INTRODUCTION
A heterogeneous group of rare neurological disorders, autosomal recessive cerebellar ataxias (ARCA), encompass a large number of diseases resulting in abnormal development (e.g., childhood cataracts) and/or degeneration of central and peripheral nervous systems (Palau & Espinós, Reference Palau and Espinós2006). Perhaps the most recognized of these is Friedreich ataxia, though there are many lesser known entities that fall into one of five broad categories based on clinicogenetic criteria: congenital, metabolic, DNA repair defect, degenerative, and ataxia associated with other features. One such ARCA falling under the metabolic category is cerebrotendinous xanthomatosis (CTX).
Disease Mechanism and Pathology
CTX is characterized by mutation in the sterol 27-hydroxylase gene (CYP27). There are roughly 50 different mutations of the CYP27 gene that have been identified in patients with CTX. The CYP27 gene plays a role in the encoding of a mitochondrial cytochrome responsible for the biosynthesis of sterols into the bile acids necessary for digestion and breakdown of fats (Cali, Hsieh, Francke, & Russell, Reference Cali, Hsieh, Francke and Russell1991; Duell et al., Reference Duell, Salen, Eichler, DeBarber, Connor, Kisanuki, Lekprasert, Malloy, Ramdhani, Ziajka, Quinn, Su, Geller, Diffenderfer and Schaefer2018; Palau & Espinós, Reference Palau and Espinós2006). In the case of CTX, a lack of key enzymes in the body’s main pathway for cholesterol removal results in an unchecked increase in circulating cholestanol, a derivative of cholesterol (Cali et al., Reference Cali, Hsieh, Francke and Russell1991). Distinctively, patients with CTX have extremely high concentrations of cholestanol found in the plasma and virtually all tissues, including nerve tissue and the brain (Bhattacharyya, Lin, & Connor, Reference Bhattacharyya, Lin and Connor2007). Compounded over time, the buildup of cholestanol is suspected to result in large-scale neural network disruption via a combination of myelin sheath degeneration, blood–brain barrier dysfunction, and cortical hypoperfusion (Inoue, Kubota, & Seyama, Reference Inoue, Kubota and Seyama1999; Mukaino, Tsuda, Yamashita, Kosaka, Wada, & Ando, Reference Mukaino, Tsuda, Yamashita, Kosaka, Wada and Ando2018). The absence of plasma cholestanoic acids, which have been found to have neuroprotective effects, also likely contributes to the neuropathology of CTX (Theofilopoulos et al., Reference Theofilopoulos, Griffiths, Crick, Yang, Meljion, Ogundare, Kitambi, Lockhart, Tuschl, Clayton, Morris, Martinez, Reddy, Martinuzzi, Bassi, Honda, Mizuochi, Kimura, Nittono, De Michele, Carbone, Criscuolo, Yau, Seckl, Schüle, Schöls, Sailer, Kuhle, Fraidakis, Gustafsson, Steffensen, Björkhem, Ernfors, Sjövall, Arenas and Wang2014).
Currently considered a rare disease impacting less than five out of every 100,000 individuals worldwide, the prevalence of CTX is nonetheless suspected to be greater considering the frequency of mutation in the CYP27 gene ranges from 1/50,000 to 1/800,000 differing significantly by ethnic groups (Berginer, Abeliovich, & Opitz, Reference Berginer, Abeliovich and Opitz1981; Lorincz, Rainier, Thomas, & Fink, Reference Lorincz, Rainier, Thomas and Fink2005). Like many of the ARCAs, CTX symptomology can begin in childhood or even infancy: jaundice with bouts of unexplained diarrhea give way to seizures and learning delays. Adolescent years bring the potential for cataracts, early cardiovascular disease, and fatty tissue deposits known as xanthomas that begin in peripheral tendons but progress over time to the central nervous system (i.e., cholesterol-rich fatty deposits dispersed throughout the brain). By adulthood, many individuals with CTX exhibit peripheral neuropathy, myopathy, ataxia, dystonia, Parkinsonism, epilepsy, neuropsychiatric issues, and frank neurocognitive impairment (Cali et al., Reference Cali, Hsieh, Francke and Russell1991; Moghadasian, Reference Moghadasian2004; Stelten, van de Warrenburg, Wevers, & Verrips, Reference Stelten, van de Warrenburg, Wevers and Verriprs2018). Unfortunately, the systemic nature of CTX results in a diversity of symptoms that frequently escape clinical detection until well into adulthood when the disease is substantially advanced. Moreover, some individuals may not experience the typical childhood ailments making it more difficult to diagnose. A review by Mignarri, Gallus, Dotti, and Federico (Reference Mignarri, Gallus, Dotti and Federico2014) reported that chronic unexplained diarrhea and frequent referral to pediatricians was present in only about half of patients later diagnosed with CTX. At present, the mean age of diagnosis is 32 years with a typical diagnostic delay of 16 years (Duell et al., Reference Duell, Salen, Eichler, DeBarber, Connor, Kisanuki, Lekprasert, Malloy, Ramdhani, Ziajka, Quinn, Su, Geller, Diffenderfer and Schaefer2018; Mignarri et al., Reference Mignarri, Gallus, Dotti and Federico2014; Salen & Steiner, Reference Salen and Steiner2017). If undiagnosed and untreated, death is anticipated in the fifth or sixth decade of life (Duell et al., Reference Duell, Salen, Eichler, DeBarber, Connor, Kisanuki, Lekprasert, Malloy, Ramdhani, Ziajka, Quinn, Su, Geller, Diffenderfer and Schaefer2018).
