Introduction
Nucleoli are the prominent contrasted nuclear structures in which ribosomal RNAs (rRNAs) are synthesized, processed, and assembled with ribosomal proteins to form ribosomes (Hernandez-Verdun, Reference Hernandez-Verdun2006). In higher eukaryotic cells, the nucleolus is assembled at the beginning of interphase, maintained during interphase, and disorganized during mitosis. Although its structural organization appears to be indissociable from its function in ribosome biogenesis, the mechanisms that govern the formation and maintenance of the nucleolus are poorly understood.
In cultured human ovary and kidney cells, up to 10 nucleoli can be observed that correspond to the number of nucleolar organizer regions (NORs) in the human karyotype (Anastassova-Kristeva, Reference Anastassova-Kristeva1977). Whilst cells that had just completed division were usually found to have eight, nine or 10 nucleoli, those progressing close to the end of interphase showed a single nucleolus. On the basis of their results, Anastassova-Kristeva (Reference Anastassova-Kristeva1977) proposed a model for the nucleolar cycle for human cells, describing the kinetics of the nucleolus within one mitotic division. In this model, several primary nucleoli first appear in telophase and then associate gradually into a single nucleolus in interphase, dissociated again in the prophase and disappear completely in metaphase (M). Similarly, in cultured human lymphocytes, the number of nucleoli per cell is also influenced by the cell cycle. Thus, mitosis led to a marked increase in the number of nucleoli, whereas in all stages of interphase a decrease occurred (Wachtler et al., Reference Wachtler, Schwarzacher and Ellinger1982). In addition, the process of formation, fusion, and dissociation of the nucleolus was also described for some plant species (Khoudoleeva et al., Reference Khoudoleeva, Lazareva, Kononenko, Chentsov and Polyakov2000). Although it has been suggested that the fusion and fragmentation of nucleoli is correlated with the metabolic activities of cells (Popp & Wachtler, Reference Popp and Wachtler1983; Wachtler et al., Reference Wachtler, Schwarzacher and Smetana1984), and therefore knowledge of nucleolar kinetics in the mitotic cycle may be useful in determining the different functional stages of both normal and pathological cells, the regulation of nucleolar dynamic changes during the cell cycle has yet to be studied.
In bovine primordial follicles, the nucleolus of an oocyte is inactive, but in the secondary follicle an active fibrillo-granular nucleolus develops and proteins involved in rDNA transcription and rRNA processing localize to it (Maddox-Hyttel et al., Reference Maddox-Hyttel, Svarcova and Laurincik2007). At the end of the oocyte growth phase, the nucleolus is inactivated again and transforms into a solid remnant, which is dissolved upon meiotic resumption. After fertilization, structures resembling the nucleolar remnant are established in the pronuclei, and these structures are referred to as nucleolus precursor bodies (NPBs). When human zygotes were observed under a phase contrast microscope, the early phase of pronuclear development was characterized by a high number of relatively small and randomly distributed NPBs in both pronuclei, while the late phase was characterized by a low number of larger NPBs accumulating near the pole at which the pronuclei attached to each other (Tesarik & Greco, Reference Tesarik and Greco1999). Electron microscopic observation also indicated that the larger NPBs arose through fusion of smaller ones (Tesarik & Kopecny, Reference Tesarik and Kopecny1989a). Although it has been shown that this early phase of nucleologenesis (consisting of the assembly, growth and mutual fusion of NPBs) is dependent on an early wave of pronuclear transcriptional activity (Tesarik & Kopecny, Reference Tesarík and Kopecný1989b, Reference Tesarik and Kopecny1990) and on the presence of ooplasmic factors produced during oocyte maturation (Tesarik & Kopecny, Reference Tesarik and Kopecny1989c), the mechanism for the movement and fusion of these nucleolar entities during the M/G1 transition is not known. In addition, information obtained using this simple model (observation under a light microscope and a relatively low magnification) will definitely shed light on the mechanisms that govern the formation and maintenance of the nucleolus in other cell types.
Maturation-promoting factor (MPF) and mitogen-activated protein kinase (MAPK) are important regulators of the cell cycle. The increase in MPF activities is necessary for the onset and maintenance of metaphase during oocyte maturation (Nurse, Reference Nurse1990; Wu et al., Reference Wu, Ignotz, Currie and Yang1997). After parthenogenetic activation or fertilization, MPF is inactivated and oocytes enter the first mitotic cell cycle (Collas et al., Reference Collas, Sullivan and Barnes1993; Liu et al., Reference Liu, Ju and Yang1998). The MAPK cascade has also been shown to play a crucial role in regulating meiotic cell cycles during oocyte maturation, metaphase arrest, and early embryonic development (Gotoh et al., Reference Gotoh, Nishida, Matsuda, Shiina, Kosako, Shiokawa, Akiyama, Ohta and Sakai1991, Reference Gotoh, Masuyama, Dell, Shirakabe and Nishida1995; Kosako et al., Reference Kosako, Gotoh and Nishida1994; Moos et al., Reference Moos, Visconti, Moore, Schultz and Kopf1995). However, the roles of these two cell cycle regulators in the formation and maintenance of the nucleolus are poorly understood. The objective of the present study was therefore to examine the roles of the MAPK and MPF activities in the fusion of NPBs during the M/G1 transition following the activation of mouse oocytes, to contribute to our understanding of the molecular mechanisms that govern the formation and maintenance of the nucleolus in cells.
