INTRODUCTION
Paleoecological studies along the north Pacific coast of North America have largely focused on inferring vegetation change since the last glacial maximum through pollen analysis of lake sediments. This research has revealed a rich paleoecological history, marked by the early to mid-Holocene establishment of temperate rain forests with some of the planet's largest stores of aboveground biomass per unit area (Pan et al., Reference Pan, Birdsey, Phillips and Jackson2013). Few studies have focused on understanding peatland dynamics in this maritime setting, despite wetlands being common landscape features and important carbon (C) stores, and even fewer have inferred long-term rates of C accumulation. At a slope bog on the north coast of British Columbia (BC), Turunen and Turunen (Reference Turunen and Turunen2003) determined that peat accumulation began 12,000 cal yr BP via forest paludification and that mean C accumulation rates (CAR) over the last 8500 years were only 8.6 g C/m2/yr (Loisel et al., Reference Loisel, Yu, Beilman, Camill, Alm, Amesbury and Anderson2014). Lacourse and Davies (Reference Lacourse and Davies2015) documented a higher mean rate (16.1 g C/m2/yr) for the last 10,000 cal yr at a flat Sphagnum bog on northern Vancouver Island that formed through terrestrialization. These CAR are similar to rates at peatlands to the north on the south coast of Alaska (9–19 g C/m2/yr; Jones and Yu, Reference Jones and Yu2010; Loisel et al., Reference Loisel, Yu, Beilman, Camill, Alm, Amesbury and Anderson2014; Nichols et al., Reference Nichols, Peteet, Moy, Castañeda, McGeachy and Perez2014), but lower than Loisel et al.’s (Reference Loisel, Yu, Beilman, Camill, Alm, Amesbury and Anderson2014) estimate for northern peatlands generally (23 g C/m2/yr), and significantly lower than those in some continental peatlands (~30 g C/m2/yr; Yu et al., Reference Yu, Vitt and Wieder2014; Zhao et al., Reference Zhao, Tang, Yu, Li, Yang, Zhao, Li and Li2014). Documenting long-term CAR, particularly in coastal BC where there are few studies, is important for understanding peatland C sequestration, improving estimates of Holocene C stocks, and clarifying the effects of climate change on peatlands and their role in global change as sinks and sources of carbon dioxide and methane (Loisel et al., Reference Loisel, van Bellen, Pelletier, Talbot, Hugelius, Karran, Yu, Nichols and Holmquist2017).
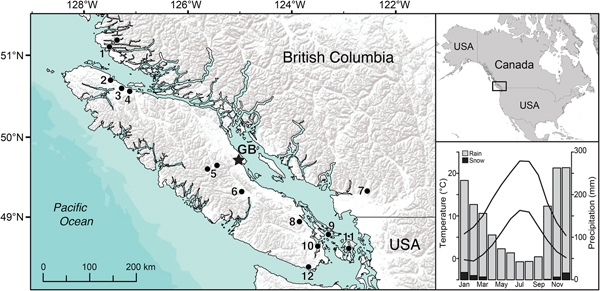
This paper focuses on the paleoecology and C accumulation of a wetland on the east coast of Vancouver Island, the largest island on the Pacific coast of North America. Vancouver Island is separated from the BC mainland by channels that range in width from as little as a few kilometers to as much as 55 km. The island is characterized by steep climatic and ecological gradients due primarily to the Vancouver Island Ranges that run the length of the island (Fig. 1), creating a rain shadow that is magnified further by the Olympic Mountains to the south in Washington. Mean annual precipitation exceeds 3000 mm on the north and west coasts of the island but is only 600 mm on the dry southeastern coast. Much of the BC coast supports closed-canopy coniferous rain forest with bog–forest complexes that are particularly abundant along the north coast. The narrow strip of lowlands on the southeast coast of Vancouver Island (Fig. 1) is characterized by long, dry summers and relatively open Pseudotsuga menziesii–dominated forest.

Figure 1. (color online) Map of Vancouver Island on the south coast of British Columbia, Canada, showing the location of Grant's Bog (star) and other paleoecological studies mentioned in the text: 1, Two Frog Lake and Tiny Lake (Galloway et al., Reference Galloway, Patterson, Doherty and Roe2007, Reference Galloway, Doherty, Patterson and Roe2009); 2, Bear Cove Bog (Hebda, Reference Hebda1983); 3, Misty Lake (Lacourse, Reference Lacourse2005); 4, Port McNeill Bog (Lacourse and Davies, Reference Lacourse and Davies2015); 5, Harris Lake Ridge Bog (Fitton, Reference Fitton2003) and Burman Pond (Mazzucchi, Reference Mazzucchi2010); 6, Turtle Lake (Fitton, Reference Fitton2003); 7, Marion Lake (Mathewes, Reference Mathewes1973); 8, Porphyry Lake (Brown and Hebda, Reference Brown and Hebda2003); 9, Roe Lake (Lucas and Lacourse, Reference Lucas and Lacourse2013); 10, Saanich Inlet (Pellatt et al., Reference Pellatt, Hebda and Mathewes2001); 11, Killebrew Lake (Leopold et al., Reference Leopold, Dunwiddie, Whitlock, Nickmann and Watts2016); 12, East Sooke Fen (Brown and Hebda, Reference Brown and Hebda2002). Top inset shows location in North America. Bottom inset shows monthly means of minimum and maximum temperature and precipitation at nearby Black Creek climate station (Environment Canada, 2018).
Here, we use multiple paleoenvironmental proxies to infer the developmental history of an ombrotrophic bog on Vancouver Island and changes in regional forest composition over the last 14,000 years. We combine pollen, non-pollen palynomorphs (NPPs), plant macrofossils, and bulk chemical analyses, including C and nitrogen (N) isotopes, to document changes in regional and local plant communities as well as hydrological and edaphic conditions. We also compare long-term rates of C accumulation with other peatland records and Holocene climate change. This study advances our understanding of wetland succession, long-term C accumulation, and peatland dynamics in a temperate maritime setting. We also further refine the paleoecological history of coastal BC by providing a new pollen record of postglacial forest dynamics from an area of the coast that has received little attention in previous research.
STUDY SITE
Grant's Bog (49°47.3′N, 125°07.6′W, 80 m above sea level) is located 7 km from the coast in the Black Creek watershed of the eastern coastal lowlands of Vancouver Island, BC (Fig. 1). The area supports coniferous forest dominated by Pseudotsuga menziesii and Tsuga heterophylla. Mean July temperature near the study site is 17.1°C, mean January temperature is 2.8°C, and the number of frost-free days is at least 280 (Black Creek weather station; Environment Canada, 2018). Mean annual precipitation is 1645 mm/yr; summers are generally dry, with most precipitation falling as rain between October and March (Fig. 1).
