INTRODUCTION
Parasites profoundly affect a variety of aspects of host biology. One of the consequences of this is that parasites have been used as biological indicators or tags providing information on their hosts (see Williams et al. Reference Williams, MacKenzie and McCarthy1992 and MacKenzie, Reference MacKenzie and Rhode2005 for reviews). In particular, parasites have often been used as tags to distinguish between host stocks (e.g. Beverly-Burton, Reference Beverley-Burton1978; Arthur and Albert, Reference Arthur and Albert1993) or to study host habitat use (e.g. Durieux et al. Reference Durieux, Bégout, Pinet and Sasal2010). The rationale for the latter studies is related to the mediating role of the environment in host parasite interactions. In other words, habitats may differ in their suitability for a given parasite, so that a host can be infected by this parasite only if it occurs at or arrives in an appropriate habitat (MacKenzie, Reference MacKenzie and Rhode2005). Parasites have been extensively used as tags for studies on aquatic hosts such as fish (Williams et al. Reference Williams, MacKenzie and McCarthy1992; MacKenzie and Abaunza, Reference MacKenzie and Abaunza1998; MacKenzie, Reference MacKenzie and Rhode2005; Timi, Reference Timi2007) and marine mammals (Balbuena et al. Reference Balbuena, Aznar, Fernández and Raga1995), while the application of parasites as tags for terrestrial hosts has been largely neglected (but see Holmstad et al. Reference Holmstad, Holstad, Karbol, Revhaug, Schei, Vandvik and Skorping2004).
Environmental mediation of interactions between parasites and terrestrial hosts is well known (Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Vatschenok and Khokhlova1997). This is especially true for ectoparasites such as fleas and gamasid mites that spend much time off-host and are strongly affected by the off-host environment (Radovsky, Reference Radovsky and Kim1985; Krasnov, Reference Krasnov2008). As a result, a substantial part of an ectoparasite community encountered on a terrestrial host is determined by its specific location and environment (Kennedy and Bush, Reference Kennedy and Bush1994), so that conspecific hosts occurring in different habitats may harbour distinct ectoparasite assemblages (Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Khokhlova and Vatschenok1998, Reference Krasnov, Stanko, Miklisova and Morand2006). Consequently, habitat occupancy of an individual host can be predicted based on composition of its ectoparasite infracommunity and relative abundance of species comprising this infracommunity. To the best of our knowledge, this has never been tested.
Furthermore, the degree of habitat dependency of ectoparasite infracommunity composition may be affected by a variety of factors. In particular, it may differ between ectoparasite taxa, host genders and seasons. Indeed, parasite taxa differ in their sensitivity to environmental factors and the ‘intimacy’ of the relationships with their hosts (Marshall, Reference Marshall1981), so that the proportion of time spent off-host differs among ectoparasite taxa (Lehane, Reference Lehane2005). Obviously, success in the prediction of host habitat occupancy is expected to be higher in parasites that spend relatively more time off-host, so that species composition of these parasites is greatly affected by the off-host environment. Behavioural differences between host individuals may also affect the probability of the success of correctly predicting habitat occupancy from ectoparasite species composition. For example, males of the majority of mammals are more mobile than females (e.g. Madison, Reference Madison1980), while females of many species rarely leave the vicinity of their burrows (e.g. Rose and Dueser, Reference Rose and Dueser1980). Consequently, males are likely ‘better’ samplers of ectoparasites characteristic for a given habitat, so that prediction of habitat occupancy based on the entire ectoparasite community composition is expected to be more successful for males than for females. Finally, the degree of climatic difference between habitats as well as between-gender difference in host spatial behaviour may vary seasonally (e.g. Korn, Reference Korn1986). As a result, the success of prediction of habitat occupancy of a host individual using ectoparasite tags may also differ between seasons.
In this study, we used data on species composition and relative abundance of 2 taxa of ectoparasitic arthropods (fleas and gamasid mites) harboured by individual hosts belonging to 8 small mammalian species (4 mice, 3 voles and 1 shrew) from central Europe. Each of these host species occurs in 2 distinct major habitat types, namely lowland habitats and mountain habitats. We tested the hypotheses that (a) species composition of ectoparasite infracommunities may be used to predict whether a host individual belongs to either a lowland or a mountain population and (b) the success in prediction of host habitat occupancy using ectoparasite tags differs between ectoparasite taxa, host genders and seasons.