Materials and methods
Chemicals and reagents used in this study were purchased from Sigma Chemical Co. unless otherwise specified.
Oocyte recovery
Mice (Kunming) were kept in a room with 14 h/10 h light–dark cycles, the dark starting from 8 p.m. The animals were handled by the rules stipulated by the Animal Care and Use Committee of Shandong Agricultural University. Female mice, 6–8 weeks after birth, were induced to superovulate with equine chorionic gonadotropin (eCG; 10 IU i.p.) followed 48 h later by human chorionic gonadotropin (hCG; 10 IU, i.p.). Both eCG and hCG used in this study were from Ningbo Hormone Product Co., Ltd, P.R. China. The superovulated mice were sacrificed at different times after hCG injection and the oviductal ampullae were broken to release oocytes. After dispersion and three washes in M2 medium, the oocytes were denuded of cumulus cells by pipetting with a thin pipette in a drop of M2 containing 0.1% hyaluronidase.
Oocyte activation
Ethanol treatment
Cumulus-denuded oocytes were treated with 10% (v/v) ethanol in M2 medium for 5 min at room temperature, washed sequentially in M2 and the Chatot-Ziomek-Bavister (CZB) medium (three times each), and cultured for 9 h in CZB medium supplemented with or without different MPF/MAPK regulators at 37.5 °C under humidified atmosphere with 5% CO2 in air.
Strontium chloride treatment
For a long Sr2+ treatment, cumulus-denuded oocytes were first cultured in Ca2+-free CZB medium supplemented with 10 mM SrCl2 and with or without different MPF/MAPK regulators for 6 h at 37.5 °C, and then cultured for 3 h in regular CZB that did not contain SrCl2 in the presence or absence of different MPF/MAPK regulators. For a short Sr2+ treatment, oocytes were first incubated for 5 min in Ca2+-free CZB containing 10 mM SrCl2, and then cultured for 9 h in regular CZB without SrCl2 but with or without different MPF/MAPK regulators.
Fertilization in vitro
Masses of dense sperm were collected from the cauda epididymis of fertile male mice and were placed at the bottom of a test tube containing T6 medium supplemented with 10 mg/ml BSA. After 3–5 min, the supernatant containing highly motile spermatozoa were removed and capacitated in the same medium under mineral oil at 37 °C for 1.5 h. Oocytes collected at 18 h post hCG injection were stripped of their cumulus cells. To facilitate sperm penetration, a hole of about 20 μM in diameter was made on the zona pellucida of the oocytes using a piezo-driven micromanipulator. After being washed in the fertilization medium (T6 containing 20 mg/ml BSA), the oocytes were placed in fertilization drops (30–35 oocytes per 100 μl drop). Capacitated sperm were added to the fertilization drops to give a final sperm concentration of about 1 × 106/ml. One hour later, oocytes were transferred with attached spermatozoa into the fertilization medium with or without MPF/MAPK regulators and cultured for 5 h. At the end of culture, oocytes were stripped of attached sperm by pipetting with a thin pipette and cultured for 6 h in regular CZB medium.
Oocyte treatment with different MPF/MAPK regulators
The MPF/MAPK regulators used in this study include roscovitine (ROS), MG132, U0126, 6-dimethylaminopurine (6-DMAP) and okadaic acid (OA). All the drugs were dissolved in dimethyl sulfoxide (DMSO) and stored in aliquots of stock solutions at −20 °C until use. Immediately before use, the stock solutions were diluted in corresponding culture medium to final concentrations (50 μM for ROS, 3 μM for MG132, 25 μM for U0126, 2 mM for 6-DMAP and 0.5 μM for OA) depending on the experimental designs.
Observation of oocyte activation and NPB development
Oocytes were observed for activation (pronuclear formation) and NPB morphology under a Leica phase contrast microscope at different times following activating stimulation or insemination. Only the oocytes showing one or two pronuclei, or two cells each having a pronucleus, were considered activated or fertilized. Most of the activated oocytes showed one pronucleus, but oocytes with two pronuclei or two cells were occasionally observed in this study. Oocytes showing two pronuclei (or two cells) were considered having completed NPB fusion only when each pronucleus showed a single fused NPB.