Grant's Bog (informal name) is part of a 70 ha wetland complex that includes 7.5 ha of marsh along the southwestern margin, which is covered by emergent Nuphar polysepala with a peripheral fringe of Dulichium arundinaceum, and a shallow, open-water pond (1.8 ha) in the southeastern corner. These shallow-water ecosystems occupy slightly deeper topographic depressions than the Sphagnum–ericad bog that characterizes most of the wetland complex. Plant cover in the bog is dominated by Sphagnum mosses (S. fuscum, S. angustifolium, S. capillifolium, S. palustre) and ericaceous shrubs (Rhododendron groenlandicum, Kalmia microphylla var. occidentalis, Vaccinium uliginosum). Other common species include Vaccinium oxycoccus, Rubus chamaemorus, Eriophorum chamissonis, Rhynchospora alba, and Drosera rotundifolia. Empetrum nigrum, Myrica gale, and stunted Pinus contorta var. contorta are present in low abundance. The water table in the bog was 16 cm below the surface at the coring location in July 2013 and mean water pH was 3.6. Golinski (Reference Golinski2004) documented mean annual water table fluctuations of 35 cm.
METHODS
A 810-cm peat and sediment core was collected from Grant's Bog in July 2013 using a Russian D-corer with a 50-cm-long and 5-cm-diameter semicylindrical chamber. We alternated between two boreholes located 25 cm apart and collected sections with 10 cm of overlap. Nine accelerator mass spectrometry (AMS) radiocarbon ages were obtained on plant macrofossils or organic lake sediment (Table 1). Six of these ages are at depths where a stratigraphic change occurs, which allows accumulation rates to be more reliably estimated than dating at systematic intervals. The IntCal13 data set (Reimer et al., Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey and Buck2013) was used to calibrate 14C ages to calendar years (cal yr BP). An age–depth model was built on calendar age probability distributions and an age of −63 cal yr BP for the top of the core, using 10,000 iterations of a smooth spline in the ‘clam’ package (Blaauw, Reference Blaauw2010) in R (R Core Team, 2017). The age at 727 cm on wood was excluded from the model, because it is out of stratigraphic order and considerably younger than the older ages immediately above and below. The ‘Bacon’ package (Blaauw and Christen, Reference Blaauw and Christen2011) was not used to build a chronology, because that approach produces a model that is more or less equivalent to linear interpolation but discounts the 14C age at 626 cm, which provides important chronological control on the transition to a terrestrial environment.
Table 1. AMS radiocarbon and calibrated ages from Grant's Bog on Vancouver Island, British Columbia.

Loss-on-ignition (LOI) was conducted on 1–2 cm3 samples taken at 2–4 cm intervals along the length of the core. Samples were dried at 105°C for 20 h and then ignited at 550°C for 4 h. C and N analyses were conducted at a resolution of <150 cal yr between samples in the peat portion of the core. Samples of 2–3 cm3 were dried for 48 h at 55°C and ground to a fine powder (<125 µm) with a Retsch MM 200 ball mill. Tin capsules (5 × 8 mm) were then packed with 3–5 mg of homogenized peat and analyzed on a Costech ECS 4010 thermal combustion elemental analyzer coupled to a Thermo Finnigan DELTAplus Advantage isotope ratio mass spectrometer. Replicate analyses were conducted on 15% of samples. Standards including acetanilide (71.09% C and 10.36% N), peach leaves (−25.95‰ δ13C and 1.88‰ δ15N), and DORM (−17.27‰ δ13C and 14.33‰ δ15N) were included in every run. Accuracy based on these standards is better than ± 1.5% for C and N, ±0.4‰ for δ13C, and ± 0.2‰ for δ15N.
Pollen and NPPs were identified in 1–2 cm3 samples (n = 102) that were treated with warm 10% KOH for 8 min, sieved through 150 µm mesh, and then treated with warm acetolysis for 2.5 min and mounted in 2000 cs silicone oil. Samples below 744 cm in the clay portion of the core were also treated with HF and sieved with 10 µm mesh. These five samples were excluded from NPP analysis because HF destroys many of these remains. One Lycopodium tablet of 18,584 ± 829 spores (batch #177745) was added to each sample to estimate palynomorph concentrations. At least 400 terrestrial pollen and spores, not including Sphagnum, were identified in each sample. Alnus pollen were identified according to May and Lacourse (Reference May and Lacourse2012). NPPs including fungal spores, algal remains, aquatic plant microfossils, and testate amoebae were identified using van Geel (Reference van Geel1978), Pals et al. (Reference Pals, van Geel and Delfos1980), Charman et al. (Reference Charman, Hendon and Woodland2000), Clarke (Reference Clarke2003), and Payne et al. (Reference Payne, Lamentowicz, van der Knaap, van Leeuwen, Mitchell and Mazei2012). Pollen percentages are based on all pollen and spores, except those from Sphagnum and obligate aquatic plants. Cluster analysis of the pollen percentage data was based on all taxa exceeding 5% of the sum, except Sphagnum and obligate aquatic taxa. Percentages were square-root transformed and then analyzed using optimal splitting by information content and a broken stick model (Bennett, Reference Bennett1996). Cluster analysis of the NPP data was based on palynomorphs present in five or more samples using the same approach.
The >150 µm fraction of pollen samples was used for estimating peat composition following a quadrat technique similar to Barber et al. (Reference Barber, Chambers, Maddy, Stoneman and Brew1994). Each sample was poured into gridded petri dishes and all remains were identified in 15 randomly selected 1-cm2 quadrats. Major peat components (herbaceous stems/leaves, moss stems/leaves, ligneous roots, ericad leaves, and unidentifiable organic material) were enumerated and are expressed as percentages of the total count in those quadrats. Other macrofossils (e.g., fungal sclerotia, Nuphar sclereids, charcoal) encountered in the same 15 quadrats were also noted.
RESULTS
Chronology, stratigraphy, and peat composition
The age–depth model for the Grant's Bog core estimated an age of 13,320 cal yr BP (13,660–12,390 cal yr BP) for the base of the organic lake sediments at 744 cm (Fig. 2). Sediment and peat accumulation rates are typically between 0.03 and 0.08 cm/yr, although rates increase between 570 and 480 cm during accumulation of peat consisting mostly of herbaceous remains (Fig. 3), reaching a maximum of 0.24 cm/yr at 525 cm (8900 cal yr BP).

Figure 2. Age–depth model for the Grant's Bog core from Vancouver Island, British Columbia. Gray bands are 95% confidence intervals based on 10,000 model runs. The age at 727 cm was excluded from the model. Glacier Peak–Dusty Creek tephra was not used to constrain the age model; its depth and modeled age of 5800 cal yr BP are shown with dashed droplines.

