Introduction
The manner in which organisms allocate resources to reproduction is a central concept within life history theory (Stearns, Reference Stearns1989; Sinervo, Reference Sinervo1990; Wilkinson & Gibbons, Reference Wilkinson and Gibbons2005). Smith & Fretwell (Reference Smith and Fretwell1974) pointed out that females face a trade-off between investment of resources in propagule size versus propagule number; they may produce either a few large offspring or many smaller ones. Clearly, individual offspring benefit whenever egg size is favoured at the expense of clutch size, but this is not always in the best interest of the parents (Stearns, Reference Stearns1992) and the actual size and number of eggs is shaped by selection acting on maternal fitness (Roff, Reference Roff1992). Consequently, egg size tends to appear relatively fixed within populations, whereas clutch size tends to vary more readily in response to environmental conditions (insects: Stewart et al., Reference Stewart, Dixon, Ruzicka and Iperti1991a,Reference Stewart, Hemptinne and Dixonb; Dixon & Guo, Reference Dixon and Guo1993; spiders: Marshall & Gittleman, Reference Marshall and Gittleman1994; fish: Einum & Fleming, Reference Einum and Fleming2000). It is often true that larger females lay more eggs than smaller ones, especially in highly fecund species such as insects (e.g. Kajita & Evans, Reference Kajita and Evans2010). Honěk (Reference Honěk1993) analyzed data on 68 insect species from ten insect orders and found that fecundity scaled with female body size in a linear manner with a slope slightly less than one. In holometabolous insects, adults may vary in body size either because of genetic differences or as a result of differential access to food during larval development. Whereas female body size determines, to some extent, the ‘capital’ available for reproduction, temporal patterns of reproductive allocation reflect a series of tradeoffs between current versus future reproductive effort over the adult lifetime (Williams, Reference Williams1966). It is known that both egg size and egg number may vary as functions of maternal body size and age (Fox & Czesak, Reference Fox and Czesak2000), but no empirical studies, to our knowledge, have yet addressed how body size and age may interact to influence maternal reproductive allocation.
The idea that species tend to evolve towards a fixed (optimal) propagule size has been challenged by observations of taxa where offspring size may vary as much as clutch size (Roff, Reference Roff1992; Stearns, Reference Stearns1992). Similarly, Bernardo (Reference Bernardo1996) argued against the use of optimality theory in analyzing the evolution of propagule size and emphasized the role of maternal effects and dynamic maternal ecologies in accounting for within-species variation. Among the most pronounced maternal effects are those deriving from a mother's size and age, although other factors such as temperature (Fischer et al., Reference Fischer, Bot, Zwaan and Brakefield2004) and maternal population density can have subtle effects (Heisswolf et al., Reference Heisswolf, Klemola, Andersson and Ruohomaki2009). Egg size plasticity can yield benefits when reproductive organisms face either spatial or temporal variation in environmental conditions over time frames shorter than their adult lifespan (amphibians: Crump, Reference Crump1981; fish: Hutchings, Reference Hutchings1991; insects: Fox & Mousseau, Reference Fox, Mousseau, Mousseau and Fox1998). Under such conditions, parental fitness is maximised by producing a range of offspring sizes, either within clutches or among them (Kaplan & Cooper, Reference Kaplan and Cooper1984).
