p.i. (P>0·05 by Kruskal-Wallis H test). In Nc-Liv-inoculated mice, the parasite burden reached more than 50 tachyzoites/μg of tissue on days 16 and 32 p.i. in most of samples, then decreased significantly on day 64 p.i. and was maintained at this level until day 155 p.i. (Fig. 1) (P<0·05 by Kruskal-Wallis H test followed by the nonparametric multiple-comparison test). When both infected groups were compared, the parasite burden in brains from Nc-Liv-inoculated mice was significantly higher than that from Nc-1-inoculated mice at day 16 p.i. (P<0·05 by Mann-Whitney U test), whereas it was significantly lower at day 64 p.i. (P<0·05 by Mann-Whitney U test) (Fig. 1).
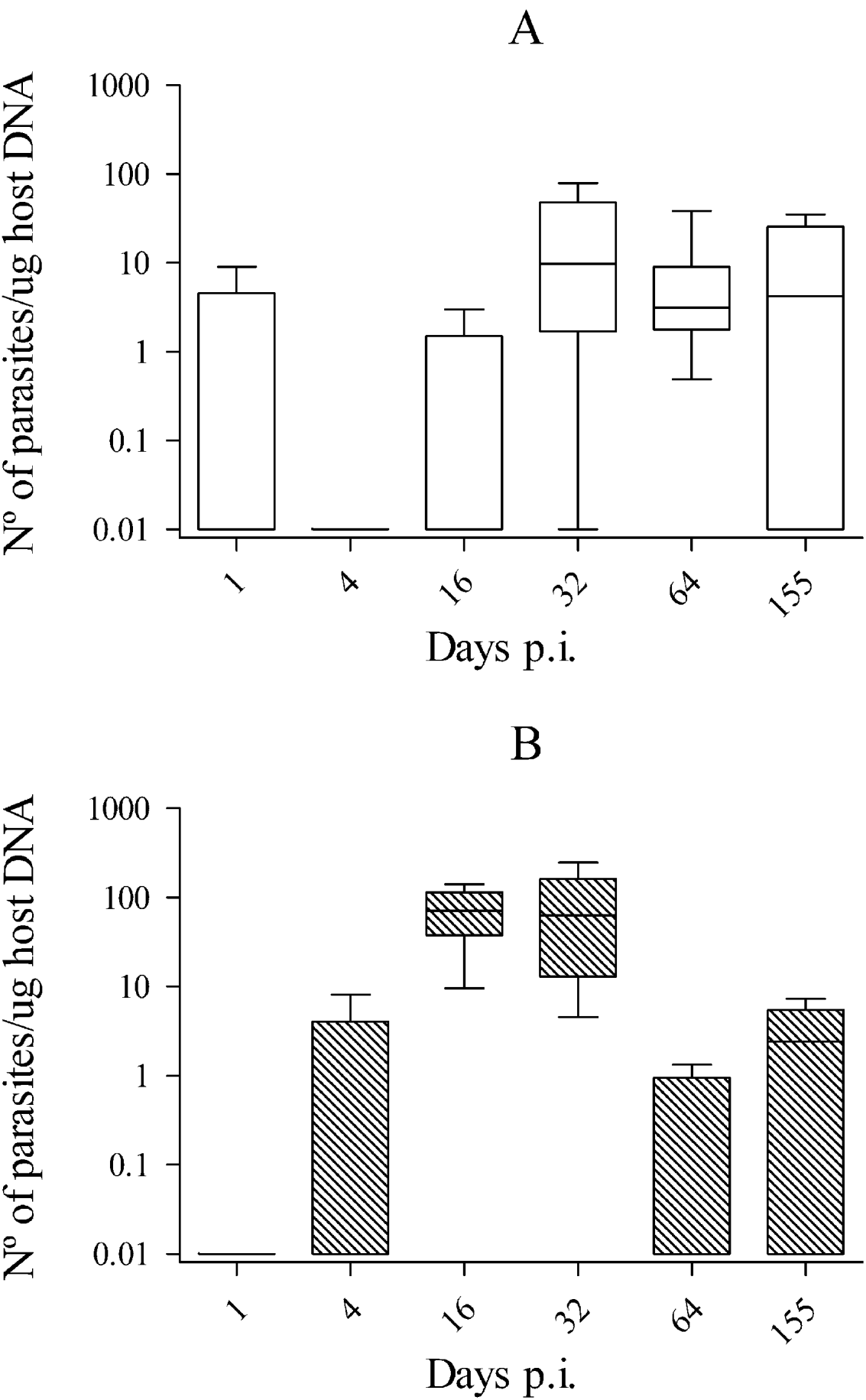
Fig. 1. Box-plot and whisker graph representing the lower and upper quartiles, median and minimum-maximum values for brain parasite burden in Nc-1 (A) and Nc-Liv-infected mice (B) during the course of the study.
