Introduction
The fine fescues (Festuca spp.) are a group of cool-season turfgrasses that are adapted to cool, dry, shaded environments and are tolerant of infertile, acidic soils and drought conditions (Beard Reference Beard1973; Hanson and Juska Reference Hanson and Juska1969; Turgeon Reference Turgeon1996). These grasses also have better overall turfgrass quality (the measure of color, density, uniformity, and texture) under lower fertility levels compared with other cool-season turfgrasses (Ruemmele et al. Reference Ruemmele, Wipff, Brilman, Hignight, Casler and Duncan2003). Fine fescues are a good choice for low-maintenance turf due the abovementioned traits. The different species of fine fescues are divided into two major groups, the red fescue (Festuca rubra L.) complex and the sheep fescue (Festuca ovina L.) complex. Within the red fescue complex there are rhizomatous or creeping growth habits as well as nonrhizomatous or bunch-type growth habits. The sheep fescue complex contains only nonrhizomatous growth habits. The three species that are most commonly used for turfgrass are hard fescue [Festuca trachyphylla (Hack.) Hack.], Chewings fescue [Festuca rubra ssp. commutata Gaudin; syn. F. rubra ssp. fallax (Thuill.) Nyman], and strong creeping red fescue (Festuca rubra L. ssp. rubra). Hard fescue is in the sheep fescue complex, and both Chewings and strong creeping red fescue are in the red fescue complex (Ruemmele et al. Reference Ruemmele, Wipff, Brilman, Hignight, Casler and Duncan2003). Chewings and hard fescue are both hexaploid (2n = 42) and strong creeping red fescue is an octoploid (2n = 56) (Ruemmele et al. Reference Ruemmele, Wipff, Brilman, Hignight, Casler and Duncan2003).
Weed control during establishment is critical for the planted species to grow without competition from invasive species (Beard Reference Beard1973). Currently, there are limited options to control broadleaf and grassy weeds during establishment for fine fescues. Previous efforts in breeding fine fescues for increased tolerance to herbicides have been successful using a recurrent-selection method. Herbicide-tolerance development in hard fescue has been demonstrated before with the nonselective herbicide glyphosate. ‘Aurora Gold’ is an advanced-generation synthetic cultivar derived from ‘Aurora’ hard fescue after using five cycles of phenotypic recurrent selection over a 10-yr period following direct applications of glyphosate at 0.8 to 1.6 kg ha−1 (Hart et al. Reference Hart, Derr, Lycan, Rose-Fricker and Meyer2005). A study conducted by McCullough et al. in 2015 determined the mechanism of resistance to glyphosate in Aurora Gold hard fescue was due to less target-site inhibition. In that study the researchers conducted laboratory experiments to evaluate shikimate accumulation in excised leaves. Aurora Gold accumulated less shikimate, which suggests differences in glyphosate activity at the target-site level. This could be due to overexpression of 5-enolpyruvylshikimate-3-phosphate synthase or target-site mutations. An aminotriazole-tolerant Chewings fescue cultivar ‘Countess’ was developed using recurrent selection (Johnston and Faulkner Reference Johnston and Faulkner1986). This provided a selective control of annual bluegrass (Poa annua L.) in those cultivars.