In general, females will benefit by increasing fecundity at the expense of egg size when environmental conditions are favourable for offspring development, whereas individual progeny typically require a larger allocation of parental resources in order to survive poor conditions, constraining the number that can be produced (Parichy & Kaplan, Reference Parichy and Kaplan1992; Fox & Mousseau, Reference Fox, Mousseau, Mousseau and Fox1998). Consequently, increased egg size has been proposed as an adaptation for coping with environmental stress. For example, in the brook trout, Salvelinus fontinalis (Salmoniformes: Salmonidae), food restriction results in the production of fewer, larger eggs that give rise to juveniles with higher survival rates (Hutchings, Reference Hutchings1991). Females of the seed beetle, Stator limbatus (Coleoptera: Chrysomelidae), lay many small eggs in the seeds of high-quality host plants and fewer, larger eggs in those of poor quality hosts, thus providing their offspring with more initial resources when they must develop on nutritionally inferior food (Fox & Mousseau, Reference Fox, Mousseau, Mousseau and Fox1998). In aquaculture populations of chinook salmon, Oncorhynchus tshawytscha (Salmoniformes: Salmonidae), there has been rapid, unintentional selection for smaller egg size, ostensibly because of enhanced juvenile survival under the benign conditions of captivity (Heath et al., Reference Heath, Heath, Bryden, Johnson and Fox2003).
There have also been many studies of changes in propagule size as a function of female age. In species with indeterminate growth, or females that continue to grow after the onset of reproduction, older mothers may be larger, possess more resources, or be more experienced in parental care, enabling them to produce offspring that are larger, of higher quality, or both, as in the painted turtle Chrysemys picta (Testudines: Emydidae) (Paitz et al., Reference Paitz, Harms, Bowden and Janzen2007). For organisms reproducing within well-defined patches of habitat, resource competition for progeny may increase predictably over time, favouring increased provisioning of eggs and reduced numbers with advancing female age, as observed in the soil mite, Sancassania berlesei (Acari: Acaridae) (Benton et al., Reference Benton, Plaistow, Beckerman, Lapsley and Littlejohns2005; Plaistow et al., Reference Plaistow, St. Clair, Grant and Benton2007). In insects, the more common pattern is for egg size to decline with female age (see Fox & Czesak, Reference Fox and Czesak2000 for a review) and most examples to the contrary occur in insect orders that lack complete metamorphosis (e.g. McLain & Mallard, Reference McLain and Mallard1991; Landa, Reference Landa1992). Reductions in egg size due to deteriorating maternal physiology may not be evident until near the end of life, but earlier declines may occur if key maternal resources become limiting (Fox & Czesak, Reference Fox and Czesak2000).
Many species of lady beetles (Coccinellidae) specialise in feeding on aphid outbreaks that present an abundance of food for a very brief period; plant suitability for aphids is typically fleeting; crowding of nymphs and/or declining host quality results in the development of alatae that disperse to colonize alternative hosts. Successful exploitation of an aphid outbreak requires that eggs be laid during the ‘oviposition window’, a brief period that coincides with the exponential growth phase of the aphid population (Kindlmann & Dixon, Reference Kindlmann and Dixon1993). This timing permits eclosing larvae to grow in parallel with aphid colonies – too early and progeny may eliminate their food supply; too late and the aphid colony may crash before larval development is complete. If conditions for progeny deteriorate over the oviposition period, and coccinellid larvae hatching from large eggs have generally better survival under conditions of food limitation (Ng, Reference Ng, Niemczyk and Dixon1988) and/or faster development (Stewart et al., Reference Stewart, Dixon, Ruzicka and Iperti1991a), an adaptive maternal strategy would be to increase egg size over the reproductive period, possibly at the expense of egg number. It has been proposed that coccinellid egg size remains near a minimum determined by the size neonate larvae require to capture their first prey item (Stewart et al., Reference Stewart, Dixon, Ruzicka and Iperti1991a; Dixon, Reference Dixon2000). However, unexplained variation in egg size among coccinellid females has been observed (e.g. Dixon & Guo, Reference Dixon and Guo1993; Honěk et al., Reference Honěk, Dixon and Martinkov2008b; Kajita & Evans, Reference Honěk, Dixon and Martinkov2010).