NcSAG4 and NcSAG1 transcription levels
Relative transcription levels of NcSAG4 and NcSAG1 could only be determined in N. caninum-PCR-positive brain tissues (as mentioned above). Moreover, it was not possible to quantify NcSAG1 and NcSAG4 transcription levels in some PCR-positive brain tissues with a very low parasite burden because the high Ct obtained on the Nc18sR normaliser gene prevented a reliable relative quantification. This was the case for 3 Nc-Liv-inoculated mice at day 155 p.i. The absence of genomic DNA in RNA samples was confirmed since no specific amplification was detected by direct real-time PCR of Nc18sR in total RNA samples.
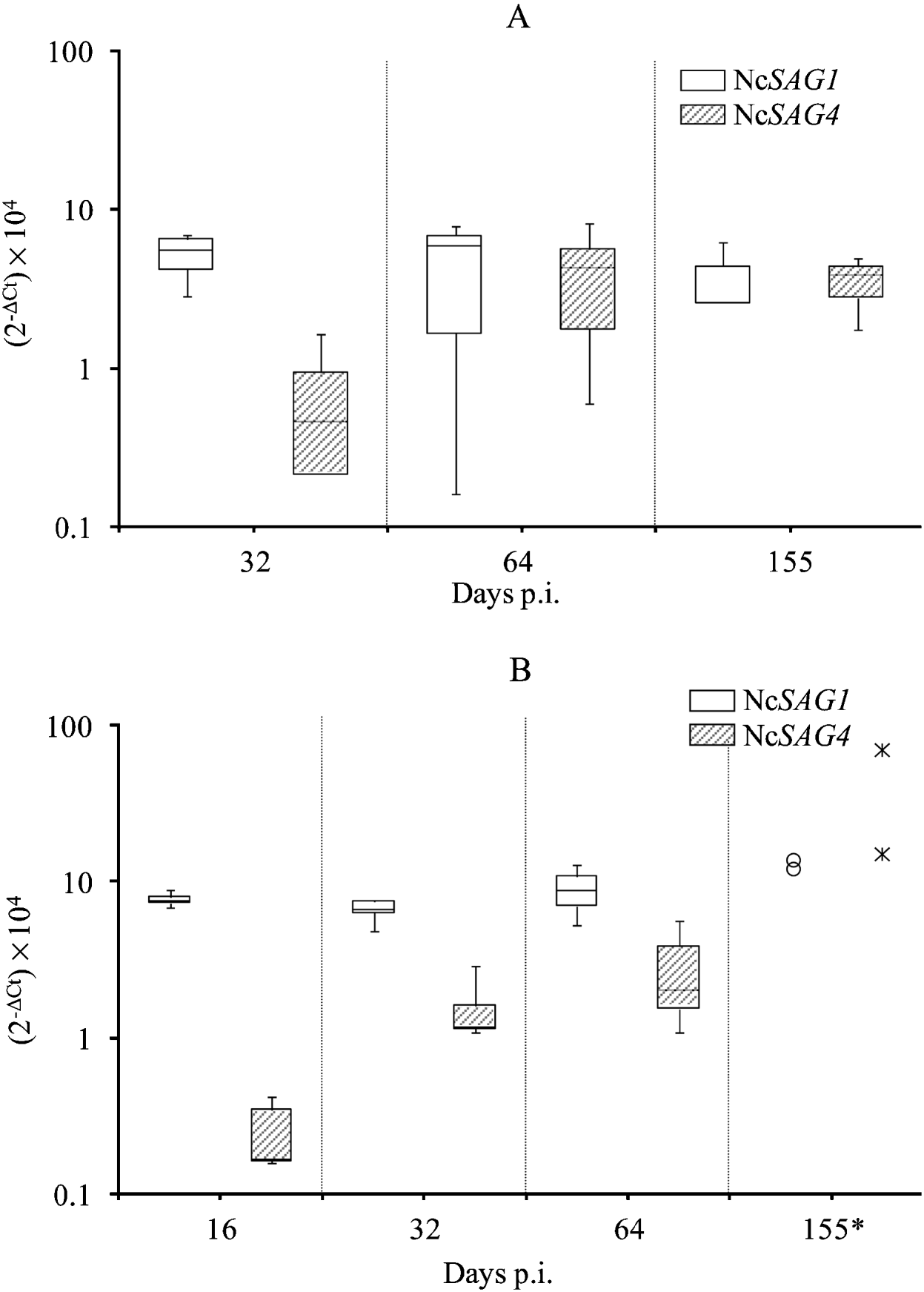
Regarding mice infected with the Nc-1 isolate, the relative NcSAG4-transcription level showed a significant increase between days 32 and 64 p.i., then remained constant thereafter until day 155 p.i. (P<0·05 by Kruskal-Wallis H test followed by the nonparametric multiple-comparison test) (Fig. 2A). Relative NcSAG1-transcription levels in mice infected with the Nc-1 isolate were similar on days 32, 64 and 155 p.i. (P>0·05 by Kruskal-Wallis H test). The statistical analysis of transcription levels of NcSAG4 and NcSAG1 over time in brains