Extracorporeal membrane oxygenation (ECMO) patients are at a higher risk of hospital-acquired infections (HAIs) than other critically ill patients.Reference Biffi, Di Bella and Scaravilli 1 Because nearly all patients on ECMO have ≥1 concurrent central venous catheters (CVCs), bloodstream infections (BSIs) in ECMO patients are often counted as central-line–associated bloodstream infections (CLABSIs) and thus contribute to penalties from the Centers for Medicare and Medicaid Services (CMS). We aimed to determine the incidence of BSI and CLABSI in ECMO patients at one of the largest ECMO centers in the United States.
We cross-referenced the ECMO patient registry and microbiology databases to identify patients who had positive blood cultures following ECMO cannulation at Duke University Hospital between January 1, 2014, and December 31, 2016. Duke University Hospital is a 957-bed tertiary-care hospital and, by volume, has been among the 5 best ECMO programs in the country since 2014. The ECMO exposure period was defined as 2 days after cannulation through 1 day after decannulation to mirror the National Healthcare Safety Network’s (NHSN) CLABSI definition. Infection preventionists adjudicated whether BSIs were primary CLABSI, secondary to another infection site, or neither (eg, single positive culture for common commensal organism), using NHSN criteria. An infectious diseases physician also reviewed each chart and abstracted clinical data using a standardized template. The Duke University Institutional Review Board approved this study.
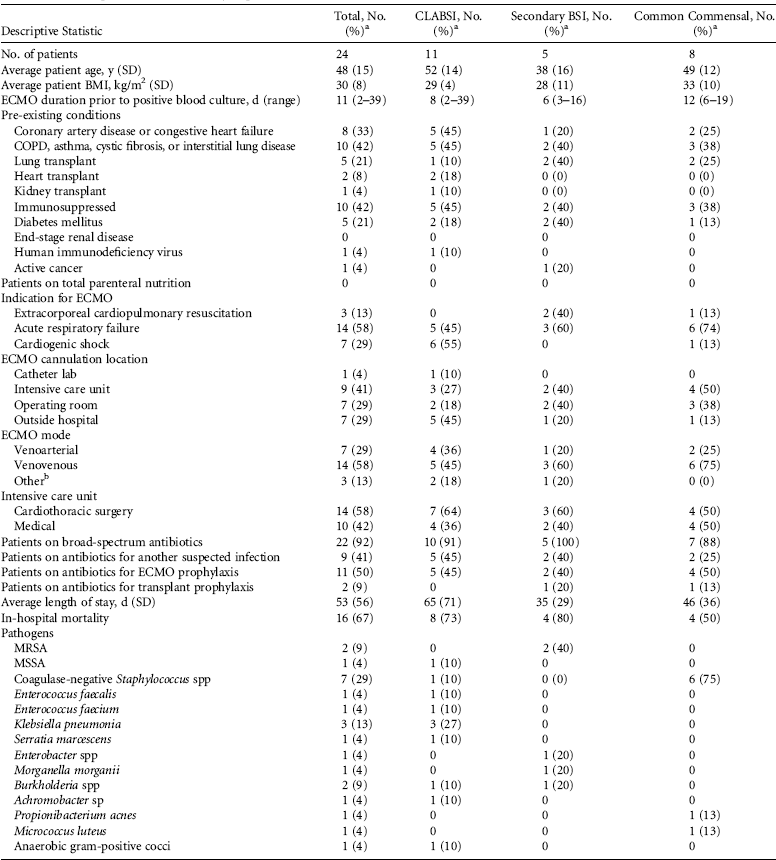
During the study period, 426 patients received 3,534 days of ECMO; 24 patients (5.6%) had a documented BSI during the ECMO exposure period (incidence rate [IR], 6.8 per 1,000 ECMO days). Overall, 11 BSIs met the criteria for CLABSI (IR, 3.1 per 1,000 ECMO days). In addition, 5 patients had BSIs secondary to pneumonia and 8 patients had single cultures positive for common commensal organisms (Table 1).
TABLE 1 Descriptive Statistics of Study Population

NOTE. CLABSI, central-line–associated bloodstream infection; BSI, bloodstream infection; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; SD, standard deviation; COPD, chronic obstructive pulmonary disease; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
a Unless otherwise specified.
b Other ECMO modes included venoarterial/venovenous to right ventricular assist device (RVAD) to oxygenator or venoarterial to left ventricular assist device (LVAD) to oxygenator.