The objective of this study was to explore the relationships between female body size, age-specific reproductive effort, and the dynamics of egg size and daily fecundity over reproductive life in a coccinellid beetle, Coleomegilla maculata (DeGeer). We produced C. maculata adults of different sizes by varying larval access to food. Females were mated, fed ad libitum as adults, and their reproductive activity monitored daily to determine effects of female body size on egg size, daily fecundity and the dynamics of these reproductive parameters as a function of female age. We hypothesized that smaller females with more limited resources should produce both fewer and smaller eggs and that reproductive effort should decline with female age. Our expectation was that general patterns of reproductive allocation should be adaptive in the ecological context of aphidophagy in which later offspring are predicted to face more severe conditions than those produced earlier.
Materials and methods
Insect colony
A colony of C. maculata was established from adult beetles collected from sorghum plants in Hays, KS, USA, in July, 2009. Insects were held in a growth chamber with L16:D8 day length at a temperature of 24±1°C. Adult females were isolated in plastic Petri dishes (5.5 cm diameter) and were fed a diet of frozen eggs of the flour moth, E. kuehniella, with water provided on a small cube of sponge, both refreshed every day. Eggs were collected daily from the inner surfaces of the Petri dishes by transferring the beetles to new dishes. Larvae of the first laboratory generation were reared on frozen eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) and offspring of the resulting adults were used in the experiment.
Experimental design
The experiment was conducted under the same physical conditions used for rearing the beetle colony. Neonate larvae (n=180) were isolated in Petri dishes upon eclosion and then assigned to one of three different treatments, representing three different levels of food availability, for rearing through to the adult stage. Eggs of E. kuehniella support normal development and successful reproduction in C. maculata (Michaud & Jyoti, Reference Michaud and Jyoti2008) and thus qualify as 'essential' (Hodek, Reference Hodek, Hodek and Honek1996) or 'adequate' (Michaud, Reference Michaud2005) food. Larvae were provided access to frozen eggs of E. kuehniella for various periods every day: (i) 30 min per day (90 larvae), (ii) 6 h per day (45 larvae) and (iii) ad libitum (45 larvae). More larvae were assigned to the 30 min regime to compensate for the relatively high mortality observed in that treatment in preliminary observations.
All insects were observed daily through the experiment and development time was tallied as the number of days from eclosion to emergence of the adult. Upon emergence, adults were weighed on an analytical balance and then isolated in a Petri dish with frozen eggs of E. kuehniella provided ad libitum and water on a sponge cube, both refreshed daily. When adults were seven days old, mating pairs were established and held together for a period of one week, whereupon males were removed and females were held in isolation for the remainder of their lives. This was necessary because preliminary observations indicated that female oviposition schedules could be disrupted by male harassment during extended periods of confinement and eggs were sometimes cannibalized by males before they could be harvested. Consequently, it is likely females became progressively sperm-limited, causing egg fertility to gradually decline in a uniform manner in all treatments from initial levels around 80%. This impeded accurate measurements of egg size data beyond 20 oviposition days because infertile eggs often collapsed before they could be measured, but egg size appeared to become asymptotic in all treatments at this point. The pre-reproductive period of each female was calculated as the number of days from adult emergence until the onset of oviposition. Observations were made daily on the number of eggs laid (daily fecundity), the linear dimensions of the eggs and the fraction that subsequently hatched. Using a stage micrometer under a stereomicroscope at 50× magnification, we measured the length and width of each egg to the nearest 0.02 mm in a sample of eggs from each daily oviposition (n=5 eggs per female per oviposition day). We assumed that eggs are ellipsoidal in shape (Takakura, Reference Takakura2004) and used these measurements to estimate egg volume using the formula:
During preliminary work, we measured a series of eggs of varying sizes (n=115) and then weighed each on a microbalance. We then used linear regression to establish the relationship between egg volume and mass, which was described by the following equation:
Since the weighing of individual eggs was very time-consuming (due to the high sensitivity of the microbalance), we henceforth measured eggs, calculated volumes and converted values to egg masses using this equation, since the latter were required to estimate females' reproductive effort as a proportion of their body mass or reproductive effort (RE).