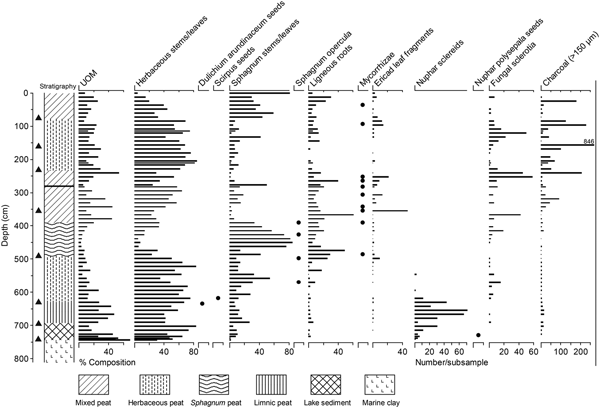
Figure 3. Stratigraphy, peat components, and plant macrofossils for the core from Grant's Bog on Vancouver Island, British Columbia. Circles indicate depth of infrequent macrofossils. Note that the charcoal scale is truncated. Glacier Peak–Dusty Creek tephra is shown as a horizontal line at 280 cm in the stratigraphic column. Triangles along the y-axis show the position of 14C ages (Table 1) used to build the age model. UOM, unidentifiable organic matter.
The base of the core (744–810 cm) consists of clay (Fig. 3). Simple wet mounts of these clays revealed marine diatoms (e.g., Thalassiosira, Campylodiscus) and Dictyocha speculum silicoflagellates below 765 cm, but both marine and freshwater algae (e.g., Thalassiosira, Campylodiscus, Trachyneis aspera, Gyrosigma, Pediastrum) between 765 and 744 cm. There is an abrupt transition at 744 cm from clay to lake sediment (693–744 cm) and then a gradual transition to limnic (possibly telmatic) peat by 693 cm. The limnic peat (628–693 cm) is composed of 40–65% herbaceous remains and 25–45% unidentifiable organic matter (UOM), but Sphagnum leaves and woody roots are also present (Fig. 3). Nuphar sclereids, likely derived from N. polysepala, are more abundant in this limnic peat than in the underlying lake sediment and are more or less absent above 618 cm. Peat consisting of 50–75% herbaceous remains and ~20% Sphagnum leaves occurs between 628 and 490 cm. Scirpus and D. arundinaceum seeds and woody remains are also present, and fungal sclerotia begin to appear more frequently (Fig. 3). Well-preserved Sphagnum-dominated peat occurs between 490 and 390 cm. This is overlain by mixed peat (234–390 cm) consisting of herbaceous, woody, and Sphagnum remains as well as a higher amount of UOM. Ericaceae leaves, mycorrhizal roots, and fungal sclerotia increase in this portion of the core. Peat dominated by herbaceous remains with abundant fungal sclerotia occurs from 234 to 78 cm. Peat near the surface (0–78 cm) is mixed in composition but marked by a notable increase in Sphagnum leaves. Macroscopic charcoal occurs throughout the core but is most abundant between 240 and 84 cm (Fig. 3).
A 1-mm tephra horizon is present at 280.5 cm. The age–depth model predicts an age of 5800 cal yr BP (5970–5410 cal yr BP) for this depth. This is within the uncertainty of the age of the Glacier Peak–Dusty Creek tephra, which was dated to 5120 ± 90 14C yr BP (5940–5750 cal yr BP) by Beget (Reference Beget1981) via charcoal embedded within a pyroclastic flow deposit near the base of Glacier Peak. Foit et al. (Reference Foit, Gavin and Hu2004) report an interpolated age range of 5880–5710 cal yr BP for this tephra in lake sediments from southeastern British Columbia. We attempted to verify the identity of the tephra using electron microprobe analysis; however, the majority of the glass shards were too small for analysis with a 5 µm beam and only two returned quantitative results (Supplementary Material). For these two shards, a similarity coefficient of 0.93 shows that the glass composition matches Glacier Peak–Dusty Creek tephra better than all other Holocene tephras documented in southern British Columbia (Supplementary Material). The Glacier Peak–Dusty Creek tephra has not been recognized previously on Vancouver Island; however, Hansen (Reference Hansen1950) hypothesized that tephra at depths of 2.8 and 3.0 m in a peat sequence at Black River Bog, ~5 km northwest of our study site, was derived from Glacier Peak. Deposition of the Glacier Peak–Dusty Creek tephra at Grant's Bog, 350 km northwest of its source, represents its most northwesterly occurrence to date.
Bulk chemical and isotopic records
Changes in organic matter content (LOI) generally follow the overall stratigraphy. LOI increases rapidly from 3% in the basal clays to 30–70% in the overlying lake sediment (Fig. 4). The limnic peat is characterized by increasing LOI from 70 to 90%. LOI in the upper 6 m of terrestrial peat is 95–99%, although there is a minor decrease to 91% at 194 cm, immediately above large pieces of charcoal (0.5–1 cm3) that were observed during subsampling. Ash-free bulk density (AFBD) is low in the basal clays (0.03 g/cm3) and then increases gradually from 0.05 to 0.13 g/cm3 between 744 and 194 cm, where it decreases abruptly to 0.07 g/cm3. AFBD remains more or less at this lower density until 97 cm and then increases toward the surface.

Figure 4. Stratigraphy and bulk chemical and isotopic records for Grant's Bog on Vancouver Island, British Columbia. See Figure 3 for stratigraphy legend. AFBD, ash-free bulk density; LOI, loss-on-ignition.
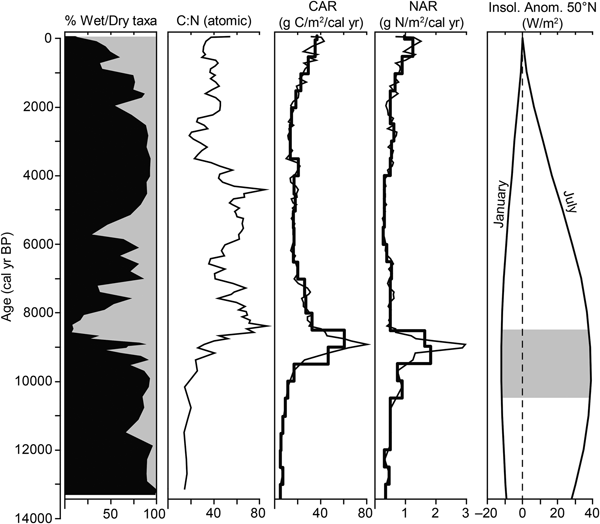
C and N also follow stratigraphic changes. Lake sediment at the base is about 30% C (Fig. 4). C increases to 40% in the limnic peat and then to about 45% in the overlying terrestrial peat. N is 2–3% in the lake sediment and limnic peat, and decreases gradually to about 1% in the terrestrial peat; however, there is a notable increase to 2–3% N between 3500 and 2300 cal yr BP. The C:N is <20 in the lake sediment and limnic peat (i.e., before 10,000 cal yr BP) and then increases gradually to 50–80 between 8700 and 3800 cal yr BP during accumulation of Sphagnum and mixed peat. Again, there is a notable decrease in C:N to 20–30 between 3500 and 2300 cal yr BP, before it increases to ~40 in the uppermost peat. δ13C values are less than −29‰ in the lake sediment and limnic peat, and increase to about −27‰ in the terrestrial peat. δ15N values are between −2‰ and 0‰ for much of the record. Sphagnum peat that accumulated 8700–7750 cal yr BP is marked by a decrease in δ15N to −3.4‰.