Adult fleas are obligate haematophages. In most flea species, pre-imaginal stages of the life cycle occur off the host, while the adults feed intermittently on the host. In contrast, gamasid mites vary enormously in their ecology and feeding modes. Although we focused on mites collected from host body surfaces, they included facultative and obligatory haemato- and/or lymphophagous as well as phoretic mites. Consequently, we expected a better prediction of host habitat occupancy for mite than for flea tags. We also expected that habitat occupancy of male hosts would be predicted more accurately than that of female hosts because of the aforementioned reasons. Regarding seasonal effects, we expected that the accuracy of prediction of host habitat occupancy using ectoparasite tags would be higher during a warmer (summer) than during a colder (winter) season due to smaller between-habitat environmental differences in winter.
In this study, we used a Random Forests (RF) algorithm of data mining (Breiman, Reference Breiman2001). This approach is based on the methodology of classification trees (Breiman et al. Reference Breiman, Friedman, Olshen and Stone1984) and has been successfully used in medical genetics (Bureau et al. Reference Bureau, Dupuis, Falls, Lunetta, Hayward, Keith and Van Eerdewegh2005), clinical epidemiology (Mazzone et al. Reference Mazzone, Hammel, Dweik, Na, Czich, Laskowski and Mekhail2007), landscape epidemiology (Furlanello et al. Reference Furlanello, Neteler, Merler, Menegon, Fontanari, Donini, Rizzoli, Chemini, Hornik, Leisch and Zeileis2003) and conservation studies (Prasad et al. Reference Prasad, Iverson and Liaw2006; Girardello et al. Reference Girardello, Griggio, Whittingham and Rushton2010; He et al. Reference He, Wang, Lek-Ang and Lek2010). Recently, it has also been introduced to studies involving communities of parasites (Perdiguero-Alonso et al. Reference Perdiguero-Alonso, Montero, Kostadinova, Raga and Barrett2008; Pérez-del-Olmo et al. Reference Pérez-del-Olmo, Montero, Fernández, Barrett, Raga and Kostadinova2010). We believe that these studies are the only examples of the application of RF to parasitological data. They used RF as a tool for discriminating populations of marine fish using data on parasites. RF has never been applied to studies of parasite communities on terrestrial hosts.
MATERIALS AND METHODS
Sampling of mammals and ectoparasites
Mammals were sampled and ectoparasites were collected between 1983 and 2001 in 18 locations across Slovakia (see details and maps reported by Stanko Reference Stanko1987, Reference Stanko1988, Reference Stanko1994 and Stanko et al. Reference Stanko, Miklisova, Gouy De Bellocq and Morand2002). Mammals were captured using snap-traps that were set following the same protocol at each of 264 trapping sessions (on average, 700 traps per session, ranging from 100 to 2000 traps and lasting from 1 to 3 nights). Each trapped animal was identified, sexed and weighed. The animal's fur was combed thoroughly, using a toothbrush, over a plastic pan and ectoparasites (fleas, mites and ticks) were collected. Trapping was done in 2 main habitat types, namely lowland and mountain habitats (see detailed description by Krasnov et al. Reference Krasnov, Stanko, Miklisova and Morand2006). In brief, lowland habitats were situated at elevations between 100 and 200 m a.s.l. and included river valleys, woodland belts, agricultural fields and shrubbery. Mountain habitats were situated at elevations from 300 to 1100 m a.s.l. and included submontane and montane brook valleys, submontane and montane forests and shrubbery patches on alpine pastures. The mean July and January temperatures in the lowlands are 20°C and −4°C, respectively; while in the mountains they are 14–17°C and −5 to −7°C, respectively (Mazur and Jakal, Reference Mazur and Jakal1982). The mean annual rainfall is 800–1000 mm in the mountains and 550–560 mm in the lowlands (Mazur and Jakal, Reference Mazur and Jakal1982).
