Alphalinolenic (ALA) and docosahexaenoic acids (DHA) are essential for the maintenance of normal health and nutrition. However, they cannot be synthesised by the body and must be supplied by the diet (Calder & Yaqoob, Reference Calder and Yaqoob2009). Intake of ALA and DHA is low in Western countries, therefore, increasing the average intake of n-3 FA is a public-health issue. To meet this goal, one strategy is to provide livestock diets supplemented with sources of n-3 polyunsaturated FA (PUFA) to enhance the n-3 FA content of meat, eggs, milk and milk products (Calder & Yaqoob, Reference Calder and Yaqoob2009).
Linseed, particularly rich in ALA [representing more than 50% of linseed total fatty acid (FA)], has been widely studied and is commonly used in monogastric animals and ruminants diets in different feed forms, i.e., whole seed, micronised, heated or extruded seed, and oil (Gonthier et al. Reference Gonthier, Mustafa, Ouellet, Chouinard, Berthiaume and Petit2005; Noblet et al. Reference Noblet, Jaguelin-Peyraud, Quemeneur and Chesneau2008). Linseed is composed of a tegument, rich in fibre, mucilage, tannins, and cyanogen compounds which surround the kernel which contains the nutritive reserves of linseed (Kadivar, Reference Kadivar2001). In monogastric animals, the tegument decreases linseed digestibility, leading to a poorer absorption of nutrients, including ALA (Noblet et al. Reference Noblet, Jaguelin-Peyraud, Quemeneur and Chesneau2008), while the whole linseed (tegument plus kernel) is digested and well used by ruminants when linseed is provided under 4% DMI with no impact on ALA transfer efficiency from feedstuff to milk (Gonthier et al. Reference Gonthier, Mustafa, Berthiaume, Petit, Martineau and Ouellet2004; Martin et al. Reference Martin, Rouel, Jouany, Doreau and Chilliard2008). In a context of increasing sustainability of feeding systems, providing monogastric animals and ruminants with linseed products adapted to their digestive systems is an important issue. By a process of sieving and sifting classically used in flour-milling industry, tegument and kernel of extruded linseed have been separated to be respectively directed to the production of feedstuff specific to ruminants and monogastric animals (Valorex, Combourtillé, France). We hypothesised that valorisation by dairy cows of sieved extruded linseed (SEL) would be as or more effective than standard extruded linseed.
However, linseed, does not contain DHA, the principal natural source of which is seafood (Calder & Yaqoob, Reference Calder and Yaqoob2009). Thus, ingredients such as microalgae have been experimentally tested in monogastric and ruminant diets (AbuGhazaleh et al. Reference AbuGhazaleh, Potu and Ibrahim2009; Stamey et al. Reference Stamey, Shepherd, de Veth and Corl2012; Baeza et al. Reference Baeza, Chartrin, Gigaud, Tauty, Meteau, Lessire and Berri2013; Bragaglio et al. Reference Bragaglio, Braghieri, Napolitano, De Rosa, Riviezzi, Surianello and Pacelli2015; De Tonnac et al. Reference De Tonnac, Karim-Luisset and Mourot2017). Even combinations between ALA and DHA sources (linseed oil and microalgae) have been studied to improve milk and meat FA profile (Angulo et al. Reference Angulo, Mahecha, Nuernberg, Nuernberg, Dannenberger, Olivera, Boutinaud, Leroux, Albrecht and Bernard2012; De Tonnac et al. Reference De Tonnac, Karim-Luisset and Mourot2017). In our experiment, DHA Gold®, obtained by drum-drying Schizochytrium algae, which contains a large amount of DHA has been used in dairy cow diets alone and in combination with SEL, specific for ruminants, to improve nutritional profile of milk. We hypothesised that the effects of ALA and DHA sources (SEL and DHA Gold®) on milk would be additive, particularly in relation to milk FA profile. Milk FA composition was measured to evaluate nutritional aspect of the milk. Milk fat globule (MFG) size and spontaneous lipolysis (SL) were also measured to evaluate suitability of milk to processing.
Material & methods
The protocol was approved by an ethics committee for animal experimentation under number 01421·02.