Statistical analysis
Treatments were compared by one-way ANOVA using PROC GLM (SAS Institute, 2008) and means separated by Tukey's HSD test. Mortality rates were analyzed by Chi Square. Linear regression was used to test the relationship between reproductive days and daily fecundity, and slopes were compared with a test for equality of slopes using PROC REG followed by PROC GLM (SAS Institute, 2008). Changes in dependent variables with female age were analyzed using PROC MIXED for repeated measures where subject effects were considered random and the response variable was calculated as a sum of terms for overall mean, treatment effect, subject effect, time effect, treatment×time interaction and random error. Sphericity was tested using PROC GLM with the response equal to the sum of terms for overall mean, treatment effect, time effect, treatment×time interaction and random error (SAS Institute, 2008).
Results
The three larval feeding regimes (30 min, 6 h and ad libitum access daily) yielded females of three different body sizes (small, medium and large, respectively) and three different developmental periods (long, medium and short, respectively; table 1). Mortality of larvae was 36% in the 30 min feeding regime, significantly more (χ2=55.43, P<0.001) than the 11% observed in the 6 h treatment, which was not significantly different (χ2=0.24, P=0.620) from the 9% observed in the ad libitum treatment.
All females that emerged as adults became reproductive. Larger females trended toward earlier onset of oviposition than smaller females, but the pre-reproductive period did not differ significantly among treatments because of high within-group variance. There was no effect of treatment on egg mass when female lifetime means were compared, nor was linear regression of egg mass on female mass significant when all females were pooled (F 1,56=1.79, P=0.186). However, small females produced fewer total eggs than did medium and large females; although they oviposited on a similar number of days, their average daily fecundity was lower. There were no differences among treatments in the percentage of eggs hatching, nor was there any indication that fertility might vary with treatment during any particular period of reproductive life. Although medium females tended to live the longest and have the greatest proportional reproductive effort, there were no significant differences among treatments in female longevity or in lifetime reproductive effort expressed as a multiple of female fresh mass at emergence (table 1).
Table 1. Life history parameters (mean±SE) of C. maculata females reared under three larval feeding regimes. As adults, each female was fed E. kuehniella eggs ad libitum and provided access to one male for the first week of adult life.

* Total egg mass as a proportion of initial female fresh weight=(fecundity×egg wt)/adult weight. Values bearing different letters were significantly different with rows (Tukey's HSD, α=0.05).
Changes in egg mass were evident as a function of female age in all treatments; egg mass increased from the first to the 20th day of oviposition by 14%, 8% and 13% for small, medium and large females, respectively. The ANOVA for repeated measures of egg mass across oviposition day revealed no significant effect of treatment (F 2,54=2.41, P=0.099); females in all treatments tended to increase egg mass in the course of the first 20 oviposition days (F 9,449=32.92, P<0.001). However, there was a significant interaction between treatment and oviposition day (F 18,449=3.43, P<0.001; fig. 1). Longitudinal comparisons of means were not justified because analysis of orthogonal components indicated non-homogeneity of variance (χ244,128=109.74, P<0.001), rendering pair-wise comparisons dependent on sphericity inappropriate. Therefore, we used linear regression to analyze changes in egg mass within treatments over the first 20 oviposition days and obtained a significant regression in all cases (small: F 1,18=101.63, P<0.001, r2=0.85; medium: F 1,18=79.85, P<0.001, r2=0.82; large: F 1,18=65.30, P<0.001, r2=0.78). A test for equality of slopes revealed treatment effects (F 2,59=3.57, P=0.035). Egg mass increased more rapidly for small females than for medium females (F 1,39=8.16, P=0.007) with large females intermediate and not significantly different from either (F 1,39=1.17, P=0.286 and F 1,39=2.35, P=0.133, respectively).