In previous research, the differences in herbicide tolerance among cool-season grasses have been frequently associated with foliar uptake and translocation (Lycan and Hart Reference Lycan and Hart2006a; Sidhu et al. Reference Sidhu, Yu and McCullough2014; Wang and Liu Reference Wang and Liu2007; Yu et al. Reference Yu, McCullough and Vencill2013, Reference Yu, McCullough and Grey2015). For example, ‘JS-501’ is a glyphosate-tolerant perennial ryegrass (Lolium perenne L.) cultivar (Hart et al. Reference Hart, Derr, Lycan, Rose-Fricker and Meyer2005), while ‘Manhattan V’ is a glyphosate-susceptible cultivar (McCullough et al. Reference McCullough, Yu, Shilling and Czarnota2015). In lab experiments, McCullough et al. (Reference McCullough, Yu, Shilling and Czarnota2015) found that JS-501 absorbed significantly less [14C]glyphosate than Manhattan V and accumulated ~50% less shikimate. Bispyribac-sodium is used to selectively control P. annua and roughstalk bluegrass (Poa trivialis L.) in creeping bentgrass (Agrostis stolonifera L.) (Lycan and Hart Reference Lycan and Hart2006b). Lycan and Hart (Reference Lycan and Hart2006a) reported that creeping bentgrass had lower amounts of foliar and root uptake and subsequent translocation of [14C]bispyribac-sodium than P. annua and P. trivialis, which contributed to the herbicide selectivity. Similarly, Yu et al. (Reference Yu, McCullough and Vencill2013) documented that amicarbazone selectivity for P. annua control in cool-season turfgrasses was associated with differential levels of absorption and translocation. The authors noted that P. annua exhibited significantly faster absorption and translocation of foliar-applied amicarbazone than creeping bentgrass and tall fescue [Lolium arundinaceum (Schreb.) Darbysh.]
Mesotrione is a 4-hydroxyphenylpyruvate dioxygenase (HPPD)-inhibiting herbicide. It works by inhibiting the HPPD enzyme. This disrupts the biosynthesis of plastoquinone, which is a cofactor of phytoene desaturase. This indirectly results in disruption of carotenoid biosynthesis (Beaudegnies et al. Reference Beaudegnies, Edmunds, Fraser, Hall, Hawkes, Mitchell, Schaetzer, Wendeborn and Wibley2009). In susceptible species, HPPD inhibition results in damage to cell membranes from free radicals. Visual symptoms are foliar bleaching and tissue necrosis (Lee et al. Reference Lee, Knudsen, Michaely, Chin, Nguyen, Carter, Cromartie, Byron, Shribbs and Fraser1998; McCurdy et al. Reference McCurdy, McElroy, Kopsell and Sams2009). Mesotrione provides effective PRE and early-POST control of many problematic broadleaf and grassy weeds, including P. annua. Mesotrione is currently only labeled for use on mature fine fescue plants (Anonymous 2008). Mesotrione is used at seeding for tall fescue, perennial ryegrass, and Kentucky bluegrass (Poa pratensis L.) with little to no turfgrass injury and control of problematic weeds such as P. annua (Askew and Beam Reference Askew and Beam2002; Dernoeden et al. Reference Dernoeden, Kaminski and Fu2008).
The development of fine fescue cultivars with improved tolerance levels to mesotrione could improve weed control during establishment. In addition to current breeding efforts, it will be important to understand the absorption and translocation of mesotrione in various Festuca spp. to better understand the factors that influence enhanced tolerance levels. The objectives of this research were to quantify the differential tolerance levels of three Festuca spp. to mesotrione and determine differences in mesotrione absorption and translocation associated with injury potential.
Materials and Methods
Plant Material
Nine individual lines (a single genotype propagated vegetatively) were used in all studies. This was done to eliminate any effects from genotypic differences in the plant material that would have been present if multiple genotypes from the same families were used. Three lines each of Chewings, hard, and strong creeping red fescue described below were used in all experiments. The plants were selected from a spaced plant nursery at the Rutgers University turfgrass breeding program research farm in Freehold, NJ. The nursery from which the individual plants were selected contained progenies of a single generation of breeding for increased tolerance to mesotrione. Plants were selected based on a range of visual injury responses following multiple applications of mesotrione. The responses were no bleaching, moderate bleaching, and severe bleaching of foliar tissue of each species. All nine plants were vegetatively propagated by tillers and were kept in a greenhouse and irrigated when required to provide adequate growth for a rate-titration study and an absorption and translocation study.