Animals
Thirty-two multiparous (n = 16) and primiparous (n = 16) Holstein dairy cows in mid-lactation were used. At the beginning of the experiment, the cows were at day 100 ± 17·5 of lactation. During the pre-experimental period, milk yield, milk-solids content, and milk monounsaturated (MUFA) and PUFA percentages were evaluated to allocate the cows into four groups. Criteria for blocking were, in order, milk yield, solids content, parity (primiparous, multiparous), lactation stage, DMI, and milk MUFA and PUFA percentages. Each group was composed of four primiparous and four multiparous cows. Mean values are presented in Supplementary Table S1. All cows were kept indoors with an average area of 6·56 m2 per cow. Cows were milked at 0700 and 1700 h in a milking parlour. The cows were weighed after milking.
Diet treatments
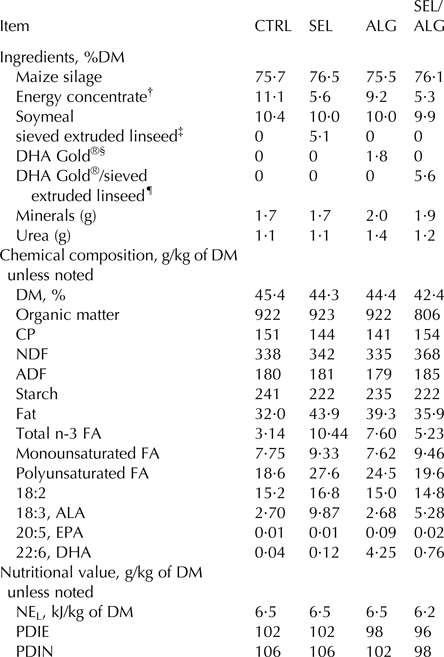
Four diet treatments were fed as total mixed rations. They were based on maize silage, a variable part of energy concentrate and n-3 FA sources, soybean meal, urea and vitamins. The new extruded linseed (EL) product was obtained by a process of linseed sieving and sifting that induced a separation between particles of different sizes. Fine particles represented the linseed kernel and the coarse particles represented the linseed teguments that were extruded. The first group received a control diet (CTRL) with no additional fat. The 3 other groups received sieved EL (SEL), microalgae DHA Gold® (ALG) and a mixture of microalgae DHA Gold® and SEL (SEL/ALG) (Table 1).
Table 1. Composition of experimental diets (CTRL: control, SEL: addition of sieved EL, ALG: addition of DHA Gold® and SEL/ALG: addition of sieved EL and DHA Gold® mixture)

FA, fatty acid; ALA, alpha linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; NEL, net energy for lactation; PDIE, protein digested in the small intestine supplied by rumen undegradable protein and by microbial protein from rumen-fermented OM (INRA, Reference Jarrige2007); PDIN, protein digested in the small intestine supplied by rumen undegradable protein and by microbial protein from rumen-degraded dietary nitrogen (INRA, Reference Jarrige2007).
† Energy concentrate on DM basis: 20% wheat, 20% corn, 20% barley, 20% beet pulp, 15% wheat bran, 3% cane molasses, 1% vegetal oil, 1% salts.
‡ sieved extruded linseed = 50% sieved extruded linseed, 50% wheat bran; Valorex, Combourtillé, France.
§ DHA Gold®; DSM, Deinze, Belgium.
¶ DHA Gold®/sieved extruded linseed = 37% sieved extruded linseed, 50% wheat bran, 13% DHA Gold®; Valorex, Combourtillé, France.
Diets were formulated to meet the energy and protein requirements, based on milk production and milk solid content measured during the pre-experimental period (Institut National de la Recherche Agronomique, Reference Jarrige2007). The ingredients, chemical composition and nutritional value of the diets are given in Supplementary Table S2 and in Table 1. The cows were fed ad libitum. The feeds were weighed and mechanically distributed twice daily at 0900 and 1830 h.
Experimental design
The experiment was conducted over a continuous period of 10 weeks. The experiment started with a covariate period of three pre-experimental weeks during which the cows were fed the CTRL diet, which was followed by one week of adaptation to the experimental diets and the six-week experimental period, from 13 January to 23 March 2014. Milk and blood were sampled during the pre-experimental period (covariate period) and during the sixth week of the experimental period.