Fig. 1. Changes in fresh mass of C. maculata eggs over the first 20 oviposition days laid by females of three different body sizes reared with three different periods of daily access to eggs of E. kuehniella: small (30 min, n=23, hatched line) medium (6 h, n=15, thin solid line) and large (ad libitum, n=19, thick solid line). Each point is the mean of n×5 eggs. The slopes of the 30 min and 6 h treatments were significantly different from one another (α<0.05), with that of ad libitum females not significantly different from either (α>0.05) (........, ○ Small, y=0.001x+0.1597; ——, ▵ Medium, y=0.0007x+0.1668; ——, □ Large, y=0.0009x+0.1625).
Linear regressions of daily fecundity versus oviposition day were significant (small females: F 1,58=73.26, P<0.001, r2=0.56; medium females: F 1,58=82.70, P<0.001, r2=0.59; large females: F 1,58=5.60, P<0.021, r2=0.08). Larval feeding treatment had a significant effect on the change in daily fecundity over time (test for equality of slopes: F 2,179=39.78, P<0.001; fig. 2). The mean daily fecundity of large females decreased over time, yielding a regression slope that was significantly different from that of medium (F 1,119=59.70, P<0.001) and small (F 1,119=42.94, P<0.001) females that both increased daily fecundity over time. Furthermore, the mean daily fecundity of medium females increased over time at a higher rate than that of small females (F 1,119=5.33, P=0.022).

Fig. 2. Changes in mean daily fecundity over the first 60 oviposition days for C. maculata females of three different body sizes reared with three different periods of daily access to eggs of E. kuehniella: small (30 min, n=23, hatched line) medium (6 h, n=15, thin solid line) and large (ad libitum, n=19, thick solid line). The slopes of all three regression lines were significantly different from each other in a test for equality of slopes (α<0.05) (........, Small, y=0.0685x+10.433, P<0.0001; ——, Medium, y=0.0998x+11.589, P<0.0001; ——, Large, y=−0.0302x+15.973, P<0.05).
When fecundity was considered in increments of ten oviposition days, there were significant effects of female body size on fecundity (1–10: F 2,56=10.45, P<0.001; 11–20: F 2,56=4.29, P=0.018; 21–30: F 2,54=3.27, P=0.045; 31–49: F 2,50=5.63, P=0.006). Medium females laid more eggs than small females from oviposition days 31–40 (Tukey's test, α=0.05). However, large females laid more eggs than medium females during the first ten oviposition days (Tukey's test, α=0.05), with no differences significant thereafter, and more eggs than small females during the first 40 oviposition days, with no differences significant thereafter. All females demonstrated a marked decline in fecundity after 80 oviposition days.
Lifetime proportional RE, calculated as total egg mass divided by female fresh mass at emergence, was highly variable and ranged from 2.66 to 28.78 multiples of female body mass. When RE was partitioned into increments of ten oviposition days, ANOVA for repeated measures revealed that all females tended to increase their RE over time (F 2,102=14.27, P<0.001), but the magnitude of the increase varied among treatments (F 2,54=3.80, P=0.028) and without significant interaction between treatment and interval (F 4,102=1.59, P=0.183). Changes in RE over the three oviposition periods did not violate the assumption of sphericity (χ22,49=2.94, P=0.22), which permitted longitudinal comparisons to reveal that both small and medium females increased their RE during this period, while large females did not (fig. 3).

Fig. 3. Changes in reproductive effort (total egg mass expressed as a multiple of female body mass at emergence) in ten-day intervals over the first 30 oviposition days for C. maculata females of three different body sizes reared with three different periods of daily access to eggs of E. kuehniella: small (30 min, n=23, open columns) medium (6 h, n=15, hatched columns) and large (ad libitum, n=19, solid columns). Values bearing the same letters were not significantly different among clutch intervals within treatments (□, Small; ![]() , Medium; ▪, Large).
, Medium; ▪, Large).