Mesotrione Rate-Titration Study
Rate-titration experiments were conducted at Rutgers University in New Brunswick, NJ. Nine individual plants of Chewings, hard, and strong creeping red fescue were divided into individual tillers, and a single tiller was planted in 3.8-cm-diameter and 20.5-cm-deep Cone-tainers™ (Stuewe and Sons, Corvallis, OR 97333). Soil was a mixture of sand and peat moss (80:20 v/v). The experiment was conducted in a growth chamber set for 25/15 C (day/night) with a 10-h photoperiod of 400 μmol m−2 s−1 (Environmental Growth Chambers, Chagrin Falls, OH 44022). Plants were kept in the growth chamber for 3 wk to acclimate before being treated. Irrigation was applied as needed to promote growth, and plants were fertigated biweekly (MacroN™ 28-7-14 Sprayable Fertilizer, LESCO, Cleveland, OH 44114). Plants were allowed to reach 7 to 10 tillers before treatment. The treatments for this experiment were 11 rates of mesotrione (0, 17.5, 35, 70, 140, 280, 560, 1,121, 2,242, 4,483, and 8,966 g ai ha−1), all of which included 0.25% v/v nonionic surfactant (Activator 90, Loveland Products, Greeley, CO 80632). All treatments were applied in a spray chamber set to deliver 260 L ha−1. Cone-tainers™ were randomized every 2 d to minimize any chamber effects.
Absorption and Translocation Experiment
Absorption and translocation experiments were conducted at the University of Georgia in Griffin, GA. Three plants of each from three fine fescue species (Chewings fescue, hard fescue, and strong creeping red fescue) were established from plugs in the greenhouse. Individual tillers were then transplanted in Cone-tainers™ with 3.8-cm diameters and 20-cm depths in a greenhouse set for 23/17 C (day/night). Soil was a mixture of sand and peat moss (80:20 v/v). Irrigation was applied as needed to promote growth, and Cone-tainers™ were fertigated weekly (MacroN™ 28-7-14 Sprayable Fertilizer, LESCO). Plants were allowed to develop 4 to 7 new tillers, and were selected for treatments based on size and population uniformity.
Root Absorption of [14C]mesotrione
Plants were removed from greenhouse pots, roots were rinsed to remove soil, and plants were grown hydroponically in a 10-L plastic tank filled with a half-strength Hoagland solution (Hoagland and Arnon Reference Hoagland and Arnon1950). Grasses were placed through holes in the plastic lid that facilitated root submergence in the solution. The tank was covered with aluminum foil to shield roots from light and then placed in a growth chamber (Percival Scientific, 505 Research Drive, Perry, IA 50220) set for 24/14 C (day/night) with a 12-h photoperiod of 350 μmol m−2 s−1. An aquarium pump (Shkerry Aqua, Shanghai Uni-Aqua, Chang Shou Road, Shanghai 200042, China) was used to provide oxygen to the solution.
After 1 wk, tap water was added to the tank to bring the volume back to 10 L. The tank was then spiked with a total of 83 kBq of [14C]mesotrione (109 µCi mg−1, phenyl-ring labeled, 99% chemical purity; Syngenta, Greensboro, NC 27419) plus 1 μM of nonlabeled mesotrione (Tenacity® (4SC), Syngenta) that equaled 3.41 mg of total herbicide (nonlabeled mesotrione = 0.339 mg L−1 or 3.39 mg total; radiolabeled mesotrione = 0.002 mg L−1 or 0.02 mg total). Plants were harvested at 72 h after treatment (HAT), and roots were blotted dry with paper towels. Roots were separated from shoots with shears, and samples were oven-dried for 7 d at 40 C. Samples were then oxidized for 2 min in a biological oxidizer (OX-500, R. J. Harvey Instrument, 11 Jane Street, Tappan, NY 10983), and radioactivity was quantified with liquid scintillation spectroscopy (LSC; Beckman LS 6500®, Beckman Coulter, Fall River, MA 02720). Absorption was determined by dividing the radioactivity recovered by sample dry weight. Translocation was determined by dividing the 14C recovered in shoots by the total radioactivity in the plant (roots plus shoots).
Foliar Absorption of [14C]mesotrione
Grasses were established as described earlier. Grasses selected for treatments were at a 4- to 7-tiller growth stage and were placed in the growth chamber used in the root absorption study. Grasses were acclimated in the growth chamber for 72 h and irrigated as needed to prevent wilting.