from Nc-Liv-inoculated mice could not be carried out because only 2 samples were available on day 155 p.i. (the 3 other PCR-positive brain samples had insufficient parasite burden). When day 155 p.i. was removed from the analysis, NcSAG4-transcription levels were statistically higher on day 32 compared to day 16 p.i. but they were similar between days 32 and 64 p.i. (P<0·05 by Kruskal-Wallis H test followed by the nonparametric multiple-comparison test). Moreover, although day 155 p.i. could not be included in the statistical analysis, the 2 samples analysed also suggested an increase of NcSAG4-transcription on day 155 p.i. (Fig. 2B). NcSAG1-transcription in Nc-Liv-inoculated mice did not vary over time (P>0·05 by Kruskal-Wallis H test).
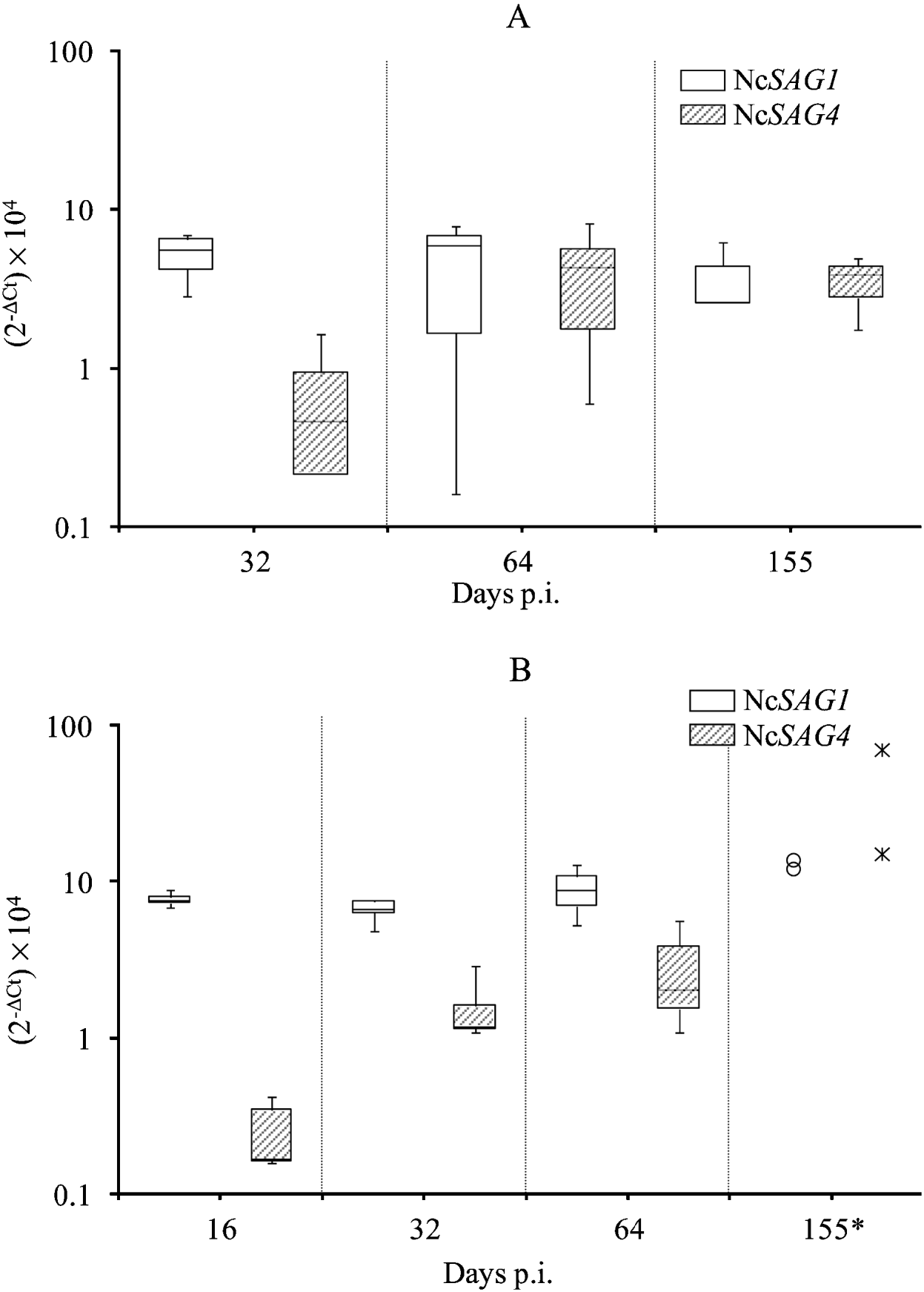
Fig. 2. Box-plot and whisker graph representing the lower and upper quartiles, median and minimum-maximum values for relative NcSAG1 and NcSAG4-transcription levels (2−ΔCt×104) in positive brain tissues from Nc-1-inoculated mice (A) or Nc-Liv-inoculated mice (B).
Despite different NcSAG4-transcription patterns over time, differences between mice inoculated with the Nc-1 or Nc-Liv isolate at each time-point were not significant (P>0·05 by Mann-Whitney U test) and neither were differences in NcSAG1 transcription levels between the two groups (P>0·05 by Mann-Whitney U test).