CAR are generally low (5–10 g C/m2/yr) in the lake sediment and limnic peat (Fig. 5), but then begin to increase dramatically at ~9700 cal yr BP during accumulation of peat consisting primarily of herbaceous remains, reaching a maximum of 81 g C/m2/yr at 8900 cal yr BP. C accumulation varies between 10 and 30 g C/m2/yr for much of the mid-Holocene and then increases in the uppermost peat to ~40 g C/m2/yr. N accumulation rates (NAR) are ~0.5 g N/m2/yr throughout most of the record (Fig. 5), but increase to 3 g N/m2/yr at 8900 cal yr BP. Mean CAR and NAR, weighted by deposition time, are 19.5 g C/m2/yr and 0.56 g N/m2/yr, respectively, in the peat portion of the record. Time-weighted mean CAR for the various peat types are as follows: 8.3 g C/m2/yr in the limnic peat; 38.6 and 16.3 g C/m2/yr in the early and late Holocene herbaceous peat, respectively; 33.3 g C/m2/yr in the mid-Holocene Sphagnum peat; and 18.2 and 31.5 g C/m2/yr in the mid- and late Holocene mixed peat, respectively.

Figure 5. Carbon (CAR) and nitrogen (NAR) accumulation rates at Grant's Bog on Vancouver Island, British Columbia. Mean accumulation rates in 500 cal yr bins are overlaid on apparent rates. Also shown are percent wet (black) and dry (gray) peat components from Figure 3, C:N, and January and July insolation anomaly at 50°N (Berger and Loutre, Reference Berger and Loutre1991). Gray box highlights the period of maximum difference between January and July insolation.
Non-pollen palynomorphs
Cluster analysis identified five statistically significant NPP assemblage zones (Fig. 6; Supplementary Material) that generally follow changes in stratigraphy and C and N measurements. NPP assemblages in the lake sediment (13,300–11,600 cal yr BP) and limnic peat (11,600–9900 cal yr BP) are dominated by freshwater diatoms and Filinia-type rotifer eggs, reflecting a freshwater environment. Closterium algae and fungal spores, including ascospores of Kretzschmaria deusta (a parasitic fungus on wood and roots), are generally more abundant in the limnic peat than the underlying lake sediment. NPP zone 2 (9800–8700 cal yr BP) coincides with accumulation of herbaceous peat and is marked by increases in Closterium algae, protists, and Type 124 fungal spores. Assemblages in zone 3 (8700–4100 cal yr BP), which corresponds with accumulation of Sphagnum and mixed peat, are characterized primarily by Assulina muscorum and Hyalosphenia subflava protists, and Entophlyctis lobata, Microthyriaceae, and Gaeumannomyces fungal remains. Fewer NPP types are present 4100–2750 cal yr BP (zone 4), when %N increases (Fig. 4); however, there are notable increases in Closterium and Zygnemataceae algae and Type 124 fungal spores at this time (Fig. 6; Supplementary Material). Testate amoebae, particularly H. subflava, increase in the uppermost NPP zone 5. Gelasinospora and E. lobata fungal remains are also common. A number of testate amoebae, including A. muscorum, Arcella discoides type, Hyalosphenia papilio, and Trigonopyxis arcula type, increase in the upper 12 cm of the core (Supplementary Material).

Figure 6. Concentrations of abundant non-pollen palynomorphs (NPP) at Grant's Bog on Vancouver Island, British Columbia. Other testate amoebae are mostly Arcella discoides type and Hyalosphenia papilio but also include Trigonopyxis arcula type, Assulina seminulum, and Hyalosphenia elegans. Note changes in scale on x-axes. Numbers in parentheses are NPP types in van Geel (Reference van Geel1978) and Pals et al. (Reference Pals, van Geel and Delfos1980). See Figure 3 for stratigraphy legend.
Pollen and spore assemblages
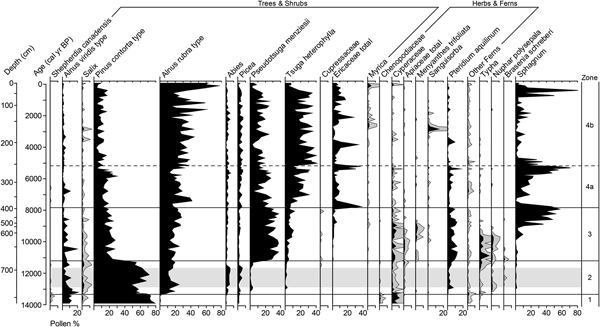
Cluster analysis identified four statistically significant pollen assemblage zones (Fig. 7). Pollen spectra between 14,000 and 13,300 cal yr BP, in the basal clays (zone 1), are 70–80% P. contorta type, 10% Alnus viridis type, and up to 10% Cyperaceae. Picea, Abies, Salix, Shepherdia canadensis, Chenopodiaceae, and Polypodiaceae are present in trace amounts. Pinus contorta continues to dominate the pollen record in the lake sediments of zone 2 (13,300–11,200 cal yr BP). Abies and Picea also increase, and P. menziesii and T. heterophylla appear for the first time, although each of these account for less than 6%. Alnus rubra type increases abruptly to account for ~15% and A. viridis type remains at ~10%. Pollen from herbaceous plants and Pteridium aquilinum spores account for up to 4% and 7%, respectively. Aquatic taxa (Typha, N. polysepala, and Brasenia schreberi) are present in low relative abundance.

Figure 7. Pollen and spore percentages from Grant's Bog on Vancouver Island, British Columbia with 5× exaggeration (gray silhouettes) for infrequent taxa. Ericaceae total includes Empetrum nigrum, Ledum, and Vaccinium, but is mostly undifferentiated Ericaceae pollen. Apiaceae is almost exclusively Angelica type. Order of taxa is based on weighted averages. Gray horizontal band marks the Younger Dryas chronozone.
Pollen zone 3 (11,200–7800 cal yr BP) is marked by a dramatic decline in P. contorta to 25% and a corresponding increase in P. menziesii to 30–40% (Fig. 7). Tsuga heterophylla increases to 5–10% and A. rubra type accounts for 20–30%. There is an overall increase in non-arboreal pollen, with Cyperaceae accounting for up to 7% and other herbaceous taxa, including Angelica type and Menyanthes trifoliata, accounting for another 3%. Pteridium aquilinum reaches its maximum abundance (12%) in zone 3. Pollen from aquatics is relatively abundant 11,500–9700 cal yr BP during accumulation of limnic peat. Ericaceae pollen begins to increase at 9700 cal yr BP, when terrestrial peat dominated by herbaceous remains begins accumulating. Sphagnum spores increase starting ~9500 cal yr BP, reaching 35–60% between 8900 and 7750 cal yr BP during accumulation of Sphagnum-dominated peat.