We used data on fleas and mites collected from individual mammals infested by at least 1 ectoparasite and belonging to 8 common host species including 7 rodents (Apodemus agrarius, Apodemus flavicollis, Apodemus uralensis, Apodemus sylvaticus, Microtus arvalis, Microtus subterraneus and Myodes glareolus) and a shrew (Sorex araneus). In total, our dataset comprised data on 16 250 individual fleas belonging to 19 species and 67 530 individual mites belonging to 92 species collected from 5701 and 7999 individual hosts, respectively (see lists of flea and mite species given by Stanko et al. Reference Stanko, Miklisova, Gouy De Bellocq and Morand2002 and Krasnov et al. Reference Krasnov, Stanko, Khokhlova, Shenbrot, Morand, Korallo-Vinarskaya and Vinarski2011; see also Mašán and Fenda, Reference Mašán and Fenda2010 for references on mites). Voucher specimens are deposited in the collection of Institute of Zoology of the Slovak Academy of Sciences (Kosice, Slovak Republic) and can be obtained from M.S. upon request. ‘Summer’ and ‘winter’ datasets included data on hosts and parasites captured and collected from April to September and from October to March, respectively. The ‘winter’ dataset did not include data on A. sylvaticus and M. subterraneus because only a few individuals were captured during the cold season.
Analytical approach
The Random Forests (RF) approach represents construction of predictive classification models by combining multiple classification trees. Detailed description of the algorithm can be found in a variety of sources (Breiman et al. Reference Breiman, Friedman, Olshen and Stone1984; Breiman, Reference Breiman2001; Cutler and Stevens, Reference Cutler and Stevens2006; Cutler et al. Reference Cutler, Edwards, Beard, Cutler, Hess, Gibson and Lawler2007; Siroky, Reference Siroky2009). In brief, a classification tree is constructed using a rule-partitioning algorithm in which the data are classified via recursive splitting of the dataset into subsets such that the homogeneity within each subset is maximized in terms of the response variable (Breiman et al. Reference Breiman, Friedman, Olshen and Stone1984). Obviously, in each tree, a class (vote) is assigned to each observation. These assignments of individual trees are combined by majority voting. In other words, each observation receives a predicted class according to the majority of votes.
In building each tree, a randomly selected subset of observations (with replacement) and a random subset of the predictor variables are used. Usually, the number of predictor variables in this subset is equal to the square root of the entire number of predictor variables. Subsets of the observation are of the same size for each tree and represent bootstrap samples. In a bootstrap sample, about two-thirds of the original observations occur at least once and are called bagged observations. The remaining observations that do not occur in a bootstrap sample represent out-of-bag (OOB) observations. Random subsets of the predictor variables and of the observations result in high diversity among the individual trees. Classification trees are fit to each bootstrap (bagged) sample, while OOB samples are then used to test the predictions obtained from the bagged samples. Estimation of the prediction error rate (=OOB error rate) is thus achieved by contrasting the OOB predictions with the bagged sample outcomes. The OOB estimation of error rate is considered highly accurate because the OOB observations are not used in the fitting of the trees.
RF has several advantages over other classification methods, although some of its limitations have been reported (Thomassen et al. Reference Thomassen, Cheviron, Freedman, Harrigan, Wayne and Smith2010). In particular, this method can handle very large datasets that include both continuous and categorical predictor variables. In addition, it is nonparametric and, thus does not require normality of the data. It is robust to overfitting, multicollinearity among predictor variables and the presence of many zero values. The latter is especially important for parasitological data.
Data analyses
We applied RF to estimate success in prediction of the occurrence of a host individual in either a lowland or a mountain habitat based on species composition and number of either flea or mite species. First, we constructed datasets separately for each host species, each parasite taxon and each season. Datasets for each host species were constructed either separately for male and female hosts or by pooling data on both genders together. Following Pérez-del-Olmo et al. (Reference Pérez-del-Olmo, Montero, Fernández, Barrett, Raga and Kostadinova2010), each data set was randomly split into a training dataset (80% of observations) and an independent validation dataset (20% of observations). The configuration for building RF models included 500 trees and default number of a randomly selected set of predictor variables (square root of the total number of predictor variables). Preliminary analyses demonstrated that an increase in the number of trees did not improve the model. We developed predictive RF models using the data from the training datasets only and estimated the error rate on (a) the internal OOB datasets (OOB error rate) and (b) the validation datasets (overall error). In addition, we estimated a model performance by calculating Area Under the Curve (AUC) of a Receiver Operating Characteristic (ROC) plot (Fielding and Bell, Reference Fielding and Bell1997) for the validation set. An ROC plots true positives against false positives. A better model is one with larger AUC. RF models were developed using the Random Forest package (4·5–36) (Liaw and Wiener, Reference Liaw and Wiener2002) implemented in R software environment (R Development Core Team, 2009) via rattle (2.5.44) Graphic User Interface (Williams, Reference Williams2009). In datasets with sharply different numbers of host individuals captured in 2 habitats, we down-sampled the larger class (with replacement) to grow each tree using the samsize option in Random Forest.