Feed and refusals
Throughout the experimental period, cows were individually fed via individual electronic gating, and all refusals were collected and weighed every day to evaluate daily intake. To determine diet chemical and nutritional composition, samples of fresh maize silage were collected five times a week and samples of energy concentrate, soybean meal, sieved EL, DHA Gold®, DHA Gold®/sieved EL mixture were collected every week throughout the experimental period. The samples were stored at −20 °C and pooled to produce one sample per type of feed and per period. The analyses of the samples are described in Supplementary Material S1.
Milk and fat characteristics
Milk yield was recorded individually every day at each milking. Milk fat, protein and lactose content, and somatic-cell score were determined for four consecutive milkings every week. These analyses were performed by mid-IR spectrometry for fat, protein and lactose content and by flow cytometry for somatic-cell score at the dairy laboratory MyLab (Châteaugiron, France). Milk samples were collected individually from milk cans from one morning milking and one evening milking and pooled at a 60:40 ratio during the pre-experimental period and the last week of the experimental period. For milk FA profile, milk was stored at −20 °C until analysis. The FA composition was determined as described in Hurtaud et al. (Reference Hurtaud, Faucon, Couvreur and Peyraud2010).
Milk fat globule size was determined as described in Vanbergue et al. (Reference Vanbergue, Delaby, Peyraud, Colette, Gallard and Hurtaud2017). Spontaneous lipolysis was determined as in Vanbergue et al. (Reference Vanbergue, Peyraud, Guinard-Flament, Charton, Barbey, Lefebvre, Gallard and Hurtaud2016) from individual milk samples from milk cans collected during the last week of the experimental period during morning milkings.
Calculation and statistical analyses
All statistical analyses were performed using SAS software (SAS 9·2 Institute Inc., Cary, NC). The statistical significance threshold was set to P < 0·05, and the trend threshold was set to P < 0·10. The normality of the data was checked using the Shapiro-Wilk test in the SAS-package univariate procedure. The effects of the diets on milk yield, milk composition (except for SL and MFG size), weight, DMI and energy and protein supplies and balances, were analysed using the GLM procedure in SAS according to the following statistical model: Y i = μ + alimi + Cov Y i + εi, where Y i is the dependent variable, μ is the mean, alimi, is the effect of the i diet treatments (CTRL, SEL, ALG, SEL/ALG), Cov Y i is the covariable associated with Y i (i.e., the value of Y i during the pre-experimental period), and εi is the residual error. For MFG size, the covariable was the fat content during the pre-experimental period and for SL, there was no covariable. For each model, comparisons were performed with LSMEANS.
Results
Intake and nutrient supply and balance
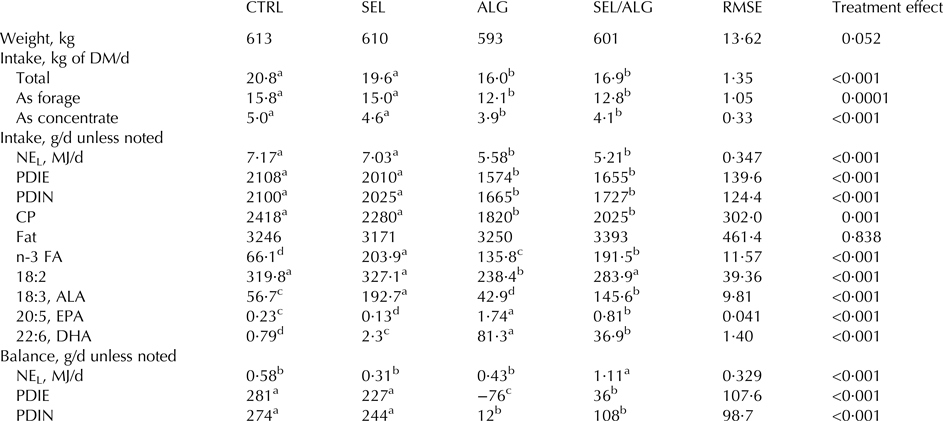
Total DMI decreased only in ALG and SEL/ALG. As a consequence, intake of net energy for lactation, PDIE (protein digested in the small intestine originating from rumen undegradable protein and by microbial protein from rumen-fermented OM; INRA, Reference Jarrige2007) and PDIN (protein digested in the small intestine originating from rumen undegradable protein and by microbial protein from rumen-degraded dietary nitrogen; INRA, Reference Jarrige2007) were lower in ALG and SEL/ALG (P < 0·001). However, net energy and metabolic protein balance stayed positive in SEL/ALG and net energy and PDIN balance stayed positive in ALG. Intake of ALA was higher in SEL and SEL/ALG and lower in ALG than in CTRL (P < 0·001). Intake of DHA was slightly higher in SEL and was higher in ALG and SEL/ALG than in CTRL (P < 0·001) (Table 2).