Pollen zone 4 (7800 cal yr BP to the present) is dominated by three main taxa: T. heterophylla and A. rubra type, which are more abundant in subzone 4b, and P. menziesii, which is more abundant in subzone 4a (Fig. 7). Pinus contorta type accounts for 10–20% and Ericaceae increases relative to zone 3, with a few increases of up to 40%. There is also an isolated increase in Sanguisorba to 15% at 2800 cal yr BP. Myrica increases over the last 2700 cal yr but does not exceed 5%. Pteridium aquilinum accounts for 5–10% in subzone 4a and is present only intermittently in subzone 4b. Sphagnum spores are also generally more abundant in 4a than 4b. The uppermost samples are marked by a large increase in the relative abundance of A. rubra.
DISCUSSION
Wetland succession and C accumulation at Grant's Bog
The Grant's Bog core begins with marine clay deposited before 13,300 cal yr BP. This agrees with Hutchinson et al.’s (Reference Hutchinson, James, Clague, Barrie and Conway2004) sea-level reconstruction based on isolation basins and 14C-dated marine shells and wood in glaciomarine deposits that infers subaerial exposure of this area at 13,500 cal yr BP. Clay deposition occurred initially in a nearshore, marine environment and then in a brackish environment as relative sea level decreased.
A freshwater lake with N. polysepala in low abundance and Typha and Cyperaceae at the margins was in place by 13,300 cal yr BP (Figs. 3 and 7), after the basin became isolated from marine waters. Brasenia schreberi was present in the lake by 12,700 cal yr BP (Fig. 7). Organic lake sediment with 2–3% N and a C:N less than 20 (Fig. 4), which is similar to most lakes (Meyers and Teranes, Reference Meyers, Teranes, Last and Smol2001), accumulated until 11,600 cal yr BP.
The gradual transition from organic lake sediment to limnic peat suggests the beginning of terrestrialization with decreasing lake levels and/or a reduction in lake area, as well as increasing organic matter accumulation and potentially floating-mat encroachment. Rotifer eggs, freshwater diatoms, Typha pollen, and N. polysepala pollen and sclereids are abundant in the limnic peat (Figs. 3, 6, and 7), suggesting the presence of standing water with emergent and floating-leaf aquatics until 9900 cal yr BP. Sclereids provide structural support and, in Nymphaeaceae, are more abundant in the petioles of erect aerial leaves than in floating lily pads (Etnier and Villani, Reference Etnier and Villani2007). The increase in Nuphar sclereids during accumulation of limnic peat (Fig. 3) likely reflects the presence of erect aerial leaves and/or an increase in the overall abundance of N. polysepala linked to decreasing water levels, as these aquatic plants tend to be most abundant in shallow-water wetlands and even occur in bog hollows on Vancouver Island today. Angelica-type pollen (Fig. 7) suggests the nearby presence of Angelica genuflexa, a species characteristic of coastal BC wetlands. The limnic peat is also characterized by relatively high N (2–3%) and low δ13C (<−30‰) due to the presence of aquatic plants and algal communities, which tend to be N rich and 13C poor (Meyers and Teranes, Reference Meyers, Teranes, Last and Smol2001; Talbot, Reference Talbot, Last and Smol2001). C and N accumulation are low during this marshy wetland stage (Fig. 5).
A small lake remains in the southeastern corner of the wetland complex today, but terrestrialization was more or less complete at the coring location by 9900 cal yr BP. δ13C values become more positive in the warm, early Holocene (Fig. 4) and remain relatively high for the rest of the record, reflecting peat accumulation in a terrestrial setting (Jones et al., Reference Jones, Peteet and Sambrotto2010; Andersson et al., Reference Andersson, Meyers, Hornibrook, Kuhry and Mörth2012). Rapidly accumulating herb-dominated peat with less UOM and C:N increasing to 25–40 (Figs. 3 and 4) suggests a short-lived poor fen stage until ~8700 cal yr BP. Given the peat composition, the fen was likely dominated by sedges, including D. arundinaceum and Scirpus (likely S. microcarpus), but Sphagnum macroremains and spores, ligneous roots, and Ericaceae, Cyperaceae, and Sanguisorba pollen indicate diverse plant communities were present. The abundance of M. trifoliata pollen, Archerella flavum (syn. Amphitrema flavum) protists, and Closterium algae between 9700 and 8600 cal yr BP (Figs. 6 and 7) suggests wet conditions and a high water table.
C and N accumulation increase dramatically during this fen stage, reaching maximum apparent rates of 81 g C/m2/yr and 3 g N/m2/yr at 8900 cal yr BP (Fig. 5). These increases are linked to high peat accumulation rates (Fig. 2) combined with increasing bulk density (Fig. 4), as opposed to high C and N content (Fig. 4). Because these high CAR and NAR are largely driven by a plateau in the age model, the early Holocene increase is better described using time-weighted mean rates, that is, 48 g C/m2/yr and 1.4 g N/m2/yr. Early Holocene increases in C and N accumulation were also found at other coastal BC peatlands (Turunen and Turunen, Reference Turunen and Turunen2003; Lacourse and Davies, Reference Lacourse and Davies2015) and are typical of northern peatlands (Loisel et al., Reference Loisel, Yu, Beilman, Camill, Alm, Amesbury and Anderson2014), including those in Alaska (e.g., Jones and Yu, Reference Jones and Yu2010). These increases coincide with high summer insolation and the interval when seasonality in temperature is maximized (Fig. 5). Warm summers and increased seasonality favor peat accumulation by enhancing primary productivity during the growing season and reducing decomposition during winter (Asada and Warner, Reference Asada and Warner2005; Yu et al., Reference Yu, Loisel, Turetsky, Cai, Zhao, Frolking, MacDonald and Bubier2013; Loisel et al., Reference Loisel, Yu, Beilman, Camill, Alm, Amesbury and Anderson2014). Although the early Holocene was drier in coastal BC relative to the present (Walker and Pellatt, Reference Walker and Pellatt2003; Brown et al., Reference Brown, Fitton, Schoups, Allen, Wahl and Hebda2006), there was still sufficient moisture to promote peat accumulation and carbon storage (Gallego-Sala et al., Reference Gallego-Sala, Charman, Brewer, Page, Prentice, Friedlingstein and Moreton2018).