For each dataset, we constructed a confusion matrix and counted the number of correctly and incorrectly classified observations (e.g. host individuals). Then, we used these counts to evaluate the combined rate of correct prediction of host habitat occupancy across host species for female hosts, male hosts or both genders together and separately for each parasite taxon, each season and each habitat type using meta-analyses. To evaluate the effects of host gender, parasite taxon and season on the accuracy of prediction of host habitat occupancy from data on ectoparasite species composition, we applied the meta-analyses of the odds ratios across host species (a) within parasite taxon and season between male and female hosts; (b) between parasite taxa within seasons and (c) within parasite taxon between seasons. The two latter analyses were carried out for datasets that combined data on male and female hosts (see Results section). All meta-analyses used a fixed effects model because we assumed that some common factors underlay habitat distribution of fleas and/or mites in every host species. Meta-analyses were carried out using computer program Comprehensive Meta-Analysis 2.2 (Biostat Inc., Englewood, NJ, USA).
RESULTS
Error rates of prediction of host habitat occupancy from data on species composition of flea and mite infracommunities using the RF approach and results of both internal (OOB error rate) and external (AUC) model validation are presented in Tables 1 and 2. In general, relatively high values of overall error and OOB error rates indicated low accuracy of predictions. Among the total of 84 RF models, the AUC values for only 32 were higher than 0·70, showing that about two thirds of the models either failed to discriminate between individuals occurring in different habitats or demonstrated only poor discriminating ability, while the performance of the remaining models could be classified as fair, good or even excellent (Swets, Reference Swets1988). In a few cases, values of AUC were below 0·50 suggesting that these models tended to predict the occurrence of an individual host in, for example, the lowlands, while in reality it was captured in the mountain habitats (Elith and Burgman, Reference Elith, Burgman, Scott, Heglund, Morrison, Haufler, Raphael, Wall and Samson2002). Furthermore, accuracy in prediction of host habitat occupancy varied among host species being highest for summer flea assemblages in A. sylvaticus and lowest for summer mite assemblages in A. uralensis.
Table 1. Error rates of prediction of host habitat occupancy from data on species composition of flea infracommunities and the results of classification validation using training (OOB error rate) and validation (AUC) datasets

Table 2. Error rates of prediction of host habitat occupancy from data on species composition of mite infracommunities and the results of classification validation using training (OOB error rate) and validation (AUC) datasets

The rate of correct prediction of host habitat occupancy varied among host species, being highest for both flea and mite assemblages in A. sylvaticus in summer and lowest for flea assemblages in M. subterraneus in summer and mite assemblages in S. araneus in winter (Figs 1 and 2). Despite this high variation, both within-host and combined rates of the correct prediction of host habitat occupancy from data on ectoparasites were significantly higher than 50%, albeit not being especially high (Table 3, Figs 1 and 2).

Fig. 1. Rate of correct classification (RCC) of host habitat occupancy from the data on species composition of flea infracommunities in summer (a) and winter (b) using Random Forests approach. CI, confidence intervals; T, correct classification; F, incorrect classification.

Fig. 2. Rate of correct classification (RCC) of host habitat occupancy from the data on species composition of mite infracommunities in summer (a) and winter (b) using Random Forests approach. CI, confidence intervals; T, correct classification; F, incorrect classification.
Table 3. Results of meta-analyses of the rate of correct classification of host habitat occupancy from data on species composition of flea infracommunities using the RF method

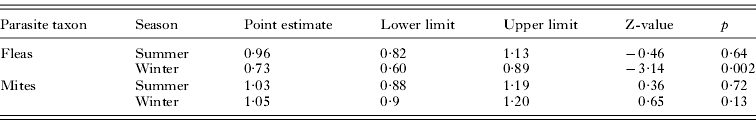
Meta-analyses of the odds ratio of correct prediction of host habitat occupancy demonstrated that the accuracy of prediction did not differ between male and female hosts when this prediction was based on species composition in flea assemblages in summer or on mite assemblages in both seasons (Table 4). However, combined odds ratio indicated slight, albeit significant, male bias in the accuracy of prediction of host habitat occupancy from the data on species composition of flea infracommunities in winter (Table 4).