Table 2. Effects of addition of sieved EL (SEL), DHA Gold® (ALG) and sieved EL/DHA Gold® mixture (SEL/ALG) on weight, DM, energy and protein intake and balance of dairy cows

RMSE, Root mean square error; NEL, net energy for lactation; PDIE, protein digested in the small intestine supplied by rumen undegradable protein and by microbial protein from rumen-fermented OM (INRA, Reference Jarrige2007); PDIN, protein digested in the small intestine supplied by rumen undegradable protein and by microbial protein from rumen-degraded dietary nitrogen (INRA, Reference Jarrige2007); ALA, alpha linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
a–cMeans in the same row with no common superscript differ (P < 0·05).
Milk yield and milk protein and fat content
Milk yield, milk fat content, milk fat and protein yield were lower in ALG and SEL/ALG (P < 0·001). Milk yield was even lower in SEL/ALG. Milk protein content was higher in SEL/ALG compared to the other treatments (P < 0·001). Lactose content tended to be higher in SEL and ALG than in CTRL and SEL/ALG (P = 0·065). Somatic-cell score tended to be higher in ALG and SEL/ALG than in CTRL (P = 0·063) (Table 3).
Table 3. Effects of addition of sieved EL (SEL), DHA Gold® (ALG) and sieved EL/DHA Gold®mixture (SEL/ALG) on milk yield and milk composition in dairy cows

RMSE, Root mean square error.
† SCS: somatic cell score = log (SCC/1 000).
a–cMeans in the same row with no common superscript differ (P < 0·05).
Milk fatty acid profile
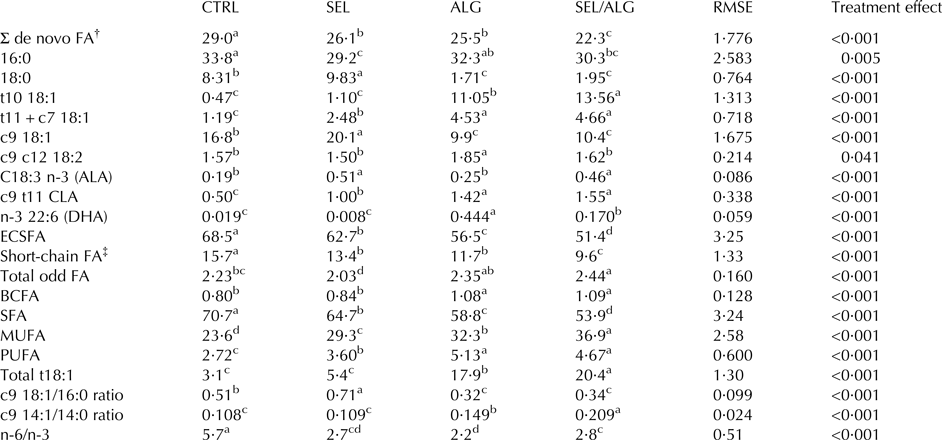
The FA profile was affected by the treatments, with the effects being much greater in ALG and SEL/ALG than in SEL. Compared with CTRL, saturated FA (SFA) percentage decreased in the order SEL, ALG, and SEL/ALG (P < 0·001), and de novo-synthesised FA percentage (4:0–15:0) was lower in SEL and lower still in ALG and SEL/ALG (P < 0·001). The percentage of 16:0 was lower in SEL and SEL/ALG (P = 0·005) but was not significantly different between ALG and CTRL. The percentage of 18:0 was higher in SEL than in CTRL, was much lower in ALG and SEL/ALG than in CTRL (P < 0·001), and did not differ between SEL/ALG and ALG. The percentages of MUFA and PUFA were higher in SEL than in CTRL, with greater differences in ALG and SEL/ALG (P < 0·001 for MUFA; P < 0·001 for PUFA) due to increase in trans-18:1 isomers. The percentage of MUFA was higher in SEL/ALG than in ALG (P < 0·001). Compared with CTRL, t10–18:1 percentage was not