The foliar mesotrione uptake was determined following a previously described procedure (McCullough et al. Reference McCullough, de Barreda and Yu2013, Reference McCullough, Yu, Shilling and Czarnota2015, Reference McCullough, Yu, Czarnota and Raymer2016; Sidhu et al. Reference Sidhu, Yu and McCullough2014; Yu et al. Reference Yu, McCullough and Grey2015). Before treatment, the second fully expanded leaf on a selected tiller was covered with Parafilm® (Bemis Company, Neenah, WI 54956). A broadcast treatment of mesotrione (Tenacity® (4SC), Syngenta) was then applied at 0.28 kg ha−1 with a CO2-pressurized sprayer calibrated to deliver 187 L ha−1. Immediately after the broadcast application, the Parafilm® was removed from the second fully expanded leaf, and two 1-μl droplets of [14C]mesotrione (109 µCi mg−1, phenyl-ring labeled, 99% chemical purity; Syngenta) were applied at 165 Bq each with a 10-μl syringe. Formulated mesotrione was added to the spotting solution at 1.5 μg μl−1 to simulate droplets of spray solution. A nonionic surfactant (Activator 90, Loveland Products) was added to the broadcast treatment and radiolabeled solution at 0.25% v/v to facilitate droplet deposition on the leaf surface.
Plants (roots plus shoots) were harvested at 24 or 96 HAT. The treated leaf was excised from shoots with shears and rinsed in a 20-ml glass scintillation vial with 10 ml of methanol. The base of the leaf was held with forceps, and rinsate was applied toward the leaf tip with a 5-ml pipette. This methodology completely removed adsorbed 14C from leaves in pilot experiments. Roots were then separated from shoots with shears, and samples were oven-dried at 40 C for 7 d.
Samples were combusted using the oxidizer and methods described earlier. The entire plant from the 24-h harvest was oxidized. Plant parts (treated leaf, nontreated shoots, and roots) were oxidized separately at the 96-h harvest to quantify translocation of radioactivity. Foliar absorption was quantified by dividing the total radioactivity recovered by the total 14C applied. Translocation was determined by dividing radioactivity recovered in plant parts (treated leaf, nontreated shoots, or roots) from the total radioactivity recovered in the plant. Methanol from leaf rinsate was evaporated from vials in a fume hood, 20 ml of scintillation fluid was then added to vials, and the adsorbed radioactivity was quantified with LSC.
Experimental Design and Data Analysis
The mesotrione rate-titration experiment was conducted as a randomized complete block design with four replications and was conducted twice. Visual percent injury ratings were taken 10, 13, 16, and 21 d after treatment (DAT). A log-logistic regression model was fit to the data, and I50 (mesotrione rate that caused 50% injury) values and 95% confidence intervals were calculated as outlined by Seefeldt et al. (Reference Seefeldt, Jensen and Fuerst1995). Foliar and root absorption experiments were conducted as completely randomized designs with five replications, and both experiments were repeated. Data were subjected to ANOVA with the PROC GLM in SAS (SAS v. 9.3, SAS Institute, Cary, NC 27513). Means were separated with Fisher’s protected LSD test at α = 0.05. Experiment by treatment interactions were not detected, and thus results were pooled over runs.
Results and Discussion
Rate-Titration Experiment
The I50 and 95% confidence intervals were calculated for each line at the 16 DAT rating date (Table 1; Figures 1–3). This date was selected because the highest injury symptoms were detected at this time point. The hierarchical rank of species for mesotrione tolerance from highest to lowest was: hard fescue > Chewings fescue > strong creeping red fescue.
Table 1. Herbicide concentrations to cause 50% (I50) injury and 95% confidence intervals (CI) for a rate titration from 0 to 8,966 g ha−1 of mesotrione on three lines of Chewings fescue, hard fescue, and strong creeping red fescue at 16 d after treatment in a growth-chamber experiment.