C and N accumulation decrease with the development of a Sphagnum-dominated peatland ~8700 cal yr BP. At most peatlands in eastern North America, the transition from fen to bog occurred later, in the mid- to late Holocene (Yu et al., Reference Yu, Loisel, Turetsky, Cai, Zhao, Frolking, MacDonald and Bubier2013). The well-preserved peat that accumulated at Grant's Bog ~8700–7750 cal yr BP is marked by an abundance of Sphagnum leaves (Fig. 3) and spores (Fig. 7). In general, fluctuations in the abundance of Sphagnum spores correlate well with changes in Sphagnum macroremains, demonstrating that spores can provide a reliable record of Sphagnum abundance in some cases (cf. Lacourse and Davies, Reference Lacourse and Davies2015). High C:N ratios of 60–80 and a notable decrease in δ15N to −3.4‰ during this early Holocene Sphagnum phase (Fig. 4) suggest a low water table (Asada et al., Reference Asada, Warner and Aravena2005), reflecting the transition to ombrotrophy and a fully terrestrialized bog ecosystem. High concentrations of A. muscorum, a testate amoeba that is often most abundant in intermediate to dry peatlands (Charman et al., Reference Charman, Hendon and Woodland2000; Payne et al., Reference Payne, Lamentowicz, van der Knaap, van Leeuwen, Mitchell and Mazei2012), and remains from saprotrophic fungi (e.g., E. lobata sporangia), which require oxic conditions to be major decomposers in peatlands, also suggest a lowering of relative water table depth compared with the preceding fen stage (Fig. 6). Ligneous roots that record colonization of the bog surface by woody plants are also present in this Sphagnum peat. Increases in Ericaceae leaf fragments (Fig. 3) and pollen (Fig. 7) suggest that ericads were abundant in the plant community after 7750 cal yr BP and that further isolation of the bog surface from the water table occurred, despite increasing precipitation through the mid-Holocene (Brown et al., Reference Brown, Fitton, Schoups, Allen, Wahl and Hebda2006).
Peat that accumulated in the mid-Holocene (7750–4700 cal yr BP) consists of a mixture of herbaceous, woody, and Sphagnum remains, with generally more UOM than before or after this time (Fig. 3). Increased decomposition in this portion of the record is also suggested by increases in mycorrhizal roots and fungal remains such as sclerotia, E. lobata sporangia, and Gaeumannomyces hyphopodia (Figs. 3 and 6). Isolated occurrences of D. rotundifolia pollen suggest nutrient-poor, acidic conditions. These various lines of evidence, along with relatively high C:N values of 40–60 (Fig. 4), indicate the site was an ombrotrophic peatland with mixed plant communities for much of the mid-Holocene. CAR are only 15–20 g C/m2/yr between 7200 and 1300 cal yr BP (Fig. 5); peat bulk density and C content (Fig. 4) are similar to the early Holocene, but accumulation rates (Fig. 2) are generally lower. Relative to the early Holocene, climate was cooler, wetter, and less seasonal in the mid- and late Holocene (Walker and Pellatt, Reference Walker and Pellatt2003; Brown et al., Reference Brown, Fitton, Schoups, Allen, Wahl and Hebda2006; Lemmen and Lacourse, Reference Lemmen and Lacourse2018). The abundance of macroscopic charcoal between ~4800 and 1000 cal yr BP (Fig. 3) suggests fire occurred on or near the peatland, despite a generally cooler, wetter climate.
Multiple proxies suggest changes in edaphic and hydrological conditions between 3500 and 2300 cal yr BP, likely as a result of disturbance by fire and subsequent flooding. The most striking change during this interval is an increase in N to 2–3% that is accompanied by a decrease in C:N to ~20 (Fig. 4), suggesting the surface of Grant's Bog was inundated. This interpretation is supported by coincident changes in NPP assemblages, including increases in Closterium algae and diatoms (zone 4 in Fig. 6). The increase in Type 124 fungal spores, probably derived from Persiciospora, suggests eutrophic to mesotrophic conditions (Bakker and van Smeerdijk, Reference Bakker and van Smeerdijk1982). A notable peak in Sanguisorba pollen and minor increases in Salix and Cyperaceae pollen (Fig. 7), as well as decreases in woody roots and ericad leaf fragments (Fig. 3), suggest a transition to plant communities more typical of fens than bogs in coastal BC. Large pieces of charcoal occur at a depth of 194–198 cm (~3600 cal yr BP), just before the increase in %N begins. Just above this, at 192–195 cm, there is a short-lived increase in ash content to 6–9% (Fig. 4), likely associated with an accumulation of combustion residues. As a visible ash layer was not present, it is unlikely that the fire spread downward to any great depth, as is the case in smoldering peat fires, which tend to leave several centimeters of ash (Zaccone et al., Reference Zaccone, Rein, D'Orazio, Hadden, Belcher and Miano2014). Together, these various lines of evidence suggest the return to wet conditions and hydroseral reversion to a poor fen was initiated by fire, which would have enhanced nutrient availability and potentially altered local hydrology, rather than by changes in climate.
Over the last 2000 cal yr, peat dominated by herbaceous remains graded into mixed peat with a C:N of ~40 (Fig. 4), indicating a return to drier surface conditions. Hyalosphenia subflava testate amoebae and Gelasinospora fungal spores, both of which are typical of dry conditions (Charman et al., Reference Charman, Hendon and Woodland2000; Chambers et al., Reference Chambers, van Geel and van der Linden2011; Payne et al., Reference Payne, Lamentowicz, van der Knaap, van Leeuwen, Mitchell and Mazei2012), increase in the late Holocene (Fig. 6). Myrica, a nitrogen-fixing shrub typical of coastal wetlands, also increases (Fig. 7). Similar increases in Myrica occur in other pollen records from coastal BC (e.g., Mathewes, Reference Mathewes1973; Brown and Hebda, Reference Brown and Hebda2002; Lacourse, Reference Lacourse2005), suggesting a region-wide expansion of these shrubs in the late Holocene. At 1000 cal yr BP, there is a marked increase in Sphagnum leaves (Fig. 3) and spores (Fig. 7), with the uppermost 72 cm of peat composed of 30–80% Sphagnum and up to 35% ligneous roots. This is consistent with plant communities at Grant's Bog today, which are dominated by ericaceous shrubs (R. groenlandicum, K. microphylla var. occidentalis, V. uliginosum) that tower above an almost complete moss cover of mostly S. fuscum, S. angustifolium, and S. capillifolium. Testate amoebae concentrations, most notably H. subflava, increase in this well-preserved peat. In the uppermost 12 cm, which corresponds with peat in the acrotelm, a number of other testate amoebae typically found in nutrient-poor peatlands but with variable water table depths (Mitchell, Reference Mitchell2004; Taylor et al., Reference Taylor, Swindles, Morris and Gałka2019) increase (e.g., A. muscorum, A. discoides type, H. papilio) or appear for the first time (e.g., T. arcula type, Hyalosphenia elegans) in the record (Fig. 6; Supplementary Material). CAR and NAR increase over the last 1500 cal yr (Fig. 5), a trend found in most long-term records from northern peatlands (Loisel et al., Reference Loisel, Yu, Beilman, Camill, Alm, Amesbury and Anderson2014) that is in part explained by a shorter interval for decomposition loss following accumulation. Recent modeling efforts suggest C sequestration at mid- to high latitudes is likely to continue to increase through the twenty-first century with further warming (Gallego-Sala et al., Reference Gallego-Sala, Charman, Brewer, Page, Prentice, Friedlingstein and Moreton2018).