different in SEL but was much higher in ALG and SEL/ALG and higher in SEL/ALG than in ALG (P < 0·001). The percentage of t11 + c7-18:1 was higher in SEL than in CTRL, and much higher in ALG and SEL/ALG (P < 0·001), and did not differ between SEL/ALG and ALG. The percentage of c9–18:1 was higher in SEL than in CTRL, was lower in ALG and SEL/ALG (P < 0·001), and did not differ between SEL/ALG and ALG. The percentage of c9 t11 CLA was higher in SEL, was much higher in ALG and SEL/ALG (P < 0·001), and did not differ between SEL/ALG and ALG. The total trans-18:1 percentage was not significantly different between SEL and CTRL but was higher in ALG and much higher in SEL/ALG (P < 0·001). Total odd FA percentage was lower in SEL than in CTRL (P < 0·001), did not differ between CTRL and ALG, was higher in SEL/ALG than in CTRL (P < 0·001), and did not differ between SEL/ALG and ALG. The percentage of ALA was higher in SEL and SEL/ALG (P < 0·001) than in CTRL, was not significantly different between ALG and CTRL, and did not differ between SEL and SEL/ALG. The percentage of DHA was not significantly different between SEL and CTRL, but was higher in SEL/ALG and much higher in ALG (P < 0·001). The ratio of n-6/n-3 FA was lower in SEL, ALG and SEL/ALG (P < 0·001) than in CTRL, and was higher in SEL/ALG and SEL than in ALG (P < 0·001). The ratio of c9–14:1/14:0 was not significantly different between SEL and CTRL but was higher in ALG and was much higher in SEL/ALG than in CTRL (P < 0·001). Transfer efficiency of ALA was 2·8% for SEL and 1·4% for SEL/ALG (P < 0·001) and transfer efficiency of DHA was 2·7% for ALG and 2·1% for SEL/ALG (P = 0·015) (Table 4).
Table 4. Effects of sieved EL (SEL), DHA Gold® (ALG) and sieved EL/DHA Gold®mixture (SEL/ALG) on milk fatty acid composition in dairy cows. Results expressed as g/100 g total FA

RMSE, Root mean square error; FA, fatty acid; ALA, alpha linolenic acid; DHA, docosahexaenoic acid; ECSFA, sum of even-chain saturated fatty acids; BCFA, sum of branched-chain fatty acids.
† Σ de novo FA: from C4 to C15.
‡ Short-chain FA = FA < 14:0.
a–dMeans in the same row with no common superscript differ (P < 0·05).
Milk fat globule size
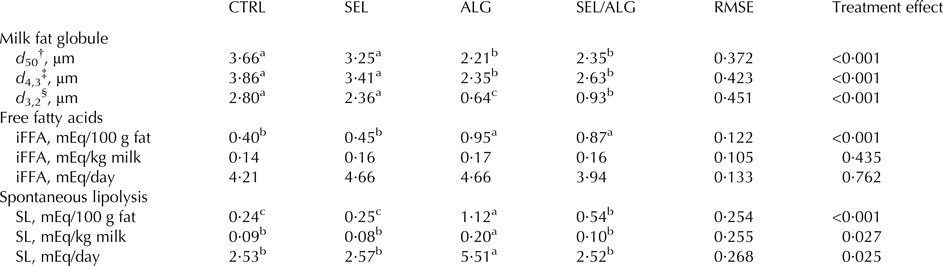
Compared to CTRL, MFG size described by median diameter d 50 and average diameters d 4,3 and d 3,2 was lower in ALG and SEL/ALG than in CTRL (P < 0·001). Average diameter d 3,2 was lower in ALG compared to SEL/ALG (P < 0·001) (Table 5).