Evaluation and Intervention
Diagnosis of CTX is made on the basis of either biochemical or clinicogenetic assessments. Typically, these assessments are supplemented with cerebrospinal fluid sampling and neuroimaging. Biochemical assay would demonstrate normal plasma cholesterol levels but abnormal serum levels of cholestanol, the derivative of cholesterol (>10.0 mg/l; Duell et al., Reference Duell, Salen, Eichler, DeBarber, Connor, Kisanuki, Lekprasert, Malloy, Ramdhani, Ziajka, Quinn, Su, Geller, Diffenderfer and Schaefer2018). Genetic testing would demonstrate the suspected mutation of the CYP27 gene (Verrips et al., Reference Verrips, Hoefsloot, Steenbergen, Theelen, Wevers, Gabreëls, van Engelen and van den Heuvel2000). Cerebrospinal fluid analysis indicates elevated levels of cholestanol as well as cholesterol, apolipoprotein B fragments, apolipoprotein A1, and albumin (Salen et al., Reference Salen, Berginer, Shore, Horak, Horak, Tint and Shefer1987). Neuroimaging is helpful for illustrating the extent of central nervous system progression. Magnetic resonance imaging (MRI) characteristically includes cerebellar atrophy as well as hyperintense white matter lesions in deep cerebellar nuclei and bilateral cerebrum. MRI evidence of dentate nuclei signal alterations is considered a strong diagnostic indicator, though it appears relatively late in disease progression (Mignarri et al, Reference Mignarri, Dotti, Federico, De Stefano, Battaglini, Grazzini, Galluzzi and Monti2017). For individuals who demonstrate significant cerebellar and/or pyramidal signs, MRI findings also reveal damage to the corticospinal tracts as they are particularly vulnerable to degeneration and metabolic damage (Mignarri et al., Reference Mignarri, Rossi, Ballerini, Gallus, Del Puppo, Galluzzi, Federico and Dotti2011). Although considered a non-specific disease marker, MR spectroscopy can be helpful and typically reveals lipid peaks between 0.9 and 1.3 ppm, which are not detected in normal tissue (Embirucu, Otaduy, Taneja, Leite, Kok, & Lucato, Reference Embirucu, Otaduy, Taneja, Leite, Kok and Lucato2010). Lipid peaks in this range are fairly non-specific and can indicate either breakdown of membrane or disease (e.g., tumor), but can also be a marker of lipid storage that occurs with CTX (Embirucu et al., Reference Embirucu, Otaduy, Taneja, Leite, Kok and Lucato2010). The interested reader is referred to Salen and Steiner (Reference Salen and Steiner2017) for an in-depth discussion of diagnostic tests.
The current first-line treatment approach for CTX involves prescribed administration of chenodeoxycholic acid (CDCA) to reduce circulating cholestanol levels. Research indicates that CDCA can achieve a threefold reduction in cholestanol levels within blood plasma as well as reduce cerebrospinal fluid levels by one-third (Salen et al., Reference Salen, Berginer, Shore, Horak, Horak, Tint and Shefer1987). CDCA is most effective at slowing disease progression and preventing neurological degeneration when initiated in childhood (Mignarri et al., Reference Mignarri, Gallus, Dotti and Federico2014). More commonly, treatments remain palliative due to delayed intervention, and substantial disease progression when diagnosis is made in adulthood. A recent multicenter review of 43 patients with CTX indicated symptom stabilization or slight improvement in 57% of cases, though 20% of cases with advanced disease continued to deteriorate (Duell et al., Reference Duell, Salen, Eichler, DeBarber, Connor, Kisanuki, Lekprasert, Malloy, Ramdhani, Ziajka, Quinn, Su, Geller, Diffenderfer and Schaefer2018). Other treatments include pravastatin, a 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitor, or a combination of both CDCA and pravastatin (Nie, Chen, Cao, & Zhang, Reference Nie, Chen, Cao and Zhang2014) in an attempt to lower sterol concentrations.