Assay of MPF and MAPK activities
Forty cumulus-free oocytes from each treatment were washed three times in the histone kinase buffer (15 mM 3-[n-morpholino] propanesulfonic acid [MOPS], pH 7.2, containing 80 mM glycerophosphate, 10 mM EGTA, 15 mM MgCl2, 0.1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 10 μg/ml cAMP-dependent protein kinase inhibitor peptide), transferred to 10 μl histone kinase buffer contained in a 1.5 ml microfuge tube, and stored frozen at –70 °C. Before kinase reactions, the frozen samples were subjected to four to five times freezing and thawing to prepare lysates. Kinase reactions were initiated by the addition of 10 μl of substrate buffer containing 2 mg/ml histone H1, 2 mM dithiothreitol and 20 μCi/ml [γ-32P] ATP to each sample, and the reactions were carried out for 50 min at 37 °C. The reaction was terminated by the addition of an equal volume of double-strength SDS sample buffer containing β-mercaptoethanol, and the mixture was boiled for 3–5 min. Kinase reaction products were then separated by 12% linear gradient SDS-PAGE. Gels were exposed to phosphor-screens. Data acquisition was the actual scanning of sample images with the Cyclone® Plus Storage Phosphor System to create an image file that can be analyzed by the OptiQuant™ Image Analysis Software. The MPF activity values of newly ovulated oocytes (recovered 13 h post hCG injection) were arbitrarily set as 100%, and the other values were expressed relative to this activity. The amount of kinase reaction product used for SDS-PAGE was strictly controlled (20 μl) for each sample, and three samples were analyzed for each treatment. The same procedures were repeated for assay of MAPK activity except that 2 mg/ml histone H1 was replaced with 1 mg/ml bovine myelin basic protein (MBP) in the substrate buffer.
Calcium measurement
Intracellular Ca2+ was measured using the Ca2+-sensitive dye fluo-3. For loading, oocytes were incubated for 20 min at 37 °C with 30 μM of the acetoxymethyl (AM) form of the dye made up in CZB with 0.02% pluronic F-127. After loading, oocytes were washed with and placed in 22.5 μl drops of calcium-free (for Sr2+ activation) or regular (for ethanol activation) M2 under paraffin oil in a Fluoro dish (World Precision Instruments, Inc.) with its base coated with phytoagglutinin. The dish was transferred to a heated stage (37 °C) of a Leica laser-scanning confocal microscope (TCS SP2; Leica Microsystems). Immediately after the oocytes were brought in focus, 2.5 μl of absolute ethanol or 100 mM SrCl2 was injected into the drop to initiate activation treatment. The 5 min activation treatment was terminated by diluting the drop with 1000 μl regular or Ca2+-free M2. Oocytes were observed using a ×10 objective. An argon laser was used for excitation at 488 nm and signals emitted at 505–540 nm were collected for 30 or 40 min by the laser scanning confocal imaging system. Traces of calcium oscillations were plotted using SigmaPlot 2000 software.
Data analysis
There were at least three replicates for each treatment. Data were analysed using SPSS 11.5 (Statistics Package for Social Sciences). Data were compared using one-way ANOVA followed by least significant difference (Duncan) post hoc tests. The percentage data were arc sine transformed, and assumptions that the transformed data were normal (Shapiro–Wilk and Kolmogorov–Smirnov tests for normality) and population variances were all equal (Levene test) were checked prior to performing the ANOVA. Differences were considered significant at p < 0.05.
Results
Appearance, fusion and disappearance of NPBs following activation of mouse oocytes
When examined under a phase contrast microscope at 3 h following ethanol activation treatment, some of the oocytes showed several small NPBs (Fig. 1A). When observed at 9 h after ethanol, however, most of the oocytes displayed a single large NPB (Fig. 1B). The fused NPBs began to disappear at about 14 h after activation (Fig. 1C). Similarly, when fertilized oocytes were examined at 6 h following insemination in vitro, some showed several NPBs (Fig. 1D), but when observed at 12 h after insemination, most showed a single fused NPB (Fig. 1E) in both the male and female pronuclei.

Figure 1 Micrographs of mouse oocytes showing the appearance, fusion and disappearance of NPBs. Following ethanol activation treatment, several NPBs appeared (A), which then fused into a single NPB (B) before disappearance (C). Likewise, several NPBs formed initially (D) and then fused into one (E) in both the male and female pronuclei in fertilized oocytes. Fusion of the NPBs was prevented when ethanol-activated oocytes were cultured in the presence of MG132 (F). Scale bar is 15 μM. NPB, nucleolus precursor body.
The time course for NPB fusion and the dynamic changes in MPF and MAPK activities following ethanol activation of mouse oocytes
To observe the time course for the appearance, fusion and disappearance of NPBs, oocytes (n = 77) were examined for NPBs every 30 min from zero to 16 h following ethanol stimulation. The dynamic changes in NPBs were classified into four stages: (A) before NPB formation; (B) formation of several small NPBs; (C) fusion of the small NPBs into a single NPB and (D) disappearance of the fused NPB. Percentages of oocytes at each stage of NPB changes were plotted in Fig. 2a, and the mean time that oocytes spent at each stage was estimated in Fig. 2b using a method reported by Sirard et al. (Reference Sirard, Florman, Leibfried-Rutledge, Barnes, Sims and First1989). The results indicated that small NPBs began to take shape at about 4 h after activation stimulus, and they took about 1.5 h to fuse with each other into a single large NPB. The fused NPB lasted for about 10 h before disappearance. Assays of the kinase activities at different times after ethanol activation indicated that while the activity of MPF went down to the basal level within 1 h, the MAPK activity did not decline until 3 h following ethanol stimulation (Fig. 3). A further observation after aceto-orcein staining indicated that the nuclear envelope appeared and disappeared at the same time as did the NPBs (data not shown). Therefore, NPBs as well as the pronuclear envelope begin to form only after both the MPF and MAPK activities drop by a considerable margin.