Table 5. Effects of addition of sieved EL (SEL), DHA Gold® (ALG) and sieved EL/DHA Gold® mixture (SEL/ALG) on milk fat characteristics (milk fat globule size, milk spontaneous lipolysis) in dairy cows

RMSE, Root mean square error; iFFA, initial free fatty acids; SL, spontaneous lipolysis.
† d 50 = the median diameter of milk fat globule.
‡ d 4,3 = Σ(Ni × di4)/Σ(Ni × di3), volume- weighted average diameter, where Ni is the number of fat globules in a size class of diameter di.
§ d 3,2 = Σ(Ni × di3)/Σ(Ni × di2), volume-surface average diameter, where Ni is the number of fat globules in a size class of diameter di.
a–cMeans in the same row with no common superscript differ (P < 0·05).
Milk spontaneous lipolysis
Initial free FA and SL, expressed in mEq/100 g fat were higher in ALG and SEL/ALG (P < 0·001). Spontaneous lipolysis was higher in SEL/ALG compared to ALG (P < 0·001). Spontaneous lipolysis, expressed in mEq/kg of milk and in mEq/day, was higher in ALG than in CTRL (respectively, P = 0·027, and P = 0·025) (Table 5).
Discussion
Sieved extruded linseed had similar effects as standard extruded linseed
Sieved EL supplementation at 2·5% of DMI, regardless of the feed form, had no deleterious effect on milk yield, milk fat and protein content or yield. In a meta-analysis, Meignan et al. (Reference Meignan, Lechartier, Chesneau and Bareille2017) showed that standard EL supplementation increased milk yield, and decreased milk protein content regardless of diets and decreased fat content only with maize silage diets. However, in individual studies, the impacts of standard EL, at a same dose, on milk production and milk traits seem to be variable in the literature, depending on ruminal conditions (Hurtaud et al. Reference Hurtaud, Faucon, Couvreur and Peyraud2010) and ruminal metabolism of lipids (Chilliard et al. Reference Chilliard, Glasser, Ferlay, Bernard, Rouel and Doreau2007). Our results are consistent with those of Ferlay et al. (Reference Ferlay, Doreau, Martin and Chilliard2013), Neveu et al. (Reference Neveu, Baurhoo and Mustafa2014) and Livingstone et al. (Reference Livingstone, Humphries, Kirton, Kliem, Givens and Reynolds2015) with similar doses of standard EL in the diet.
As expected, SEL led to a decrease in SFA percentage and an increase in MUFA and PUFA percentages. The decrease in SFA was similar to that reported by Hurtaud et al. (Reference Hurtaud, Faucon, Couvreur and Peyraud2010) under similar condition with standard EL. The supplementation of SEL increased ALA as expected. The overall effect was an improved n-6/n-3 FA ratio in the milk. The enrichment in ALA and the transfer efficiency was consistent with Hurtaud et al. (Reference Hurtaud, Faucon, Couvreur and Peyraud2010) under similar conditions with standard EL (2·8% in the current study vs. 2·2 and 3·5%).
The supplementation of SEL had no impact on MFG size and SL. Hurtaud et al. (Reference Hurtaud, Faucon, Couvreur and Peyraud2010) found no impact of standard EL at 2·1% of DM on MFG size but higher level of SL. Knowing that SL is variable with milking time (Vanbergue et al. Reference Vanbergue, Delaby, Peyraud, Colette, Gallard and Hurtaud2017), this difference is possibly due to the fact that in the current study, samples were collected during morning milkings, whereas in Hurtaud et al. (Reference Hurtaud, Faucon, Couvreur and Peyraud2010), samples were collected during the morning and evening milkings and pooled in a 60:40 ratio.
Sieving and sifting EL is as efficient as standard EL and can be used in ruminants’ diets formulation and thus increase their sustainability, although the transfer rate from diet to milk is still low.