Detection of specific immunoglobulins
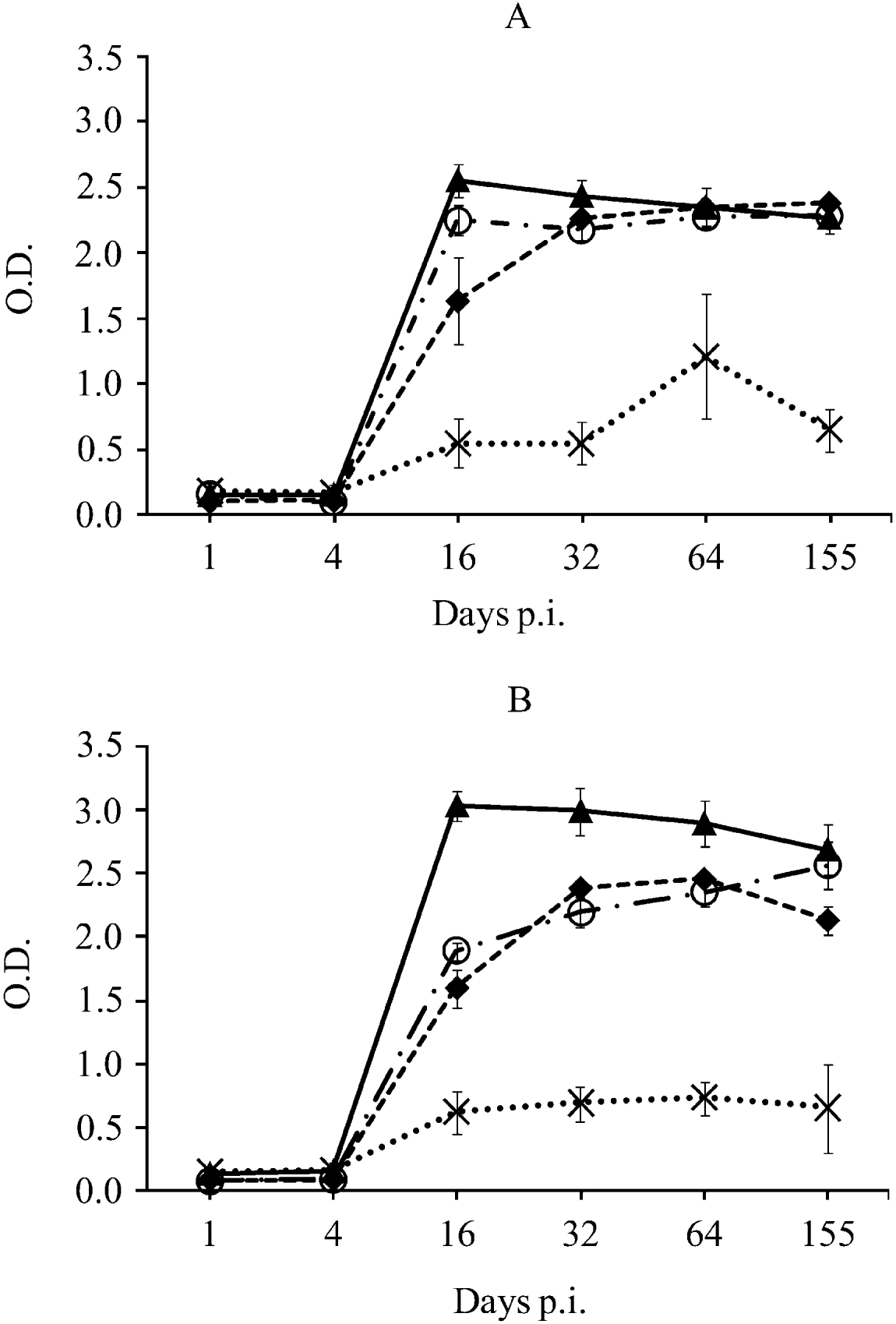
Neospora caninum-specific IgG1, IgG2a and rNcGRA7 and rNcSAG4-specific IgGs were detected from day 16 p.i. until the end of the experiment in both groups of mice. Neospora caninum-specific IgG2a levels were higher than IgG1 levels on day 16 p.i. (P<0·0001 by one-way ANOVA test followed by the Tukey's multiple-comparison test) and similar IgG1/IgG2a-based responses were observed thereafter in both groups of mice (Fig. 3). rNcGRA7-specific antibodies peaked on day 16 p.i. and their levels slightly decreased over time in both groups (P<0·05 by one-way ANOVA test followed by the Tukey's multiple-comparison test). Moreover, rNcGRA7-specific IgG levels were statistically higher than N. caninum-IgG1 and/or IgG2a on day 16 p.i. in both groups of mice and on days 32 and 64 p.i. in Nc-Liv-inoculated mice (P<0·0001 by one-way ANOVA test followed by the Tukey's multiple-comparison test) (Fig. 3).
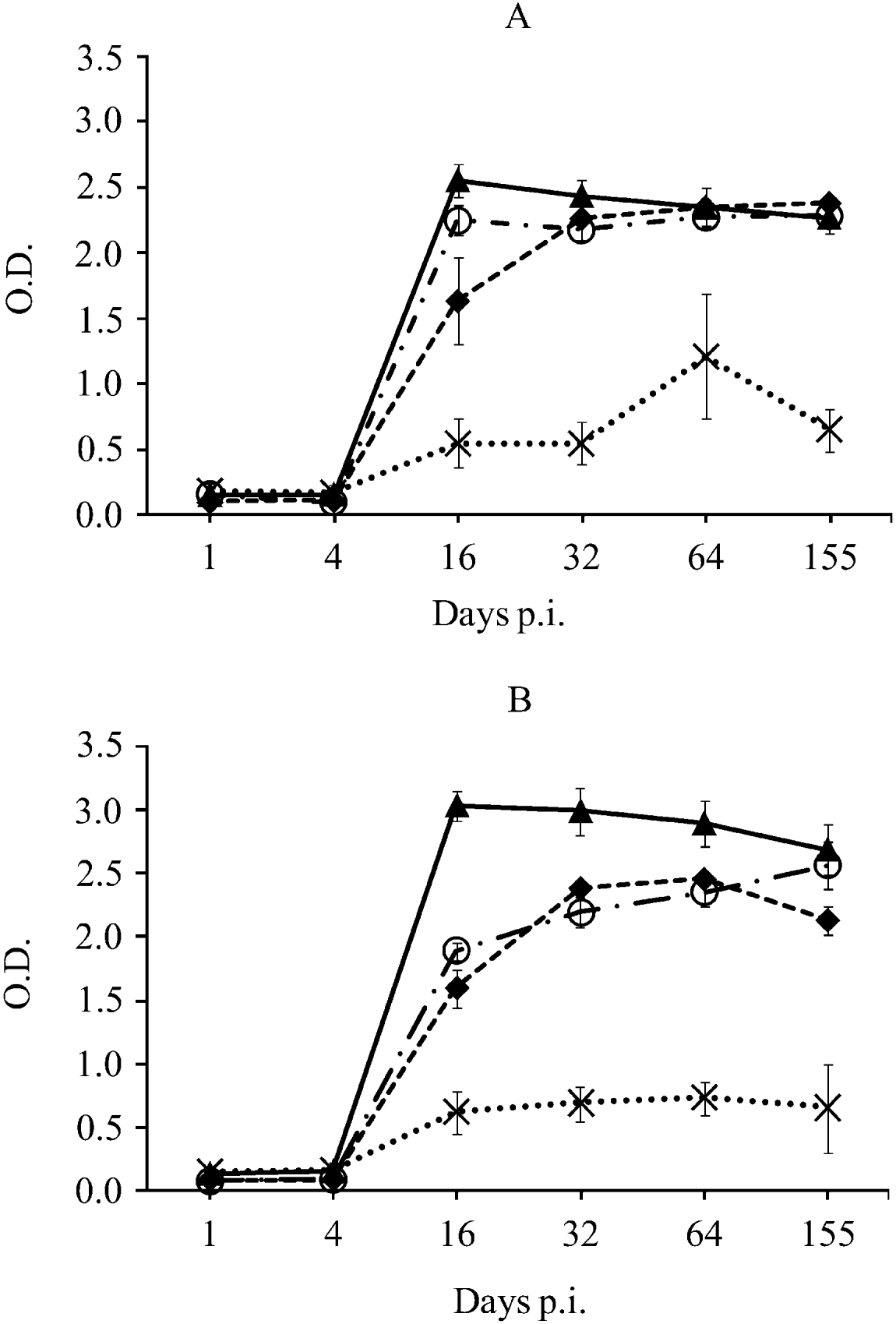