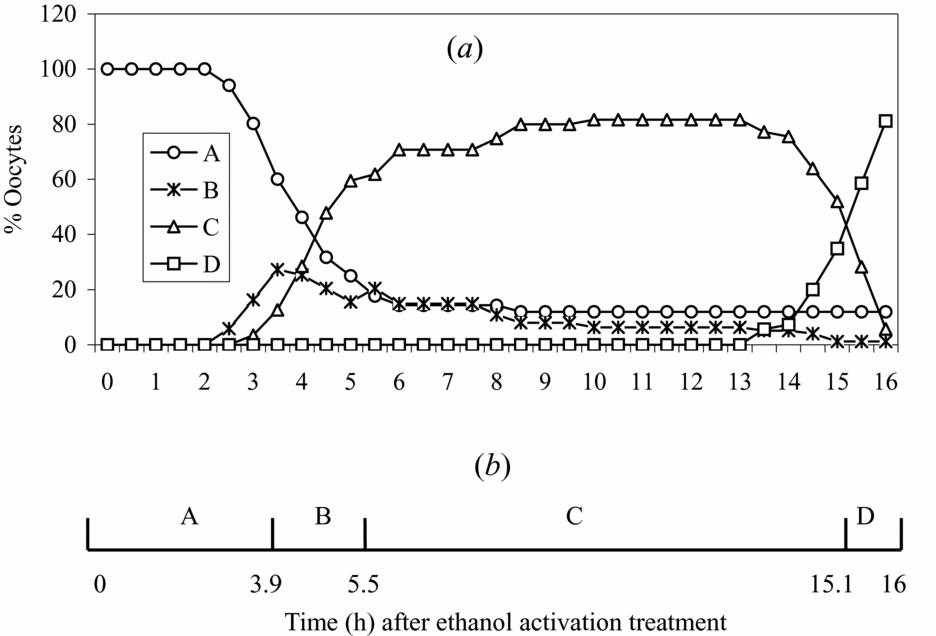
Figure 2 (a) Percentages of mouse oocytes at each stage of NPB changes after ethanol activation: A, before NPB formation; B, formation of several small NPBs; C, fusion of the small NPBs into a single NPB; and D, disappearance of the fused NPB. (b) The areas under the four curves in (a) were computed into a timetable, as reported by Sirard et al. (Reference Sirard, Florman, Leibfried-Rutledge, Barnes, Sims and First1989). The areas under the four curves of panel (a) were added to represent 16 h. This computation allowed an estimate of the mean time that oocytes spent at each stage of NPB changes. NPB, nucleolus precursor body.

Figure 3 Relative activities of MPF and MAPK after mouse oocytes were cultured in regular CZB for different times after ethanol treatment. a–cValues that are not followed by a common letter differ significantly (p < 0.05) within the MPF or MAPK activities. C, control oocytes assayed for kinase activities immediately after collection at 13 h post hCG injection. MAPK, mitogen-activated protein kinase; MPF, maturation-promoting factor.
Effects of different MPF/MAPK regulators on the fusion of NPBs after ethanol activation of mouse oocytes
To characterize the roles of MPF and MAPK in regulating the formation and fusion of NPBs, effects of different MPF and MAPK regulators were observed. Oocytes were cultured in regular CZB supplemented with different MPF/MAPK regulators following ethanol treatment, and morphology of NPBs was examined at 9 h of culture. Rates of oocyte activation (pronuclear formation) were high after treatments with 6-DMAP, ROS or U0126 at the routine concentrations (Table 1) but low when treated with 5 μM MG132 (45.8 ± 3.4%). When the MG132 concentration decreased to 3 μM, however, rates of oocyte activation increased to 90% (Table 1). Fusion of NPBs was prevented in the presence of MAPK inhibitor U0126 and the protein kinase inhibitor 6-DMAP, but not in the presence of the MPF inhibitor ROS. However, the MPF activator, MG132, efficiently prevented NPB fusion. As it is known that inhibition of protein kinases by 6-DMAP accelerates the transition to interphase in activated mouse oocytes (Szöllösi et al., Reference Szöllösi, Kubiak, Debey, Pennart, Szöllösi and Maro1993), the results suggest that that NPB fusion is regulated by MPF and MAPK activities and that the formation and fusion of NPBs are two separate events that can be uncoupled by manipulating the proteasome activity.
Table 1 Effects of MPF or MAPK regulators on the fusion of NPBs after ethanol activation of mouse oocytes

a –cValues without a common letter in their superscripts differ (p < 0.05) in the same column. Oocytes were treated with 10% ethanol contained in M2 for 5 min before culture in CZB medium containing different regulators. NPBs were examined at 9 h of culture.
6-DMAP, 6-dimethylaminopurine; MAPK, mitogen-activated protein kinase; MPF, maturation-promoting factor; NPB, nucleolus precursor body; ROS, reactive oxygen species.
Effects of MPF and MAPK activities on NPB fusion in aged oocytes
Oocytes recovered at different times after ovulation were treated with ethanol for activation or assayed for kinase activities. Both MPF and MAPK activities decreased with time after ovulation (Table 2). While rates for NPB fusion declined significantly when the MAPK activity dropped to 70%, rates for pronuclear formation did not decline until the MAPK activity decreased to 25% of that of control oocytes. This finding suggested that the MAPK activity became the determining factor for NPB fusion when MPF activity was low because the already decreased MPF activity would have declined further after activation of the aged oocytes. Furthermore, when aged oocytes collected 30 h post hCG were treated with 0.5 μM OA for the first 2 h after ethanol activation, rates for NPB fusion (87.8 ± 5.9) increased significantly compared to untreated controls (59.4 ± 2.4), confirming that a preactivation decline in MAPK activities impaired NPB fusion after pronuclear formation.