Microalgae DHA Gold® increased milk DHA content but induced milk fat depression
ALG and SEL/ALG led to a sharp decrease in milk yield due to a decrease in DMI and to a drastic drop in fat content and fat and protein yields. Our results are consistent with those of Boeckaert et al. (Reference Boeckaert, Vlaeminck, Dijkstra, Issa-Zacharia, Van Nespen, Van Straalen and Fievez2008), AbuGhazaleh et al. (Reference AbuGhazaleh, Potu and Ibrahim2009) and Angulo et al. (Reference Angulo, Mahecha, Nuernberg, Nuernberg, Dannenberger, Olivera, Boutinaud, Leroux, Albrecht and Bernard2012). These effects are similar to the effects of fish oils (Chilliard et al. Reference Chilliard, Glasser, Ferlay, Bernard, Rouel and Doreau2007). Indeed, milk t10–18:1 sharply increased (+10·6%) indicating a change in ruminal fermentation and the production of fat synthesis inhibitors. The observed decrease in protein yield in ALG and SEL/ALG might be explained by the reduction in energy and protein intake.
As expected, ALG and SEL/ALG treatments led to a decrease in SFA percentage and an increase in MUFA and PUFA percentages. These changes were very significant mainly due to the significant decrease of 18:0. The decrease in SFA is consistent with results of Boeckaert et al. (Reference Boeckaert, Vlaeminck, Dijkstra, Issa-Zacharia, Van Nespen, Van Straalen and Fievez2008) and Angulo et al. (Reference Angulo, Mahecha, Nuernberg, Nuernberg, Dannenberger, Olivera, Boutinaud, Leroux, Albrecht and Bernard2012) (except for 18:0). Fast rate of FA release into the rumen from microalgae DHA Gold® would explain higher production of trans FA, to the detriment of 18:0 production in the rumen, leading to greater inhibition of de novo mammary lipogenesis (Chilliard et al. Reference Chilliard, Martin, Rouel and Doreau2009; Ferlay et al. Reference Ferlay, Doreau, Martin and Chilliard2013). The short FA percentage was lower with the ALG treatment than with CTRL (AbuGhazaleh et al. Reference AbuGhazaleh, Potu and Ibrahim2009). DHA percentage was increased by 23-fold in ALG and by 9-fold in SEL/ALG. Recovery of DHA is consistent with Boeckaert et al. (Reference Boeckaert, Vlaeminck, Dijkstra, Issa-Zacharia, Van Nespen, Van Straalen and Fievez2008) (3·1% of recovery). With protected microalgae, Stamey et al. (Reference Stamey, Shepherd, de Veth and Corl2012) reported 3·4% of transfer efficiency and Bragaglio et al. (Reference Bragaglio, Braghieri, Napolitano, De Rosa, Riviezzi, Surianello and Pacelli2015) did not detect significant change in FA profile.
ALG and SEL/ALG induced a strong decrease in MFG size. Briard-Bion et al. (Reference Briard-Bion, Juaneda, Richoux, Guichard and Lopez2008) found that t10–18:1 was negatively correlated with MFG size (R 2 = 0·87) and related to reduced milk fat. Smaller MFG have previously been associated with a decrease in fat content (Hurtaud et al. Reference Hurtaud, Faucon, Couvreur and Peyraud2010). The decrease in milk fat content would induce synthesis of smaller MFG, as also reported by Couvreur & Hurtaud (Reference Couvreur and Hurtaud2017). ALG and SEL/ALG also increased initial FFA and SL, expressed in mEq/100 g of fat, compared to CTRL. We showed that SL increased sharply beyond a certain threshold of microalgae DHA Gold® supplementation because SL (in mEq/kg of milk) did not differ between SEL/ALG and CTRL. According to Cartier & Chilliard (Reference Cartier and Chilliard1990), MFG membrane integrity is an important factor determining SL susceptibility. Both ALG and SEL/ALG were associated with a drastic reduction in MFG size, although only ALG was associated with an increase in SL (in mEq/kg of milk). The long-chain FA profile of the MFG membrane could have differed between ALG and SEL/ALG due to differences in the FA profile in the diet. These differences could lead to differences in MFG membrane integrity.