Fig. 3. Kinetics of anti-Neospora caninum tachyzoite antigen and anti-recombinant protein antibodies in Nc-1-inoculated mice (A) or Nc-Liv-inoculated mice (B).
- - -◆- - - N. caninum-specific IgG1, –·○·– N. caninum-specific IgG2a, –▴– rNcGRA7-specific IgG, ![]() rNcSAG4-specific IgG.
rNcSAG4-specific IgG.
The anti-rNcSAG4 IgG response followed different kinetics; its levels slightly increased over time, reaching maximum levels on day 64 p.i. in both inoculated groups, and slightly declined on day 155 p.i. (Fig. 3). However, anti-rNcSAG4 IgG levels were always statistically below levels of anti-N. caninum IgG1, IgG2a and anti-rNcGRA7 IgGs in both groups of mice (P<0·0001 by one-way ANOVA test followed by the Tukey's multiple-comparison test). A significant temporal increase of anti-rNcSAG4 IgG was only found among Nc-1-inoculated mice on day 64 p.i. compared to the rest of the days (P<0·05 by one-way ANOVA test followed by the Tukey's multiple-comparison test) (Fig. 3A).
Regarding the comparison of the antibody response to each isolate, anti-rNcGRA7 antibody levels among Nc-Liv-inoculated mice were statistically higher than those among Nc-1-inoculated mice during the entire study (P<0·01 by two-way ANOVA test). However, anti-rNcSAG4 antibody levels were only statistically different on day 64 p.i., when Nc-1-infected mice showed higher levels than Nc-Liv-infected mice (P<0·01 by two-way ANOVA test) (Fig. 3).
DISCUSSION
We have studied for the first time, transcription levels of the N. caninum bradyzoite-specific NcSAG4 gene during the course of infection in a cerebral mouse model. Transcription levels of the NcSAG1 gene, which is specifically expressed in tachyzoites, were also measured as a control for the presence of tachyzoites in the brain. Our initial hypothesis was that quantifiable variations in expression levels of stage-specific genes could be related to the phase of the infection, since serology based on recombinant NcSAG4 protein provided new insights into the predominant phase of infection in naturally infected cattle (Aguado-Martinez et al. Reference Aguado-Martínez, Álvarez-Garcia, Fernández-García, Risco-Castillo, Arnaiz-Seco, Rebordosa-Trigueros, Navarro-Lozano and Ortega-Mora2008). Thus, to evaluate whether this gene could be employed as a molecular marker of chronic infection, mRNA levels of NcSAG4 were quantified by real time RT-PCR in brain tissue samples from experimentally-infected mice. NcSAG1 mRNA levels were also quantified by this technique in the same samples.