Table 2 Fusion of NPBs and activities of MPF and MAPK after ethanol activation of aging mouse oocytes
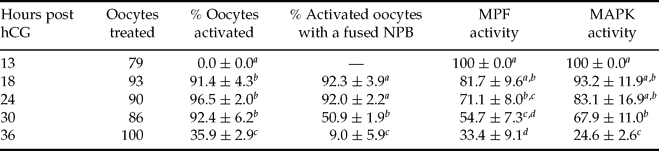
a –dValues without a common letter in their superscripts differ (p < 0.05) in the same column.
hCG, human chorionic gonadotropin; MAPK, mitogen-activated protein kinase; MPF, maturation-promoting factor; NPB, nucleolus precursor body.
Temporal window for U0126 or MG132 inhibition of NPB fusion after ethanol activation of mouse oocytes
To specify how long the high MAPK activity should persist and at what time the MPF activity should decline following oocyte activation in order for NPB fusion to take place, oocytes were incubated for different times with U0126 or MG132 starting from different times after ethanol stimulation. Activation and NPB fusion were examined at 9 h following ethanol stimulation. While U0126 treatments starting from the third or fourth hour of activation had no effect, those that were started from the first hour and lasted for two or more hours inhibited NPB fusion significantly (Table 3). While the MG132 treatments starting from the fourth hour onwards showed no effects, treatments that were initiated from the first hour and lasted for four or more hours inhibited NPB fusion significantly (Table 4). The results suggested that a high MAPK activity during the first 2 h and a low MPF activity during the first 3–4 h after activation were essential for NPB fusion after pronuclear formation.
Table 3 Temporal window for U0126 inhibition of NPB fusion after ethanol activation of mouse oocytes

a –cValues without a common letter in their superscripts differ (p < 0.05) in the same column.
NPB, nucleolus precursor body.
Table 4 Temporal window for MG132 inhibition of NPB fusion after ethanol activation of mouse oocytes

a –eValues without a common letter in their superscripts differ (p < 0.05) in the same column.
NPB, nucleolus precursor body.
While showing a crucial role for the maintenance of a low MPF activity during the first 4 h following oocyte activation in subsequent fusion of NPBs, the above results also raised some questions. As the MPF activity drops to the basal level within 1 h after activation during culture without MG132 (Fig. 3), with about 90% of NPB fusion, why did NPB fusion still occur in 45% of the oocytes cultured for 3 h in the presence of MG132 (Table 4), and why could MG132 treatment initiated from the third hour still inhibit NPB fusion in 50% of the oocytes? We hypothesized that: (i) for NPBs to fuse the MPF activity must be always low during the first 3–4 h after activation; (ii) the MPF activity would decline gradually in the presence of MG132; and (iii) inactivated MPF could be reactivated in the presence of MG132. To test this hypothesis, MPF activity was assayed after oocytes were treated with MG132 for different times starting from different hours following ethanol activation. The results showed that the MPF activity declined gradually in the presence of MG132 and to the basal level by 6 h after ethanol stimulation (Fig. 4A). The MPF activity increased significantly after oocytes were cultured with MG132 for 2 h whether the treatment was started at the second or third hour post ethanol, while incubation with MG132 for 1 h induced only a mild elevation of the MPF activity (Fig. 4B). The elevated MPF activity decreased again 2 h later despite the presence of MG132.

Figure 4 Relative activities of H1 kinase (MPF) after mouse oocytes collected 18 h post hCG injection were cultured with or without MG132 for different times after ethanol treatment. (A) MPF activities after oocytes were cultured continuously in the presence of MG132. (B) MPF activities after oocytes were cultured for 1 h in CZB (CZB1), 4 h in MG132 (MG4), 1 h in CZB followed by 2 h in MG132 (CZB1MG2), 1 h in CZB followed by 4 h in MG132 (CZB1MG4), 2 h in CZB followed by 2 h in MG132 (CZB2MG2) or 3 h in CZB followed by 1 h in MG132 (CZB3MG1). a–cValues that do not have a common letter above their bars differ significantly (p < 0.05). MAPK, mitogen-activated protein kinase; MPF, maturation-promoting factor.
Effects of MPF/MAPK regulators on the fusion of NPBs in fertilized oocytes
To improve synchronization of fertilization, oocytes were inseminated following zona drilling. The inseminated oocytes were treated for 6 h with U0126 or MG132 in CZB starting from the first hour after insemination, and NPB fusion was examined at 12 h after insemination. While MG132 significantly reduced NPB fusion in both male and female pronuclei, U0126 showed no significant effect (Table 5), suggesting that inhibition of MAPK activity does not affect NPB fusion when the repetitive intracellular Ca2+ rises are induced after fertilization.