Clinical features of patients with BSI are shown in Table 1. Importantly, 8 patients (33%) were solid-organ transplant recipients, and 2 patients were immunosuppressed following receipt of chemotherapy and a tumor necrosis factor (TNF)-α inhibitor, respectively. The median numbers of days on ECMO prior to a positive blood culture were 8 days (range, 2–39 days) for CLABSI, 6 days (range, 3–16 days) for secondary BSI, and 12 days (range, 6–19 days) for common commensal organisms (Figure 1). Of 11 total CLABSIs, 5 (45%) occurred between days 2 and 5 of ECMO exposure, which suggests the possible introduction of bacteria with ECMO cannulae insertion.

FIGURE 1 Number of positive blood cultures versus days on extracorporeal membrane oxygenation (ECMO), stratified by type of bloodstream infection.
Most identified pathogens causing CLABSI and secondary BSI were gram-negative organisms (Table 1). Furthermore, 2 (92%) patients were receiving broad-spectrum antibiotics at the time of positive blood culture, either as prophylaxis or as treatment of another infection,. Of the 11 identified CLABSI pathogens, 8 were resistant to broad-spectrum antibiotics, 2 were sensitive to concurrent antibiotics, and 1 occurred in a patient not receiving antimicrobials. Of the 11 CLABSI patients, 6 had femoral ECMO cannulae and 4 had a femoral CVC insertion site.
Although the rate of total BSI among ECMO patients in our cohort was comparable to those of other published reports,Reference Biffi, Di Bella and Scaravilli 1 the incidence rate of CLABSI among ECMO patients was 3 times higher than nationally reported rates for medical and cardiothoracic ICUs at academic teaching hospitals.Reference Dudeck, Weiner and Allen-Bridson 2 Furthermore, ECMO patients accounted for 18% of CLABSIs occurring in our cardiothoracic and medical intensive care units (ICUs), despite representing only 8% of CVC days in these ICUs. While the risk of infection in ECMO patients has been well described,Reference Biffi, Di Bella and Scaravilli 1 our study is among the few publications to specifically report on the impact of ECMO utilization and CLABSIs. We were not able to determine the definitive BSI source from chart review, but we suspect that at least a portion of “CLABSIs” were not associated with CVCs and may have originated from the ECMO cannulae insertion sites. Moreover, ECMO cannulae are often inserted at femoral sites, and maintenance of sterile dressings is challenging due to the large caliber of the cannulae and high incidence of bleeding at the insertion site.
We believe that many of the CLABSIs identified in our study were not preventable with existing CVC bundles. Additional research is needed to better understand how to prevent BSI in this high-risk patient population. The Extracorporeal Life Support Organization (ELSO) specifies that cannulation be performed with full sterile preparation but does not comment on cannula maintenance practices. Additionally, the role of prophylactic antibiotics for patients on ECMO is unclear. At our institution, all patients in the cardiothoracic ICU are placed on vancomycin, cefuroxime, and fluconazole unless they have other indications for antibiotics. In the medical ICU, ECMO patients are only placed on antibiotics if this approach is indicated based on suspicion or diagnosis of an infection or if prophylaxis is required for another indication such as following organ transplant. Of all patients in our study, 92% were on broad-spectrum antibiotics, either for prophylaxis or for treatment of another infection, when they developed a BSI. Additional studies are needed to determine whether prophylactic antibiotics provide benefit or harm.
This study has limitations inherent in a single-center, retrospective study. Our findings may not be applicable to other centers with different patient populations. Data were obtained by retrospective chart review, and it is possible that we misidentified BSI events as CLABSI that were secondary to another site of infection. Also, we could not reliably determine whether blood cultures were drawn from an indwelling line, which may have increased the risk of blood culture contamination.
In conclusion, we support the 2018 proposed NHSN CLABSI definition change that excludes BSI occurring in patients on ECMO. 3 This recommendation will improve the reliability of interhospital comparisons and reduce potential CMS penalties for centers with ECMO populations.
ACKNOWLEDGMENTS
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.