Postglacial forest dynamics near Grant's Bog
The pollen record from Grant's Bog provides insight into local changes in wetland vegetation but is primarily dominated by trees (Fig. 7), as would be expected given the high pollen productivity and effective pollen dispersal of conifers, which have dominated vegetation communities in the region since the late Pleistocene. There are few pollen records from central Vancouver Island (Hansen, Reference Hansen1950; Heusser, Reference Heusser1960; Fitton, Reference Fitton2003; Mazzucchi, Reference Mazzucchi2010) to compare with the lowland record from Grant's Bog. Most of these are from high elevations, have low sample resolution, and/or are poorly dated. In general, the record from Grant's Bog provides a history of compositional changes in forests that corresponds with expectations based on these and the many records to the south (e.g., Mathewes, Reference Mathewes1973; Pellatt et al., Reference Pellatt, Hebda and Mathewes2001; Brown and Hebda, Reference Brown and Hebda2002, Reference Brown and Hebda2003; Gavin et al., Reference Gavin, Brubaker and Greenwald2013; Leopold et al., Reference Leopold, Dunwiddie, Whitlock, Nickmann and Watts2016) and north (e.g., Hebda, Reference Hebda1983; Lacourse, Reference Lacourse2005; Galloway et al., Reference Galloway, Patterson, Doherty and Roe2007, Reference Galloway, Doherty, Patterson and Roe2009; Lacourse and Davies, Reference Lacourse and Davies2015).
During and following the late-glacial decrease in relative sea level about 14,000 cal yr BP, vegetation near Grant's Bog consisted primarily of P. contorta, as was the case along much of the northeast Pacific coast at this time (Peteet, Reference Peteet1991; Lacourse, Reference Lacourse2005; Lacourse et al., Reference Lacourse, Mathewes and Fedje2005; Galloway et al., Reference Galloway, Doherty, Patterson and Roe2009; Gavin et al., Reference Gavin, Brubaker and Greenwald2013; Leopold et al. Reference Leopold, Dunwiddie, Whitlock, Nickmann and Watts2016). Alnus viridis, Salix, and Shepherdia canadensis shrubs were also present, making these early vegetation communities near Grant's Bog similar to those that occurred at about the same time at low elevations on northern Vancouver Island (Lacourse, Reference Lacourse2005) and the southeastern BC mainland (Mathewes, Reference Mathewes1973). Macroscale climate at this time was cool and likely drier, relative to the present (Heusser et al., Reference Heusser, Heusser and Peteet1985; Kienast and McKay, Reference Kienast and McKay2001; Lemmen and Lacourse, Reference Lemmen and Lacourse2018).
Pinus contorta continued to dominate plant communities along much of the northeast Pacific coast until the beginning of the Holocene. At Grant's Bog, high relative abundance of Pinus pollen and moderate organic matter content suggest the presence of open P. contorta forests until about 11,200 cal yr BP (Figs. 4 and 7). Pollen from A. rubra and more shade-tolerant conifers, including Abies and Picea, increase during this interval, suggesting an increase in forest density at least regionally, as some portion of these probably derive from long-distance transport. Pseudotsuga menziesii first appears in the Grant's Bog record just before 13,000 cal yr BP. Given the short dispersal distance of P. menziesii pollen (Tsukada, Reference Tsukada1982), it is likely that scattered individuals occurred near Grant's Bog by this time. Pteridium aquilinum ferns, which often occur in association with P. menziesii in modern forests, also appeared at this time.
The increase in P. contorta pollen in the Grant's Bog record between 12,700 and 11,700 cal yr BP is accompanied by minor increases in Abies and Picea and a decrease in A. rubra (Fig. 7). There is also a notable decrease in organic matter content (Fig. 4), indicative of lower overall productivity. The timing of these changes suggests a link to Younger Dryas–related climate change. Some paleoecological records from the northeast Pacific coast suggest cooling during the Younger Dryas chronozone (Engstrom et al., Reference Engstrom, Hansen and Wright1990; Mathewes, Reference Mathewes1993; Lacourse, Reference Lacourse2005; Galloway et al., Reference Galloway, Patterson, Doherty and Roe2007, Reference Galloway, Doherty, Patterson and Roe2009; Gavin et al., Reference Gavin, Brubaker and Greenwald2013), while others show little evidence of cooling at this time (Brown and Hebda, Reference Brown and Hebda2003; Lacourse et al., Reference Lacourse, Mathewes and Fedje2005, Reference Lacourse, Delepine, Hoffman and Mathewes2012; Leopold et al., Reference Leopold, Dunwiddie, Whitlock, Nickmann and Watts2016). A chironomid-based temperature reconstruction from the south coast of BC suggests a decrease of as much as 3°C, relative to modern, during the Younger Dryas (Lemmen and Lacourse, Reference Lemmen and Lacourse2018), which is consistent with other paleotemperature records from the northeast Pacific (Kienast and McKay, Reference Kienast and McKay2001; Gavin et al., Reference Gavin, Brubaker and Greenwald2013). It is unlikely that the Younger Dryas on the northeast Pacific coast was cool and dry, as it was at many locations around the North Atlantic and in northeast Asia (Björck, Reference Björck and Elias2007). Some of the strongest terrestrial evidence for cooling in the northeast Pacific is an increase in Tsuga mertensiana (Mathewes, Reference Mathewes1993; Lacourse, Reference Lacourse2005), an indicator of cool and moist climate. Recent modeling efforts suggest the Younger Dryas chronozone in the northeast Pacific was characterized by an increase in moisture (Renssen et al., Reference Renssen, Goosse, Roche and Seppä2018).