Table 5 Effects of MPF or MAPK regulators on the fusion of NPBs after in vitro fertilization of mouse oocytes

a ,b Values without a common letter in their superscripts differ (p < 0.05) in the same column.
MAPK, mitogen-activated protein kinase; MPF, maturation-promoting factor; NPB, nucleolus precursor body.
Effects of U0126 and MG132 on NPB fusion of mouse oocytes after SrCl2 treatment for different times
Why U0126 could not inhibit NPB fusion in fertilized eggs while it could inhibit in ethanol-activated oocytes? It is known that fertilization induces repetitive intracellular Ca2+ rises, but ethanol stimulation causes a monotonic increase in the intracellular Ca2+. We therefore hypothesized that the multiple Ca2+ rises during fertilization would probably overcome the inhibition by U0126 and induce NPB fusion in its presence. To test the hypothesis, we first observed the Ca2+ transients after mouse oocytes recovered 18 h post hCG injection that were treated either with Sr2+ for 5 or 40 min or with ethanol for 5 min. The results demonstrated that while repetitive intracellular Ca2+ increases were induced following a 40 min Sr2+ treatment, only a single Ca2+ rise was observed after ethanol or Sr2+ treatment for 5 min (Fig. 5).

Figure 5 Ca2+ oscillations in mouse oocytes after treatment for activation with ethanol or Sr2+ for 5 or 40 min. Activation treatment was initiated by injecting SrCl2 or ethanol at 0 min and was terminated in 5 min by dilution with large amount of M2 medium for the 5 min ethanol or Sr2+ activation group. The fluorescence signals were monitored and recorded for 30 min in the 5 min groups but for 40 min in the 40 min Sr2+ group.
We then examined NPB fusion after oocytes were treated with Sr2+ for 6 h or 5 min. For the 6 h Sr2+ treatment, mouse oocytes were first cultured for 6 h in the activating medium (Ca2+-free CZB with 10 mM SrCl2) supplemented with 25 μM U0126 or 3 μM MG132, and then for 3 h in regular CZB supplemented with or without U0126, respectively. For a 5 min Sr2+ treatment, oocytes were incubated in the activating medium for 5 min before culture for 9 h in regular CZB containing U0126 or MG132. Fusion of NPBs was examined at the end of culture. As observed in fertilized oocytes, while MG132 inhibited NPB fusion significantly, U0126 showed a mild effect on NPB fusion in oocytes activated by a 6 h Sr2+ treatment (Table 6). Increasing U0126 concentration from 25 μM to 50 μM could not improve inhibition of NPB fusion but caused oocyte degeneration (data not shown). In oocytes activated by a 5 min Sr2+ treatment, however, both MG132 and U0126 prevented NPB fusion efficiently, a situation similar to that observed in ethanol-activated oocytes.
Table 6 Effects of U0126 or MG132 on NPB fusion of mouse oocytes after SrCl2 treatment for 6 h or 5 min

a –cValues without a common letter in their superscripts differ (p < 0.05) in the same column within Sr2+-treatment duration.
NPB, nucleolus precursor body.
Discussion
For the first time, this study has observed the timing of NPB formation and fusion after oocyte activation. The results showed that several small NPBs began to appear about 4 h after ethanol activation of mouse oocytes, and they took about 1.5 h to fuse with each other into a single large NPB. The fused NPB lasted for about 10 h before disappearance. The nuclear envelope appeared and disappeared at the same time as did the NPBs. While the activity of MPF went down within 1 h, the MAPK activity did not decline until 3 h following ethanol stimulation. This timing for pronuclear formation and disappearance and the kinase dynamics are consistent with those reported previously. Verlhac et al. (Reference Verlhac, Kubiak, Clarke and Maro1994) reported that the second polar body (Pb2) began to extrude about 45 min, pronuclei were clearly visible 4 h later and 70% of the eggs reached metaphase by 14 h after ethanol activation of mouse oocytes. Kubiak et al. (Reference Kubiak, Weber, Géraud and Maro1992) observed NPBs (the fused ones) in activated mouse oocytes 6 h after ethanol treatment. Both Verlhac et al. (Reference Verlhac, Kubiak, Clarke and Maro1994) and Kubiak et al. (Reference Kubiak, Weber, Géraud and Maro1992) observed a dramatic drop of histone H1 kinase activity during extrusion of the Pb2, which took place within 1 h after ethanol activation. In contrast, the MAPK activity remained high even 1 h after Pb2 extrusion, but reduced to 50% by 3 h and to basal level by 5 h after Pb2 extrusion (Verlhac et al., Reference Verlhac, Kubiak, Clarke and Maro1994). Likewise, in fertilized mouse eggs, whereas MPF activity fell to negligible levels by 90 min post-insemination, a decrease to negligible levels of MAPK was not observed until about 7 h post-insemination (Moos et al., Reference Moos, Visconti, Moore, Schultz and Kopf1995). In addition, the present results indicated that a preactivation decline in the MAPK activity was associated with decreased NPB fusion following activation of aged oocytes, and that NPB fusion was improved significantly when aged oocytes were treated with OA to increase MAPK activities. Furthermore, our analysis of the temporal windows for U0126 and MG132 action on NPB fusion indicated that a high MAPK activity during the first 2 h and a low MPF activity during the first 4 h after activation stimulus were both essential for NPB fusion after pronuclear formation.