Table 4. Results of meta-analyses of the odds ratio of host gender differences in the accuracy of prediction of host habitat occupancy from the data on species composition of ectoparasite infracommunities
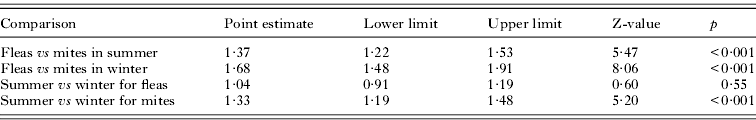
Comparison of the accuracy of prediction of host habitat occupancy between ectoparasite taxa using meta-analyses showed that this accuracy was significantly higher for flea than for mite assemblages in both seasons (Table 5). The effect of season on the accuracy of prediction was found in mites but not in fleas with the accuracy of prediction being significantly higher in summer than in winter assemblages (Table 5).
Table 5. Results of meta-analyses of the odds ratio of differences (a) within-parasite taxon between seasons and (b) within-season between parasite taxa in the accuracy of prediction of host habitat occupancy from the data on species composition of ectoparasite infracommunities
DISCUSSION
Our predictions were only partly supported. Host habitat occupancy appeared to be predictable from data on species composition of ectoparasite infracommunities, although the accuracy of the prediction was low. As we predicted, host habitat occupancy was more accurately predicted for male than for female hosts, although this was true for data on flea assemblages in winter only and for summer than for winter mite, but not flea assemblages. Contrary to our prediction, host habitat occupancy was more accurately predicted for flea than for mite data.
Can ectoparasites be used as tags to distinguish between populations of small mammals?
Earlier application of the RF approach to parasitological data demonstrated high accuracy in distinguishing fish hosts belonging to different populations (80–85% in Perdiguero-Alonso et al. Reference Perdiguero-Alonso, Montero, Kostadinova, Raga and Barrett2008 and 56–94% in Pérez-del-Olmo et al. Reference Pérez-del-Olmo, Montero, Fernández, Barrett, Raga and Kostadinova2010). Our application of RF produced much less encouraging results. On the one hand, the accuracy of prediction of host habitat occupancy was generally higher than 50%. On the other hand, it was generally lower than 70%. In other words, host individuals residing in different habitats harbour somewhat different ectoparasite communities. However, the degree of difference between these communities was not sufficiently high for successful use of ectoparasites as habitat markers. For example, we earlier demonstrated that species composition of flea assemblages in a given small mammalian host species in a given habitat was determined by both host identity and habitat identity, although host identity was a more important factor affecting structure of a flea assemblage compared to habitat identity (Krasnov et al. Reference Krasnov, Stanko, Miklisova and Morand2006). The effect of habitat identity on ectoparasite species composition is likely to be associated with between-habitat differences in environmental variables, such as air temperature, relative humidity, and substrate structure. All these components have a strong effect on survival and development of both fleas and mites (Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001a,Reference Krasnov, Khokhlova, Fielden and Burdelovab; Reference Krasnov, Khokhlova, Fielden and Burdelova2002a,Reference Krasnov, Khokhlova, Fielden and Burdelovab for fleas and Zemskaya, Reference Zemskaya1973 for gamasid mites). Furthermore, different (even closely-related) ectoparasite species often demonstrate differential environmental preferences (e.g. Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001a,Reference Krasnov, Khokhlova, Fielden and Burdelovab). The difference in elevation and, consequently, in air temperature between mountain and lowland habitats may thus be a reason behind different species composition of ectoparasite infracommunities.
Nevertheless, some ectoparasites might be highly host-specific and occur on their host species independent of the habitat where the host resides (Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Vatschenok and Khokhlova1997). In addition, individual small mammals often re-locate between habitats (e.g. Ylönen et al. Reference Ylönen, Altner and Stubbe1991). Obviously, both of these phenomena promote ‘mixing’ of flea assemblages and likely result in some degree of between-habitat within-host similarity in species composition of ectoparasite infracommunities.