There were also treatment effects on the time frame of daily reproductive bouts (table 2). Large females produced their first 20 clutches in a significantly shorter period than did medium females, with small females intermediate and not different from either, although after 30 oviposition days, no differences among treatments remained significant.
Table 2. Mean (±SE) periods of adult life (days) required to obtain 10, 20 and 30 oviposition days from C. maculata females of three different body sizes generated by three different daily larval feeding regimes (30 min, 6 h, and ad libitum access to eggs of E. kuehniella).
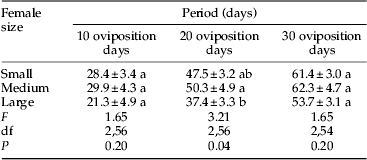
Mean values bearing different letters were significantly different within columns (Tukey's HSD, α=0.05).
Discussion
The largest effect of maternal body size was on daily fecundity, reflecting a consistent trend across iteroparous animals in general (Roff, Reference Roff1992), and insects in particular (Honěk, Reference Honěk1993). Similarly, Santos-Cividanes et al. (Reference Santos-Cividanes, dos Anjos, Cividanesm and Dias2011) observed diminishing female fecundity with increasing larval food deprivation when rearing C. maculata on the same food source. Although the smallest females, obtained by severely restricting larval access to food, did not produce smaller eggs than larger females, as hypothesized, they did produce fewer of them. Specifically, large females adopted a ‘front-loaded’ reproductive strategy and produced their largest clutches early in adult life when they are expected to contribute most to maternal fitness, their daily fecundity gradually decreasing over time (fig. 2, table 2). In contrast, small and medium-sized females obtained from 30 min and 6 h feeding regimes, respectively, gradually increased their daily fecundity over time, possibly because they required a period of adult feeding to compensate for larval food deprivation. Daily fecundity declined in small and medium females only near the end of reproductive life (data not shown). Note that small females fed for more than a week longer, on average, than did medium females before initiating oviposition, although treatment means were not significantly different due to the large variation among females.
The egg size of all females increased over the course of the first few weeks of oviposition and converged on a similar mass around the 20th oviposition day. The slope of the increase varied among treatments (fig. 1); small females laid the smallest eggs initially but increased egg size more rapidly than larger females. Since the fecundity of small and medium females also increased during this period (fig. 2), there was no indication of an egg size-number tradeoff in temporal trajectories of reproductive effort. Furthermore, there was no significant correlation between maternal body size and mean egg mass when all females were pooled, suggesting that egg mass was more affected by maternal age than maternal body size.
Although eggs of E. kuehniella may be considered a factitious diet in comparison to aphids, C. maculata is a relatively polyphagous species that is known to prey on eggs of Lepidoptera (e.g. Musser & Shelton, Reference Musser and Shelton2003). Furthermore, eggs of E. kuehniella may be more nutritious than aphids for coccinellids (Specty et al., Reference Specty, Febvay, Grenier, Delobel, Piotte, Pageaux, Ferran and Guillard2003), and studies that have compared E. kuehniella eggs to natural prey such as greenbug, Schizaphis graminum (Rondani), have found the former to be a superior diet for C. maculata (Michaud & Jyoti, Reference Michaud and Jyoti2008; da Silva et al., Reference da Silva, Cruz, Figueiredo and Tavares2010). Nevertheless, natural populations most often develop as larvae in highly ephemeral patches of aphid prey (e.g. Wright & Laing, Reference Wright and Laing1980). Within genetically determined limits, egg size is a maternal effect (Bernardo, Reference Bernardo1996), and the consequences of variation in egg size are typically most pronounced in adverse environments (Fox & Mousseau, Reference Fox, Mousseau, Mousseau and Fox1998). Aphidophagous habits have been theorized to exert very strong selection on the placement of eggs and the size, number and timing of their production (Honěk et al., Reference Honěk, Dixon and Martinkov2008a; Kindlmann & Dixon, Reference Kindlmann, Dixon, Kindlmann, Dixon and Michaud2010). The food supply for developing