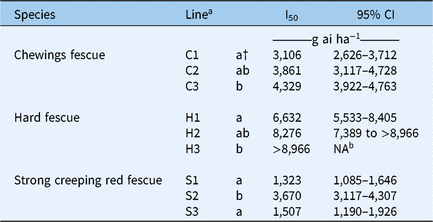
a Lines within species followed by the same letter are not considered statistically different according to Fisher’s protected LSD at α = 0.05.
b Unable to calculate 95% CI due to I50 being greater than the highest rate in the experiment.

Figure 1. Foliar percent injury response curves of three lines of Chewings fescue to 11 rates of mesotrione 16 d after treatment in a growth-chamber experiment.

Figure 2. Foliar percent injury response curves of three lines of hard fescue to 11 rates of mesotrione 16 d after treatment in a growth-chamber experiment.

Figure 3. Foliar percent injury response curves of three lines of strong creeping red fescue to 11 rates of mesotrione at 16 d after treatment in a growth-chamber experiment.
Differential levels of mesotrione tolerance were detected among lines within each species (Figures 4–6). The I50 values for hard fescue lines were >8,966, 8,276, and 6,632 g ha−1 for H3, H2, and H1, respectively (Table 1). The results agreed with the observations made in the field to broadcast applications made to the breeding germplasm nursery in the initial screen and the treatment of the first-generation germplasm. There were a greater number of hard fescue plants with less injury than Chewings and strong creeping red fescue. Chewings and strong creeping red fescue had the greatest number of plants with bleaching injury. The I50 values for the Chewings fescue lines were 4,329, 3,861, and 3,106 g ha−1 for C3, C2, and C1, respectively. Strong creeping red fescues had the most injury, and the I50 values measured 3,670, 1,507, and 1,323 g ha−1 for the S2, S3, and S1 lines, respectively. The wide range of I50 levels indicated that there was some tolerance to mesotrione present in the germplasm. Having variation and higher tolerance present in the germplasm is an indication that the tolerance to mesotrione can be increased using recurrent selection, based on previous research published on increasing fine fescue tolerance to other herbicides.

Figure 4. Foliar injury symptoms at 13 and 21 d after treatment (DAT) of the three lines of Chewings fescue treated with 11 rates of mesotrione from 0 to 8,966 g ai ha−1 + 0.25% nonionic surfactant in a growth-chamber experiment.
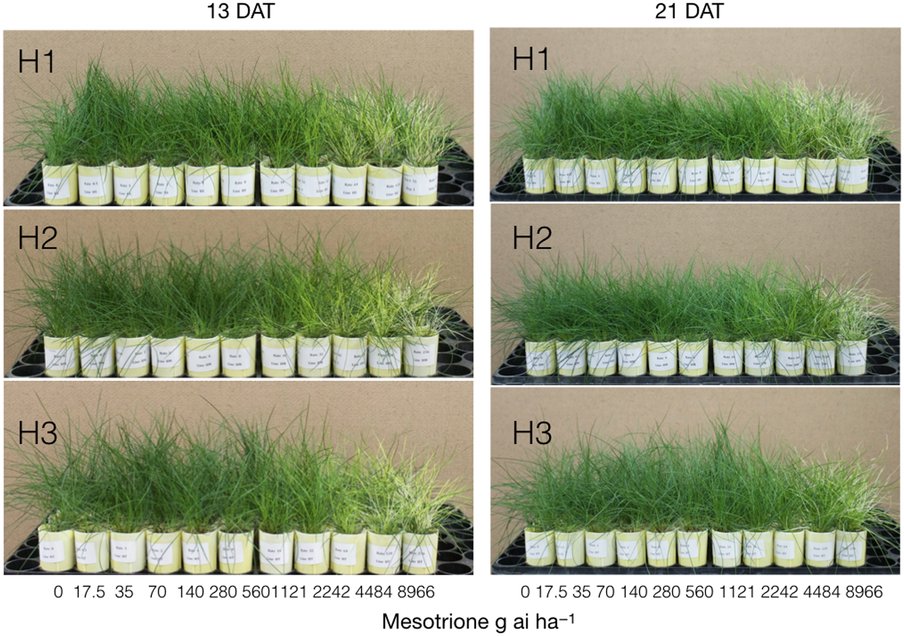
Figure 5. Foliar injury symptoms at 13 and 21 d after treatment (DAT) of the three lines of hard fescue treated with 11 rates of mesotrione from 0 to 8,966 g ai ha−1 + 0.25% nonionic surfactant in a growth-chamber experiment.