On one hand, a statistically significant temporal increase of relative NcSAG4-mRNA levels was found in brain from both Nc-1 and Nc-Liv-infected mice during chronic infection, suggesting that tachyzoite-to-bradyzoite conversion took place at this stage. However, different expression patterns were observed between mice receiving each isolate. These two patterns could be due to a difference in virulence between the two isolates. Nc-1 is a moderately virulent isolate in mice with less brain tropism than Nc-Liv (Collantes-Fernández et al. Reference Collantes-Fernández, López-Pérez, Álvarez-García and Ortega-Mora2006). In our study, these previous results were corroborated as Nc-1-inoculated mice displayed significantly lower parasite burden in brain during the early chronic infection (days 16 and 32 p.i.) than Nc-Liv-inoculated mice. Thus, tachyzoites of the Nc-1 isolate could replicate slower in brain, or may reach the brain later or with a lower dose than Nc-Liv. Consequently, conversion to bradyzoite stage could be also delayed compared to Nc-Liv isolate. This could explain the observation that NcSAG4-transcription in brain from Nc-1-inoculated mice peaked during the late chronic infection (at day 64 p.i.), whereas the first significant increase of NcSAG4-RNA from Nc-Liv-inoculated mice occurred earlier, on day 32 p.i. Brain samples from the 2 remaining Nc-Liv-inoculated mice on day 155 p.i. also showed a relative increase of NcSAG4 transcription, compared to day 64 p.i., which could be associated with spontaneous reactivation of bradyzoites to tachyzoites. However, a greater number of samples are required to confirm this NcSAG4-mRNA increase. In the same way, TgSAG4 gene amplification has been previously associated with Toxoplasma-encephalitis relapse in human patients by RT-PCR (Cultrera et al. Reference Cultrera, Seraceni and Contini2002); supporting the theory that NcSAG4 is a bradyzoite-specific marker of reactivation. Further evaluation of these issues could be undertaken by experimental induction of N. caninum reactivation in mice.
It is also unknown whether levels of NcSAG4-mRNA would decrease at some point after day 155 p.i. It would be complicated to show this using this mouse model due to the low parasite burden found in the brain beyond day 64 p.i. Some authors have suggested that the majority of TgSAG4 expression (and other T. gondii developmentally-regulated genes) takes place during the tachyzoite-to-bradyzoite conversion; expression then decreases once encystation and/or chronic infection have been established (Gross et al. Reference Gross, Bohne, Lüder, Lugert, Seeber, Dittrich, Pohl and Ferguson1996; Cleary et al. Reference Cleary, Singh, Blader, Brewer and Boothroyd2002; Contini et al. Reference Contini, Giuliodori, Cultrera and Seraceni2006). Thus, Cleary et al. (Reference Cleary, Singh, Blader, Brewer and Boothroyd2002) developed a microarray analysis of T. gondii cDNA in which they confirmed bradyzoite-specific expression of TgSAG4A. However, they observed that this gene not only is highly expressed at the end of in vitro conversion assay (day 4 post-stress), but also earlier (days 2 and 3 post-stress).
Regarding the tachyzoite-specific NcSAG1 (Fuchs et al. Reference Fuchs, Sonda, Gottstein and Hemphill1998), 3 different possible causes could explain the lack of decrease in its transcription levels during chronic infection. (1) The presence of a significant burden of tachyzoites when other tachyzoites start the conversion to the bradyzoite stage, or even co-existence with mature cysts containing bradyzoites. (2) The presence of intermediate zoites co-expressing both tachyzoite and bradyzoite-specific genes for a significant period of time during the tachyzoite-to-bradyzoite conversion. (3) Spontaneous reactivation of some bradyzoites to the tachyzoite stage. These 3 possibilities, which could all take place simultaneously, are supported by in vitro and in vivo assays. Thus, the asynchronous process of in vitro conversion of N. caninum and T. gondii tachyzoites to bradyzoites with simultaneous presence of both stages within the same parasitophorous vacuole, or even simultaneously with mature cysts, is a well-known phenomenon (Soete et al. Reference Soete, Fortier, Camus and Dubremetz1993; Vonlaufen et al. Reference Vonlaufen, Muller, Keller, Naguleswaran, Bohne, McAllister, Bjorkman, Muller, Caldelari and Hemphill2002; Risco-Castillo et al. Reference Risco-Castillo, Fernández-García and Ortega-Mora2004). Moreover, microarray analysis of TgSAG1 cDNAs showed a significant transcription decrease during in vitro bradyzoite development on days 3 and 4 post-bradyzoite induction, but not on day 2 post-stress, suggesting the relative expression level of this gene in the beginning of tachyzoite-to-bradyzoite conversion (Cleary et al. Reference Cleary, Singh, Blader, Brewer and Boothroyd2002). In addition, the detection of an intermediate stage co-expressing both tachyzoite and bradyzoite-specific genes during a significant period of the infection course has been previously shown with in vitro assays of both N. caninum and T. gondii bradyzoite differentiation (Tunev et al. Reference Tunev, McAllister, Anderson-Sprecher and Weiss2002; Radke et al. Reference Radke, Guerini, Jerome and White2003; Risco-Castillo et al. Reference Risco-Castillo, Fernández-García and Ortega-Mora2004). An in vivo study of T. gondii stage conversion in intermediate hosts also described intermediate organisms displaying specific-tachyzoite and bradyzoite molecular markers during the transition to chronic infection in brain lesions by electron microscopy and immunocytochemistry (Ferguson, Reference Ferguson2004). Although Contini et al. (Reference Contini, Giuliodori, Cultrera and Seraceni2006) did not measure RNA transcription level; they observed a different profile for some stage-specific genes measured by quantitative real-time PCR in polymorphonuclear blood monocytic cell samples from patients with toxoplasmic lymphadenitis. In this study, despite the fact that TgSAG1 is a major tachyzoite-specific antigen, it continued to be detected all through the acute infection and persisted thereafter during the convalescent phase. These observations corroborated our findings regarding the persistence of NcSAG1-RNA during N. caninum chronic infection in mice.