It is generally accepted that the decrease in MPF activity is involved in the initiation of egg activation (the exit from MII), whereas the decrease in MAPK activity correlates with onset of pronuclear formation (Liu et al., Reference Liu, Ju and Yang1998; Fan & Sun, Reference Fan and Sun2004). Thus, low activities of both MAPK and CDC2A kinases were found essential for normal meiotic completion and pronuclear formation in parthenogenetically activated porcine oocytes (Ito et al., Reference Ito, Shimada and Terada2004). Treatment with OA, a specific inhibitor of protein phosphatase 1 and 2A, increased MAPK activity and overcame pronuclear formation of mouse oocytes activated by various stimuli (Moos et al., Reference Moos, Visconti, Moore, Schultz and Kopf1995; Sun et al., Reference Sun, Lax, Rubinstein, Chen and Breitbart1999). In this study, pronuclear and NPB formation was partially and completely prevented when oocytes were cultured with 5 μM and 8 μM MG132 (data not shown), respectively, but was allowed when the MG132 concentration decreased to 3 μM. However, the NPB fusion was inhibited in the presence of 3 μM MG132. It could be that the mechanism regulating pronuclear formation was more responsive to the decline in the MPF activity than the mechanism controlling NPB fusion, and thus, more MG132 was needed to maintain higher MPF activities to inhibit pronuclear formation than to prevent NPB fusion. Alternatively, pronuclear formation requires, in addition to cyclin B, an ubiquitin-conjugated protein(s), which is more sensitive to proteasomes than the cyclin B. In addition, while rates for NPB fusion declined significantly when the MAPK activity dropped to 70%, rates for pronuclear formation did not decline until the MAPK activity decreased to 25% in aging oocytes, suggesting that NPB fusion was more sensitive than pronuclear formation to a preactivation decline in MAPK activities. Furthermore, while showing no effect on pronuclear formation, treatments with U0126 or 6-DMAP impaired NPB fusion of activated oocytes. For Hela cells proceeding through M/G1 transition, whilst ROS treatment blocked relocalization of Nop52 and impaired formation of nucleoli, treatment with U0126 caused no obvious effect on nucleologenesis (Sirri et al., Reference Sirri, Hernandez-Verdun and Roussel2002). Together, these results strongly suggest that pronuclear (also NPB) formation and fusion of NPBs are two separate events that are controlled by different mechanisms.
A possible role of MAPK in the maintenance of a metaphase state during the MI to MII transition when MPF is inactive has been proposed (Verlhac et al., Reference Verlhac, Kubiak, Clarke and Maro1994)). The delayed MAPK inactivation after the onset of MPF inactivation in activated oocytes has been found to have a crucial role for Pb2 emission and sperm chromatin decondensation to accomplish the transition from meiosis to mitosis (Sun et al., Reference Sun, Lax, Rubinstein, Chen and Breitbart1999; Tatemoto & Muto, Reference Tatemoto and Muto2001). For the first time, this study demonstrated that a high MAPK activity during the first 2 h and a low MPF activity during the first 4 h after activation were both essential for subsequent NPB fusion during pronuclear development. The results have undoubtedly revealed another role for the high MAPK but low MPF activities during the MII–interphase transition. It is interesting to show that a low MPF activity is the prerequisite for MAPK to function properly and that a cellular event that occurs prior to pronuclear formation dictates the cellular processes 2 h later during pronuclear growth. It was shown in porcine oocytes that retardation of MAPK inactivation via delayed phorbol myristyl acetate (PMA) treatment resulted in improved release of the Pb2 (Ito & Shimada, Reference Ito and Shimada2005). In somatic cells, the small GTPase RhoA, which is activated by the guanine nucleotide exchange factor (GEF) ECT2, is known to orchestrate the constriction of the contractile ring, enabling cytokinesis. A recent study in mouse oocytes showed that MPF inactivation at the onset of anaphase induces ECT2 dephosphorylation, and the dephosphorylated ECT2 enables a ring-shaped accumulation of RhoA at the ingression site of the cortex, which allows contractile ring formation and leads to the completion of the first meiotic division (Elbaz et al., Reference Elbaz, Nevo, Galiani and Dekel2009). Furthermore, our study on chemical-induced cytokinesis of goat oocytes indicated that the constriction of the actomyosin ring occurred when the MPF activity decreased while both MAPK and RhoA activities were high (data to be published). Although this event provides some explanation for the role of a high MAPK activity for Pb2 extrusion during the MII–interphase transition when the MPF activity is low, we do not know what happens during this same period that controls subsequent NPB fusion after pronuclear formation.