At ~11,200 cal yr BP, there was a rapid transition to Pseudotsuga menziesii forest with abundant Pteridium aquilinum ferns in the understory. Pseudotsuga continued to dominate forests near Grant's Bog until about 7000 cal yr BP. The expansion of P. menziesii populations is a well-documented feature of Holocene vegetation change along the south coast of BC. During the last glacial maximum, P. menziesii occurred south of the Cordilleran Ice Sheet in western Washington and Oregon (Tsukada, Reference Tsukada1982; Gugger and Sugita, Reference Gugger and Sugita2010). Populations migrated north as the ice sheet receded, reaching southern Vancouver Island as early as 14,000 cal yr BP (Brown and Hebda, Reference Brown and Hebda2003), east-central Vancouver Island by 13,000 cal yr BP (this study), and northern Vancouver Island by ~11,000 cal yr BP (Lacourse, Reference Lacourse2005; Lacourse and Davies, Reference Lacourse and Davies2015), at an approximate rate of 120 m/yr. Early Holocene warming allowed P. menziesii to become the dominant conifer on southern (Pellatt et al., Reference Pellatt, Hebda and Mathewes2001) and central (this study) Vancouver Island by 11,000 cal yr BP. It continues to be abundant near Grant's Bog today and to the south, but it has been uncommon on northern Vancouver Island since about 7500 cal yr BP (Lacourse, Reference Lacourse2005; Lacourse and Davies, Reference Lacourse and Davies2015), when a cooler and moister climate lead to contraction of its northern limit (Gugger and Sugita, Reference Gugger and Sugita2010).
Alnus rubra and T. heterophylla pollen increase at Grant's Bog in the early Holocene, but this is probably a reflection of their increasing populations throughout the region, rather than an indication of high local abundance. Only a few Cupressaceae pollen grains, likely from Thuja plicata, are present between 11,500 and 8000 cal yr BP (Fig. 7). Cupressaceae is otherwise absent from the Grant's Bog record. This is unusual compared with Holocene pollen records to the south (Pellatt et al., Reference Pellatt, Hebda and Mathewes2001; Lucas and Lacourse, Reference Lucas and Lacourse2013; Leopold et al., Reference Leopold, Dunwiddie, Whitlock, Nickmann and Watts2016) and north (Lacourse, Reference Lacourse2005; Galloway et al., Reference Galloway, Patterson, Doherty and Roe2007), which contain small but consistent amounts of Cupressaceae in the early Holocene. Furthermore, most pollen records show increasing relative abundance of Cupressaceae in the mid- to late Holocene as precipitation increased across the region (Brown et al., Reference Brown, Fitton, Schoups, Allen, Wahl and Hebda2006). However, there are a few records, mostly from eastern Vancouver Island in the rain shadow of the Vancouver Island Ranges (Brown and Hebda, Reference Brown and Hebda2003; Mazzucchi, Reference Mazzucchi2010; Lacourse and Davies, Reference Lacourse and Davies2015), where Cupressaceae pollen is infrequent throughout the Holocene. Thuja plicata is generally intolerant of dry conditions and is most abundant in wet coniferous forests, although it can also occur in drier P. menziesii forests, at least on moister sites. Allen et al. (Reference Allen, Brown and Hebda1999) found little Cupressaceae pollen in modern surface samples from P. menziesii forests, except at moist sites close to T. heterophylla–dominated forest. The low relative abundance of Cupressaceae in the Grant's Bog record suggests it was not abundant near the site. Rain shadow effects, including dry summers in particular, likely created conditions with insufficient moisture to support T. plicata.
Increasing precipitation and decreasing temperature in the mid- and late Holocene (Walker and Pellatt, Reference Walker and Pellatt2003; Brown et al., Reference Brown, Fitton, Schoups, Allen, Wahl and Hebda2006) facilitated the expansion of T. heterophylla near Grant's Bog and throughout much of the region. Mid-Holocene forests near Grant's Bog were composed primarily of P. menziesii and T. heterophylla, with P. aquilinum ferns continuing in the understory. By 5000 cal yr BP, the abundance of T. heterophylla and A. rubra increased further and P. menziesii and P. aquilinum decreased, suggesting an increase in forest density and relatively closed canopies in these T. heterophylla–P. menziesii forests. The uppermost pollen assemblages at Grant's Bog are characterized by an increase in A. rubra to 60−75%. A similar increase was found at Port McNeill Bog (Lacourse and Davies, Reference Lacourse and Davies2015), approximately 160 km to the northwest of Grant's Bog. Given this species’ tendency to colonize disturbed sites, it is likely that at least some portion of this increase is linked to increased human activity and logging in the region.
CONCLUSIONS
A complete terrestrialization sequence is recorded at Grant's Bog, starting with isolation of the basin from marine waters by 13,300 cal yr BP due to decreases in relative sea level. Transition from an oligotrophic lake to a shallow, marshy wetland with aquatic plants occurred by ~11,600 cal yr BP. This was followed by autogenic development of a poor fen by 9900 cal yr BP and then a drier ombrotrophic bog by 8700 cal yr BP. Changes in multiple paleoenvironmental proxies since the mid-Holocene point toward fluctuating edaphic and hydrological conditions and local plant community composition, including hydroseral reversion to a poor fen 3500–2300 cal yr BP following disturbance by fire.
Long-term mean CAR at peatlands on the northeast Pacific coast (Turunen and Turunen, Reference Turunen and Turunen2003; Jones and Yu, Reference Jones and Yu2010; Nichols et al., Reference Nichols, Peteet, Moy, Castañeda, McGeachy and Perez2014; Lacourse and Davies, Reference Lacourse and Davies2015; this study) are low compared with some continental peatlands (Yu et al., Reference Yu, Vitt and Wieder2014; Zhao et al., Reference Zhao, Tang, Yu, Li, Yang, Zhao, Li and Li2014), suggesting that seasonality plays an important role in constraining peatland C sequestration. Mild year-round temperatures on the coast lead to long growing seasons and enhanced primary productivity, but also to greater decomposition and lower net C storage compared with inland peatlands with more seasonal climates (Asada and Warner, Reference Asada and Warner2005). This pattern is also true on long time scales at many sites including Grant's Bog, where the highest CAR occurred during the early Holocene when summers were warmer and seasonality was maximized. C accumulation was high in the early Holocene but comparatively low in the cooler, less seasonal late Holocene, despite accumulation of peat that is similar in composition. Macroscale climate appears to be a more dominant control on long-term C accumulation in peatlands than local, site-specific factors such as peat composition or vegetation type. Further research comparing a large number of sites is needed to confirm this general pattern.
The pollen record from Grant's Bog indicates that open P. contorta–dominated communities were present by 14,000 cal yr BP. Pseudotsuga menziesii forests with abundant Pteridium ferns were established in the early Holocene and dominated coastal lowlands until ~7000 cal yr BP. Tsuga heterophylla and P. menziesii formed closed-canopy forests in the mid-Holocene. In contrast to most other pollen records from coastal BC, Cupressaceae (T. plicata) appears never to have been abundant in forests near Grant's Bog. Additional pollen records from eastern Vancouver Island should clarify the spatial extent of this pattern.
ACKNOWLEDGMENTS
We thank C. Grondahl, M. Davies, and M. Adeleye for help in the field and lab; J.A. Antos and R.J. Hebda for insightful discussions; and D.M. Peteet and M.E. Edwards for peer-review comments. This research was supported by research grants to TL from the Natural Sciences and Engineering Research Council of Canada (342003) and Canada Foundation for Innovation (17214). Pollen data are archived in the Neotoma Paleoecology Database.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2018.146