Effects of host gender and season
The most likely reason behind higher accuracy in prediction of male than female habitat occupancy from data on ectoparasites is gender difference in spatial behaviour. In many small mammal species, males occupy larger home ranges than females (Ylönen and Mappes, Reference Ylönen and Mappes1995; Horvath and Trócsányi, Reference Horváth and Trócsányi1998; Shenbrot et al. Reference Shenbrot, Krasnov and Khokhlova1997; Gromov et al. Reference Gromov, Krasnov and Shenbrot2000; Stradiotto et al. Reference Stradiotto, Cagnacci, Delahay, Tioli, Nieder and Rizzoli2009; Haapakoski and Ylönen, Reference Haapakoski and Ylönen2010). Males also move over longer distances than females in both summer and winter (Crawley, Reference Crawley1969; Attuquayefio et al. Reference Attuquayefio, Gorman and Wolton1986; Tew and Macdonald, Reference Tew and Macdonald1994; Madison, Reference Madison1980; Fleming and Nicolson, Reference Fleming and Nicolson2004). One of the results of higher mobility and larger home ranges of males is that they are exposed to a larger number of ectoparasite species than females (Brinck-Lindroth, Reference Brinck-Lindroth1968; Lang, Reference Lang1996; Morand et al. Reference Morand, Gouy de Bellocq, Stanko and Miklisova2004; Krasnov et al. Reference Krasnov, Morand, Hawlena, Khokhlova and Shenbrot2005a; Matthee et al. Reference Matthee, McGeoch and Krasnov2010; Krasnov and Matthee, Reference Krasnov and Matthee2010). This, as well as broadly overlapping male home ranges (Ylönen and Mappes, Reference Ylönen and Mappes1995; Stradiotto et al. Reference Stradiotto, Cagnacci, Delahay, Tioli, Nieder and Rizzoli2009), may facilitate horizontal transmission and exchange of ectoparasites among individuals. In other words, ectoparasite infracommunities in male hosts may be more representative samples of an entire ectoparasite assemblage characteristic of a given habitat. In contrast, females are often spatially conservative and, thus, could ‘under-sample’ an ectoparasite assemblage of a habitat. Species-poor infracommunities of female hosts may, thus, confound predictive models.
However, host gender differences in the accuracy of prediction were revealed only for winter datasets and only for fleas. The reason why host gender differences were found for fleas but not for mites is likely related to the finding that fleas represent better markers for host habitat occupancy than mites, as discussed in the next subsection. The reason why host gender differences were found in winter but not in summer might be envisaged from Table 3. In fact, values of meta-analytical estimation of the accuracy of prediction suggests that significant gender difference in predictability of habitat occupancy in winter is a result of generally low predictability in female habitat occupancy in winter as compared to summer, while season had no effect on predictability of male habitat occupancy. This may be a result of interplay between 3 independent patterns. First, environmental similarity between habitats increases in winter (see below), so that flea infracommunities, at least in females, become more similar than is the case in summer. Second, strictly winter-active fleas (e.g. Atyphloceras nuperus, Peromyscopsylla silvatica, Rhadinopsylla integella) occur in mountain but not lowland habitats, although abundance and prevalence of these species is low (Stanko, Reference Stanko1987, Reference Stanko1988). Third, despite a general decrease in size, home ranges of male hosts in winter are still larger than those of female hosts (e.g. Attuquayefio et al. Reference Attuquayefio, Gorman and Wolton1986). Consequently, males may still be better samplers of fleas that females, and thus have higher chances than females to encounter rare fleas such as the above-mentioned winter-active mountain species. Taken together, these patterns explain why (a) predictability of female, but not male, habitat occupancy from flea data differed between seasons and (b) gender differences in the accuracy of prediction of host habitat occupancy was found for winter, but not for summer datasets. However, we recognize that this explanation is highly speculative and requires further investigation.
Higher predictability of host habitat occupancy from summer than winter data may be explained by higher similarity of environmental conditions experienced by ectoparasites during the colder than warmer season. This is because in winter, small mammals (and their ectoparasites) of a temperate zone spend much time in the subnivean space (i.e. between the snow cover and the ground) (Fuller, Reference Fuller1967). Due to poor thermal conductivity of snow, air temperature in the subnivean zone is around 0°C, does not usually depend on the air temperature above snow cover (Davenport, Reference Davenport1992) and thus is similar in both habitat types. The potential reason why seasonal difference in predictability of host habitat occupancy was found for fleas but not mites is explained in the next section.