aphid predators follows a predictable, sigmoidal trajectory ultimately terminating in the dispersal of alate aphids and intense competition among later-developing larvae for the remaining prey (Kindlmann & Dixon, Reference Kindlmann and Dixon1993). A gradual increase in egg size after onset of oviposition would seem an adaptive maternal strategy because progeny survival will increase as a function of their size at hatching under the stress of late-cycle conditions, as will their rate of development (Stewart et al., Reference Stewart, Dixon, Ruzicka and Iperti1991a). Larger neonates will be better equipped to pursue increasingly scarce prey (Sloggett & Lorenz, Reference Sloggett and Lorenz2008) and will have lower susceptibility to cannibalism and intraguild predation, mortality risks that likely escalate as aphid outbreaks enter the decline phase (Michaud, Reference Michaud, Hodek, Emden and Honěk2012). It has been argued that coccinellid egg size is held close to a minimum determined by the ability of newly-eclosed larvae to capture their first prey item (Dixon, Reference Dixon1958; Stewart et al., Reference Stewart, Dixon, Ruzicka and Iperti1991a; Dixon & Guo, Reference Dixon and Guo1993). However, Dixon & Guo (Reference Dixon and Guo1993) observed variation in egg size among C. septempunctata females that was not explained by differences in maternal body size. Similarly, Honěk et al. (Reference Honěk, Dixon and Martinkov2008b) collected egg masses of Coccinella septempunctata and Propylea quatordecimpunctata (Coleoptera: Coccinellidae) from the field and observed average egg size to “change in parallel with trophic conditions experienced before oviposition”, an effect they described as “puzzling”. These observed variations in egg size may be partially or fully explained by effects of female age.
Since the fecundity and fertility of coccinellid females typically decline with age after an early peak (Dixon & Agarwala, Reference Dixon and Agarwala2002; Michaud & Qureshi, Reference Michaud and Qureshi2006), selection acts most strongly on early reproductive bouts. Furthermore, few coccinellid females in nature are likely to achieve the longevity or lifetime fecundity of our laboratory females; these enjoyed ad libitum food and protection from mortality risks and were forced to conserve energy (i.e. they did not fly nor expend normal amounts of effort foraging or seeking oviposition sites). Consequently, the observed lifetime means of reproductive parameters are likely exaggerated artifacts of confinement rather than realistic values achievable in nature.
If the medium females in our study can be considered average within the range of possible body sizes, then small and large females represent outliers that might develop under conditions of prey scarcity or abundance, respectively. Note that medium females expressed the highest lifetime RE, even though treatment means did not separate significantly (table 1). If maternal body size is subject to stabilizing selection, both small and large females are likely to experience some fitness costs related to their extreme size, and these are likely to be more evident under field conditions. In this experiment, small females experienced significant stress as larvae that resulted in high mortality, slow development and reduced fecundity in the experiment, similar to effects observed in C. maculata females fed only every third day as larvae by Santos-Cividanes et al. (Reference Santos-Cividanes, dos Anjos, Cividanesm and Dias2011). However, the costs of being large will not necessarily be apparent under laboratory conditions, especially with ad libitum adult food. Large females likely incur higher energy costs during flight, possibly resulting in reduced dispersal ability, shorter longevity or other impacts on fitness not measurable in these experiments. Contrary to our hypothesis, the RE of females did not decline with advancing age, at least over the course of the first 30 oviposition days. Whereas the RE of large females did not change, that of small and medium females increased, possibly because the ad libitum adult diet afforded some compensation for the food deprivation these females experienced during larval development (fig. 3). We infer that the front-loaded reproductive strategy of large females appears well suited to maximise maternal fitness on scarce patches of ephemeral prey, especially if any costs of large body size accrue over time.