Figure 6. Foliar injury symptoms at 13 and 21 d after treatment (DAT) of the three lines of strong creeping red fescue treated with 11 rates of mesotrione from 0 to 8,966 g ai ha−1 + 0.25% nonionic surfactant in a growth-chamber experiment.
Absorption and Translocation Experiment
Total recovery in foliar absorption experiments was 94% (±1.8 SE) of the applied radioactivity. There was no significant effect of line and there was no significant interaction of species by line for foliar absorption (Table 2). There was also no effect for species, line, or species by line interaction for translocation of the foliar-applied [14C]mesotrione. There was a significant effect of species for absorption at both 24 (P < 0.0001) and 96 HAT (P < 0.0001). Chewings fescue absorbed the highest percentage of applied [14C]mesotrione compared with hard fescue and strong creeping red fescue. Foliar absorption for all species at 96 HAT was higher than the levels at 24 HAT. At 24 HAT, hard fescue absorbed 3.1%, strong creeping red fescue absorbed 6.1%, and Chewings fescue absorbed 14.2% of the applied 14C-labeled mesotrione. At 96 HAT, hard fescue absorbed 3.5%, strong creeping red fescue absorbed 6.9%, and Chewings fescue absorbed 19.3% of the applied 14C-labeled mesotrione. Low levels of foliar uptake in hard fescue, compared with the other species in this experiment, may be associated with higher tolerance levels to broadcast mesotrione applications observed in the rate titration, but other factors not tested in this study, such as metabolism and binding-site affinity, need to be evaluated. Low foliar absorption of mesotrione in fine fescue could be associated with leaf surface morphology, such as the thin, rolled nature of the leaves, which may limit retention of spray droplets, cuticular wax thickness, or epicuticular waxes, but further studies would be needed to determine whether the absorption in fine fescues is due to these traits. Previous studies clearly documented that herbicide foliar uptake is associated with leaf properties such as cuticular wax thickness, epicuticular waxes, leaf maturity, and number of stomata (Chachalis et al. Reference Chachalis, Reddy, Elmore and Steele2001; Hess Reference Hess and Duke1985; Sanyal et al. Reference Sanyal, Bhowmik and Reddy2006; Wang and Liu Reference Wang and Liu2007). In addition, researchers have reported that turfgrass maturity (Yu and McCullough Reference Yu and McCullough2016), temperatures (Johnson and Young Reference Johnson and Young2002), and humidity (Johnson and Young Reference Johnson and Young2002; Ramsey et al. Reference Ramsey, Stephenson and Hall2005) influenced mesotrione foliar uptake. In the present study, the low foliar uptake of mesotrione in fine fescues might be associated with these properties and might have influenced the differential tolerance levels among the turfgrasses observed.
Table 2. Foliar absorption and translocation of 14C-labeled mesotrione on three lines each of Chewings fescue, hard fescue, and strong creeping red fescue at 24 and 96 h after treatment (HAT) in a growth-chamber experiment. a

a An asterisk (*) indicates a significant difference at α = 0.05. NS, not significant at α = 0.05.