Fleas versus mites as tags for host habitat occupancy
Host habitat occupancy was consistently better predicted based on flea than on mite data. This was despite substantial difference between fleas and mites in the pattern of their relationships with a host with mites compared to fleas being presumably more strongly affected by the off-host environment. Indeed, many gamasid mites feed not only on the resources extracted from hosts such as blood or lymph but also on small arthropods and nematodes in the host's burrow/nest (Radovsky, Reference Radovsky and Kim1985; Murphy and Sardar, Reference Murphy, Sardar, Schuster and Murphy1991). Moreover, some gamasids that are considered to be obligatory haematophages do not exploit a host directly. They may obtain host's blood via predation on other haematophages (e.g. other mites or larvae of ixodid ticks) (Tagiltsev, Reference Tagiltsev1957; Radovsky, Reference Radovsky and Kim1985). Tagiltsev (Reference Tagiltsev1957) argued that the gnathosoma of some mites did not allow them to pierce skin of a mammal host, whereas they could penetrate the thin cuticle of, for example, an ixodid larva and use the blood extracted from a host by a tick. These mites may also satisfy their requirements for blood by consuming dried blood as well as dead lice or fleas that previously fed on a host (Radovsky, Reference Radovsky and Kim1985; Dowling, Reference Dowling, Morand, Krasnov and Poulin2006). In contrast, adult fleas exploit resources (blood) that they extract directly from hosts. However, larval fleas (except for a few species) feed mainly on organic debris found in the nest/burrow of the host. Furthermore, immature fleas are strongly affected by environmental factors such as air and soil temperature and relative humidity and often respond to subtle differences in these factors (Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001a,b; 2002a,b; 2005b). Obviously, an infracommunity of fleas on a given host individual in a given location is composed of species that not only, as imago, find this host to be suitable but also, as pre-imago, find an environment of this location to be tolerable. In other words, a relatively high accuracy of prediction of host habitat occupancy from data on flea species composition could be associated with strong environmental effects on immature fleas.
An additional reason behind the weaker predictability of host habitat occupancy from data on mite species composition may be related to their dispersal. Despite the limited abilities of gamasid mites for active dispersal their passive dispersal (e.g. phoresy on various mammals, birds and insects) supposedly helps them to overcome rather long distances (Tagiltsev, Reference Tagiltsev1967; Mašán and Halliday, Reference Mašán and Halliday2010). The possibility of aerial dispersal similar to that reported for other mite taxa (e.g. Jung and Croft, Reference Jung and Croft2001) cannot be ruled out, although this has never been reported for ectoparasitic mite species. Consequently, mites supposedly rely on their hosts to a lesser degree than adult fleas and are able to move passively over long distances, crossing between habitats. As a result, this would make mites inferior tags compared to fleas with respect to host habitat occupancy despite that members of some mite taxa (e.g. Laelapidae) have been found to demonstrate some degree of habitat preference (Mašán and Fenda, Reference Mašán and Fenda2010). In addition, weaker ties between hosts and mites compared to adult fleas suggest that mites are more exposed to the off-host environment than imago fleas. This may explain why (a) host gender differences in predictability of host habitat occupancy were found for flea but not mite data and (b) seasonal differences in this predictability were found for mite but not flea data.
CONCLUSION
In general, results of this study suggested that ectoparasites were not especially useful as biological markers for distinguishing host populations in different habitats in temperate zones of Europe. However, ectoparasites could be more useful habitat tags in other geographical areas. Indeed, among-habitat differences in flea assemblages within a host species in central Europe are much less pronounced than those in Middle Eastern deserts (Krasnov et al. Reference Krasnov, Stanko, Miklisova and Morand2006vs Krasnov et al. Reference Krasnov, Shenbrot, Medvedev, Khokhlova and Vatschenok1998). Flea assemblages of some desert hosts in different habitats were found to be composed, at least seasonally, of completely different species, while flea assemblages of the same host in different habitats of Slovakia differed by relative abundances of fleas rather than by their species assortment. Consequently, we believe that applicability of ectoparasites as biological markers should be tested in other biomes.
ACKNOWLEDGEMENTS
Alan Degen, Lajos Rozsa and an anonymous referee read the earlier version of the manuscript and made helpful comments. This study was supported by the Slovak Research and Development Agency (grant SK-FR-0007-09 for M.S.), the French-Slovak PHC STEFANIK ‘Ecology of mammal ectoparasites’ for Slovakia (for M.S. and S.M.) and partly by the Israel Science Foundation (grant 27/08 to B.R.K.). This is publication no. 720 of the Mitrani Department of Desert Ecology.