The in vivo developmental regulation of the NcSAG4 gene observed in this work was supported by the study of specific antibody production in mice. The N. caninum-specific IgG1/IgG2a response showed a similar kinetic pattern to that found previously (Collantes-Fernández et al. Reference Collantes-Fernández, López-Pérez, Álvarez-García and Ortega-Mora2006). On one hand, we analysed specific antibodies against rNcGRA7, an immunogenic antigen shared by both tachyzoites and bradyzoites but associated with active infection in bovines (Huang et al. Reference Huang, Liao, Zhang, Lee, Nishikawa and Xuan2007; Aguado-Martínez et al. Reference Aguado-Martínez, Álvarez-Garcia, Fernández-García, Risco-Castillo, Arnaiz-Seco, Rebordosa-Trigueros, Navarro-Lozano and Ortega-Mora2008). Specific anti-rNcGRA7 antibody levels were high at the time of seroconversion (day 16 p.i.), and remained high during chronic infection despite a slight decrease at the end. This kinetic pattern is similar to the relative NcSAG1-transcription levels, which remained constant from day 16 to day 155 p.i. in both groups of mice. Here, we found again a clear difference between both isolates. Thus, the faster replication of tachyzoites from the Nc-Liv isolate compared to Nc-1, could produce a more intense and prolonged exposure to the NcGRA7 protein and, consequently, a major production of specific anti-rNcGRA7 immunoglobulins as we observed in this study. Levels of rNcSAG4 specific antibodies, which have been associated with chronic infection in bovines (Aguado-Martínez et al. Reference Aguado-Martínez, Álvarez-Garcia, Fernández-García, Risco-Castillo, Arnaiz-Seco, Rebordosa-Trigueros, Navarro-Lozano and Ortega-Mora2008), remained low after seroconversion at day 16 p.i. and increased slightly over time. In fact, anti-rNcSAG4 antibody levels elicited by Nc-1-inoculated mice peaked on day 64 p.i. coinciding with the significant increase of NcSAG4 transcription levels. Although this increase on NcSAG4 transcription was detected on day 64, it could have produced several days before (between day 32 and 64 p.i.), allowing time for induction of the anti-rNcSAG4 immune response detected on day 64 p.i. These observations support the temporal increase of NcSAG4 gene transcription found in parasitized brain tissues during the course of the infection.
In conclusion, we have analysed for the first time the transcription kinetics of the bradyzoite-specific gene, NcSAG4 in mice experimentally infected with N. caninum. Whereas the relative transcription of the tachyzoite-specific NcSAG1 did not show significant fluctuations during chronic infection in N. caninum-infected brains, our results show that NcSAG4 could be a promising candidate as a molecular marker for monitoring the establishment of chronic infection or reactivation by the different isolates. In the future, other stage-specific genes such as NcBSR4 (Risco-Castillo et al. Reference Risco-Castillo, Fernández-García, Zaballos, Aguado-Martínez, Hemphill, Rodriguez-Bertos, Álvarez-García and Ortega-Mora2007) could be studied in the same way.
We gratefully acknowledge Vanesa Navarro-Lozano for her excellent technical assistance. This work was supported by the AGL2004-02529/GAN project from Ministerio de Educación y Ciencia (MEC). Adriana Aguado-Martínez was financed by a fellowship from MEC.