There was a significant interaction of species and line (P = 0.0045) for root absorption (Table 3). For Chewings fescue, the C1 line (most susceptible) absorbed 38% more 14C-labeled mesotrione than the C2 and C3 lines, which had greater tolerance levels. Similarly, the S1 lines of strong creeping red fescue absorbed 33% more radioactivity (Bq g−1) from root absorption than the more tolerant S2 and S3 lines. Greater absorption of the root-applied 14C-labeled mesotrione in the most susceptible line of Chewings and most susceptible line of strong creeping red fescue do correlate, but further studies are needed to determine whether the differences in root absorption observed in this study are causing the greater bleaching injury to those individual plants. For hard fescues, differences detected among lines for root absorption had dissimilar trends to tolerance levels noted in the rate-titration experiment. The hard fescue H1 had the most injury in the rate-titration study and the lowest absorption of root-applied 14C-labeled mesotrione. Hard fescue generally had the best tolerance levels among the three species, and root absorption does not appear to be associated with trends in injury potential for hard fescue based on these data.
Table 3. Root absorption of 14C-labeled mesotrione on three lines of Chewings fescue, hard fescue, and strong creeping red fescue in a growth-chamber experiment.

a An asterisk (*) indicates a significant difference at α = 0.05. NS, not significant at α = 0.05.
The main effect of species was significant (P = 0.0067) for translocation of root-absorbed radioactivity, while no significant effect of line or interaction of species and line was detected (Table 4). Strong creeping red fescue and Chewings fescue translocated 58% and 56% of absorbed 14C to shoots, respectively, while hard fescue only translocated 44%. Perhaps reductions in acropetal movement of radioactivity from root-absorbed [14C]mesotrione in hard fescues are associated with reduced bleaching and injury potential compared with the more susceptible species, Chewings and strong creeping red fescue, but further studies into the fate and binding affinity of the herbicide once absorbed into the plants is needed before that can be concluded.
Table 4. Translocation of root-absorbed of 14C-labeled mesotrione on three lines of Chewings fescue, hard fescue, and strong creeping red fescue in a growth-chamber experiment.

a An asterisk (*) indicates a significant difference at α = 0.05. NS, not significant at α = 0.05.
Implications for Breeding Mesotrione-Tolerant Fine Fescues
In this study, fine fescues had a wide range of tolerance to mesotrione. The hard fescues had I50 values that ranged from greater than 16X to 11.8X the high label rate of 560 g ha−1 of mesotrione, the Chewings fescues had I50 values that ranged from 7.7X to 5.5X, and strong creeping red fescues had I50 values that ranged from 6.5X to 2.4X. This study demonstrated that after just a single generation of breeding for increased tolerance to mesotrione, the progeny population contained multiple plants of each species with high levels of mesotrione tolerance. Based on these results, we were encouraged that tolerance levels could be further increased using recurrent selection. Previous research demonstrated that recurrent selection to increase tolerance to various herbicides had been successfully implemented with glyphosate in hard fescue (Hart et al., Reference Hart, Derr, Lycan, Rose-Fricker and Meyer2005) and aminotriazole in Chewings fescue (Johnston and Faulkner Reference Johnston and Faulkner1986). Less foliar uptake of mesotrione may be associated with enhanced tolerance of hard fescue to broadcast applications compared with Chewings and strong creeping red fescues, but further studies are needed to determine the fate and binding affinity of the absorbed herbicide before any conclusions about the mechanism of increased tolerance can be made. Root uptake appears to be less consequential for tolerance levels among the species evaluated, but could have a stronger association with injury potential among lines of individual species. Reductions in acropetal movement after root uptake could also be associated with enhanced tolerance levels in fine fescues, such as hard fescue, compared with more susceptible species. Further research is needed to evaluate differential levels of metabolism and target-site inhibition of mesotrione in fine fescues.
Recurrent selection and breeding efforts will continue following the testing of these individual plants from the first generation. The overall goal is for tolerance to be increased to a level where mesotrione applications can be made at seeding without reducing or slowing establishment and not causing bleaching injury in the seedlings. Mesotrione-tolerant Chewings, hard, and strong creeping red fescue cultivars would greatly increase the utility of these grasses, because they would provide an option for controlling Poa annua and many other problematic grassy and broadleaf weeds during establishment.
Acknowledgments
The authors would like to thank the following agencies for funding this research project: Rutgers Center for Turfgrass Science and the New Jersey Agricultural Experiment Station. No conflicts of interest have been declared.