Sieved extruded linseed and microalgae combination had a stronger impact on milk fatty acid profile and milk fat characteristics
Although the dose of DHA Gold® in SEL/ALG was half the dose used in ALG (156 vs. 340 g/d), the SEL/ALG treatment led to a larger decrease in milk yield and fat yield (although non-significant for fat yield) compared to ALG. The percentage of t10–18:1 was effectively higher, which would explain the more dramatic down-regulation of milk fat synthesis (Shingfield et al. Reference Shingfield, Bernard, Leroux and Chilliard2010). The short and SFA percentages were lower and the MUFA percentages were higher with SEL/ALG than with ALG. This could be explained by the increased in trans-18:1 as previously discussed, and by the increase in the 14:1/14:0 ratio that could reflect an increase of Δ9 desaturase activity. Based on the literature available about non-protected DHA Gold® used at different doses (Boeckaert et al. Reference Boeckaert, Vlaeminck, Dijkstra, Issa-Zacharia, Van Nespen, Van Straalen and Fievez2008; AbuGhazaleh et al. Reference AbuGhazaleh, Potu and Ibrahim2009; Angulo et al. Reference Angulo, Mahecha, Nuernberg, Nuernberg, Dannenberger, Olivera, Boutinaud, Leroux, Albrecht and Bernard2012), we assumed that the relation between the dose of DHA Gold® and milk fat depression was non-linear. Boeckaert et al. (Reference Boeckaert, Vlaeminck, Dijkstra, Issa-Zacharia, Van Nespen, Van Straalen and Fievez2008) noted a fat depression of 520 and 750 g/d respectively for 382 and 195 g/d of DHA Gold® in the same conditions. The higher milk fat depression in SEL/ALG could be explained by the higher n-3 FA intake (+55·7 g/d) compared to ALG.
The d 3,2 was lower for SEL/ALG than for ALG. This could be explained by the difference in the long chain FA profile as also observed by Lu et al. (Reference Lu, Argov-Argaman, Anggrek, Boeren, van Hooijdonk, Vervoort and Hettinga2016). Indeed, DHA and EPA were respectively 2·6 and 1·9 lower in SEL/ALG compared to ALG. Spontaneous lipolysis was also lower in SEL/ALG compared to ALG. Milk fat globule structure in relation to FA profile could explain the observed difference (Vanbergue, Reference Vanbergue2017).
Conclusion
Sieved extruded linseed had a positive impact on the milk FA profile, despite the low transfer efficiency of beneficial FA from the diet to the milk. Sieved extruded linseed supplementation at a dose of 2·5% of DMI had no deleterious effect on milk mineral and protein composition, and fat characteristics. So, it could replace standard EL to increase the sustainability of dairy cows’ diets providing adapted linseed feedstuff to ruminants. Microalgae supplementation (340 and 156 g/d) had deleterious effects on milk composition, milk FA, and fat characteristics, and the effect was even more deleterious when microalgae were mixed with sieved EL. Lipid supplementation of the dairy cow diet can increase levels of valuable FA in milk if protected from rumen biohydrogenation. Protected microalgae and the doses of microalgae in the diet should be further investigated to prevent FA modification in the rumen and the consequent deleterious effects on milk fat.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029918000390
Mathieu Guillevic (Valorex, Combourtillé, France) and Jacques Mourot (PEGASE, INRA, Agrocampus Ouest, 35590, Saint-Gilles, France) are to be thanked for their valuable scientific collaboration. The authors also thank all the staff of the experimental farm of Méjusseaume (INRA, F-35650 Le Rheu, France): G. Boulet, A. Cozien, J.B. Eon, A. Eveno, M. Fargetton, M. Guilloux, J. Lassalas, A. Mottin, J. Orinel, J. Parois, F. Pichot, P. Pichot, D. Sidaner, M. Texier, and G. Théaud for herd management and for their assistance with milk sampling. We are grateful to P. Debournoux, N. Huchet, Y. Jaguelin-Peyraud, C. Perrier, S. Philau, and T. Le Mouël, for their technical assistance. Thanks are also due to the Joint Technical Unit of Research and Engineering on dairy farming (UMT RIEL, Rennes, France) for the constructive exchanges. The authors also thank Jane Williams and American Journal Experts for their fine work correcting the English in the manuscript.
This research received financial support from the ANR ALID 12-0003-01 AGRALID programme.