Human nucleolar phosphoproteins p130 and hNopp140 are nuclear proteins that oscillate during the cell cycle, and both proteins were found to be involved in nucleologenesis (Pai et al., Reference Pai, Chen, Sheu and Yeh1995; Tsai et al., Reference Tsai, Lin, Chen, Lee, Hsu, Yang and Yeh2008). Extracts of mitotic cells had lower concentrations of p130 compared with those of interphase cells, and all the remaining p130 in mitotic cells was further phosphorylated, resulting in increase in its solubility, and its dispersion throughout the entire cytoplasm. At telophase, p130 reappeared and aggregated into granular structures, resembling the pre-nucleolar bodies. M-phase cell extract provided significant kinase activity for incorporation of phosphate into the interphase p130 and the hyperphosphorylation of p130 during mitosis was abolished when cells were treated to inhibit the CDC2A activity (Pai et al., Reference Pai, Chen, Sheu and Yeh1995). The increase in solubility of p130 due to hyperphosphorylation at the onset of mitosis is similar to the case of lamins, the well characterized substrates of cdc2 kinase (Gerace & Blobel, Reference Gerace and Blobel1980; Peter et al., Reference Peter, Nakagawa, Dorée, Labbé and Nigg1990). Reassembly of the nuclear lamina during telophase appears to be associated with lamin dephosphorylation (Burke & Gerace, Reference Burke and Gerace1986). Furthermore, the hNopp140 molecule was also found to contain CDC2A/cyclin B phosphorylation sites (Tsai et al., Reference Tsai, Lin, Chen, Lee, Hsu, Yang and Yeh2008). Together, we propose that a decrease in the MPF activity during the telophase II of the activated oocytes would dephosphorylate the related nucleoproteins, which would enable not only the formation but also the fusion of the NPBs.
This study showed that while U0126 inhibited NPB fusion in ethanol- or 5-min Sr2+-activated oocytes, it had a mild effect on fertilized and 6-h Sr2+-activated oocytes. Although sperm entry induces repetitive intracellular Ca2+ rises during normal fertilization (Kline & Kline, Reference Kline and Kline1992), most parthenogenetic agents including ethanol cause a monotonic increase in the intracellular Ca2+ (Colonna et al., Reference Colonna, Tatone, Malgaroli, Eusebi and Mangia1989; Jellerette et al., Reference Jellerette, He, Wu, Parys and Fissore2000; Grupen et al., Reference Grupen, Nottle and Nagashima2002). However, extended Sr2+ treatments were found to induce oocyte activation with repetitive intracellular Ca2+ increases (Kline & Kline, Reference Kline and Kline1992; Bos-Mikich et al., 1995). In the present study, while repetitive intracellular Ca2+ increases were induced following a 40 min Sr2+ treatment, only a single Ca2+ rise was observed after ethanol or Sr2+ treatment for 5 min. We therefore hypothesize that it could be the multiple Ca2+ rises during fertilization that overcame the inhibition by U0126 and induced NPB fusion in its presence. As various studies, including the present one, indicated that MPF activity decreased to the basal level soon after activation or fertilization as discussed above, we suggest that inhibition of MAPK activity does not affect NPB fusion when the repetitive intracellular Ca2+ rises are induced after fertilization.
The present results indicated that in the presence of a low concentration (3 μM) of MG132, the MPF activity declined gradually after activation of mouse oocytes, permitting the formation of nuclear envelope and NPBs and NPB fusion in most and some of the activated oocytes, respectively. However, pronuclear and NPB formation was completely blocked in the presence of a higher concentration (8 μM) of MG132. Josefsberg et al. (Reference Josefsberg, Galiani, Dantes, Amsterdam and Dekel2000) reported that intervention of proteasomal action with 10 μM MG132 resulted in a gradual accumulation of cyclin B and elevation of MPF activity during maturation of rat oocytes. Furthermore, the present study also showed that the inactivated MPF could be reactivated to some extent in the presence of 3 μM MG132 and prevented NPB fusion in some activated oocytes. This finding confirms both cyclin B accumulation and the availability of activatable CDC2A during the MII–interphase transition after oocyte activation. The mechanism for the concentration-dependent effect of MG132 on the dynamics of MPF activities is worth further investigation.
In summary, we have studied fusion of NPBs and its regulation by MAPK and MPF using the chemical-activated mouse oocyte model. The results suggest that: (i) the MAPK and MPF activities at the initial stage of activation regulate NPB fusion after pronuclear formation; (ii) pronuclear assembly and NPB fusion are two separable events that might be controlled by different mechanisms; and (iii) high MAPK activity and low MPF activity at the initial stage of activation is essential for NPB fusion when only one calcium rise is induced by ethanol; whilst inhibition of MAPK activity does not affect NPB fusion when the repetitive intracellular Ca2+ rises are induced after fertilization. The chemical-activated mouse oocyte model has several advantages to facilitate studies on nucleolar formation and fusion. For example, oocytes are much larger than somatic cells and the nucleolar morphology is thus easier to observe under a light microscope; the mouse oocytes are more transparent than oocytes from large animals; and pronuclear development was more synchronized in chemical-activated oocytes than in fertilized oocytes. To our knowledge, this is the first report on the signalling regulation of nucleolar fusion, which is important because this study will probably evoke further work and provide a new avenue of investigation for studying the mechanisms of nucleologenesis.
Acknowledgements
This study was supported by grants from the National Basic Research Project of the China Ministry of Science and Technology (Nos. 2007CB947403 and 2006CB944003), the China National Natural Science Foundation (Nos. 30771556 and 30972096), the National “863” Project of the China Ministry of Science and Technology (Nos. 2008AA10Z160 and 2008AA101003) and the China Special Project for Transgenic Research (No. 2008ZX08010–001).