Our results can be fit quite well to the model developed by Parker & Begon (Reference Parker and Begon1986), which assumes that larval fitness is determined by three components: the absolute investment in the egg, the number of larval competitors and the size of the egg relative to the average size of other eggs. Within their categories (see their table 1) aphidophagous coccinellids are likely subject to the ‘hierarchy effect’ which favours the production of larger clutches by larger females, particularly early in the aphid cycle when sib competition is likely more important than non-sib competition. Later in the cycle, a competition intensifies among larvae, including non-sibs, and the production of larger eggs is favoured. This is consistent with observed increase in egg size by C. maculata females over the course of the first 20 oviposition days and with the general concept that increasing competition selects for more investment in individual offspring to ensure their survival (Brockelman, Reference Brockelman1975). Similarly, Plaistow et al. (Reference Plaistow, St. Clair, Grant and Benton2007) reasoned that an increase in egg size with female age in soil mites served to improve the survival of later-produced offspring that are forced to compete with older siblings.
An ability to vary offspring size will be adaptive when the resources available for offspring fluctuate over short periods (Fox & Czesak, Reference Fox and Czesak2000; Benton et al., Reference Benton, Plaistow, Beckerman, Lapsley and Littlejohns2005, Reference Benton, St. Clair and Plaistow2008), and this is true for aphidophagous coccinellids. Fischer et al. (Reference Fischer, Taborsky and Kokko2011) reasoned that the more reliably mothers can estimate environmental quality for their offspring, the more plasticity in offspring size should be favoured. However, the C. maculata females in our experiment did not increase egg size in response to deteriorating conditions as they were all provided ad libitum food continuously; changes in egg size appeared to be a de facto effect of oviposition sequence. Thus, in C. maculata, egg size plasticity does not hinge on a maternal response to changing environmental conditions but rather reflects a heritable program of dynamic change likely triggered at the onset of oviposition. Räsänen & Kruuk (Reference Räsänen and Kruuk2007) argued for the importance of a genetic basis for maternal effects and their significant impact on evolution at ecological time scales. An alternative, non-adaptive, explanation is that physiological constraints associated with ovariole development prevent females from producing their largest eggs initially, so that ‘optimum’ egg size is only achieved after a period of maturation.
Stearns (Reference Stearns1992) emphasized that even small differences in body size at birth may have important consequences later in life, especially for arthropods such as holometabolous insects whose body size is determined during the larval stage. To the best of our knowledge, this is the first study of egg size dynamics as a function of female age in an insect species in which the constraints imposed by maternal body size are clearly illustrated. The life history consequences of larval size at eclosion have not been adequately explored in coccinellids and warrant further study. The production of small eggs early in the reproductive cycle begs the question of whether the resulting neonates may compensate for their small initial size through larval feeding, or whether they are destined to develop more slowly or become smaller adults. Oosorption in response to resource depletion is a phenomenon that has only recently been described in aphidophagous coccinellids (Osawa, Reference Osawa2005; Kajita & Evans, Reference Kajita and Evans2009), and its potential impacts on subsequent partitioning of RE merits investigation. Studies that address these questions will further our understanding of the adaptive significance of dynamic reproductive allocation schedules in holometabolous insects and their life history consequences.
Acknowledgements
The authors are grateful to Mary E. Strong, Xiaoli Wu, Brandon Boccia and Brian Nechols for technical assistance. We also thank Leigh Murray, Department of Statistics, KSU, for help with the analyses and Jeremy Marshall for reviewing an early version of the manuscript. This work was made possible, in part, by financial support for the first author by the Colombian Sugarcane Research Center (CENICAÑA), the Fulbright Program, the Colombian Institute for the Development of Science and Technology (COLCIENCIAS) and the National Planning Department of Colombia (DNP). Voucher specimens of C. maculata are deposited at the Prairie Museum of Arthropod Research in Manhattan, Kansas under Lot Number 217. This is contribution no. 11-361-J of the Kansas State Agricultural Experiment Station.