Recognition of clinical neuropsychology as a valued healthcare profession continues to grow, resulting in increased referrals from specialty clinics as well as increased opportunities to participants on transdisciplinary treatment teams. Low awareness of CTX within the neuropsychology community increases risk in the differential diagnostic process as these patients could easily be mistaken for a range of other etiologies, including Alzheimer’s disease, frontotemporal lobar degeneration, Parkinson-plus syndromes (e.g., corticobasal degeneration, multiple systems atrophy), multiple sclerosis, or even psychiatric illness.
Neurodegeneration is a recognized manifestation of CTX; however, there is scant literature to characterize the nature of cortical symptoms and even less detailing of its associated neurocognitive and neuropsychiatric manifestations (Mukaino et al., Reference Mukaino, Tsuda, Yamashita, Kosaka, Wada and Ando2018). Neuropsychologists could contribute much to the study and care of this population. Based on the current lack of representation of CTX in current neuropsychological literature, we sought to present a case seen within our own clinic. It is our hope that this not only spurs awareness of this condition but increases interest in a more systematic approach to research and clinical care of this population.
METHODS
The present study involves an N = 1 single-case design. This individual, hereafter referred to as Mr. X, provided written informed consent for case study presentation. All consenting procedures and documentation were conducted in adherence with our institutional review board and professional ethical standards. Mr. X was diagnosed with CTX by his neurologist and immediately referred for a comprehensive neuropsychological evaluation to assess his current cognitive functioning, assist with treatment recommendations, and direct education efforts with the patient and his family. As part of the evaluation process within our transdisciplinary section, patients may be identified as appropriate for other aspects of care such as neuropsychiatry and/or individual psychotherapy referral. This was the case for Mr. X, which allowed continuity of care as well as longitudinal observation up until his untimely death at age 41.
Case History
Mr. X was a 39-year-old, right-hand-dominant, Caucasian gentleman who was referred for outpatient neuropsychological evaluation at the request of his treating neurologist. He was monolingual, with the primary language being English. He obtained a master’s degree in special education, and he described himself as a “fair” student without any history of learning disorder or intellectual concerns. His occupational background involved serving as a special educational aide, which allowed for a more flexible schedule to take care of his children. Mr. X was unemployed after having been let go due to concerns for inappropriate behavior (e.g., standing in too close proximity to others, including small children). He was serving as the primary caretaker for his two young children, both well under the age of 10.
Biochemical and genetic analysis
A formal diagnosis of CTX was ascertained after biochemical analysis revealed a cholestanol level of 33.7 mg/ml. This is well over the cutoff for abnormality (i.e., >10.0 mg/l; Duell et al., Reference Duell, Salen, Eichler, DeBarber, Connor, Kisanuki, Lekprasert, Malloy, Ramdhani, Ziajka, Quinn, Su, Geller, Diffenderfer and Schaefer2018) and nearly 10 times the normal reference (0.0–0.50 mg/ml). Serology analysis was consistent with a sterol-27-hydroxylase defect, and further genetic testing confirmed CYP27A1 mutation. He was subsequently placed on 1000 mg CDCA, which inhibits cholestanol synthesis, to help normalize plasma cholestanol levels.
Neuroimaging
An initial MRI completed in 2012 demonstrated symmetric hyperintense T2/FLAIR areas surrounding the bilateral inferior cerebellum and in the bilateral frontal white matter (Figure 1). Also noted were a series of cysts near the anterior left lateral ventricle and left middle cranial fossa, and right frontal and parietal scalp fibromas. MR spectroscopy revealed lipid peaks ranging from 0.9 to 1.3 ppm. A follow-up MRI completed in 2013 was deemed stable. Note that Mr. X underwent neuropsychological evaluation in late 2016, the findings of which are outlined below. A subsequent MRI in 2017 was commensurate with evaluation findings, indicating substantial progression from his last study (Figure 1). Later findings were remarkable for cerebellar volume loss and xanthoma predominantly in and around the dentate nuclei, with additional FLAIR signal abnormalities noted in deep corticospinal tract, midbrain, thalami, bilateral frontal lobes, periventricular, and subcortical white matter.

Figure 1. Neuroimaging for Mr. X. (a) FLAIR/T2 imaging from 2012 demonstrates symmetric hyperintense signal abnormalities surrounding the bilateral inferior cerebellum and in the bilateral frontal white matter. (b) FLAIR/T1 imaging from 2017 demonstrates cerebellar volume loss predominantly around the dentate nuclei with additional FLAIR signal abnormalities noted in the deep corticospinal tract, midbrain, thalami, bilateral frontal lobes, periventricular, and subcortical white matter.
Record review
Medical background included infantile jaundice, essential hypertension, obstructive sleep apnea with CPAP adherence, vitamin D deficiency, peripheral neuropathy, and chicken pox. Psychiatric history was denied, though he had recently been involved in marital counseling due to significant marital problems related to his behavioral presentation. Substance use or abuse was denied. Childhood developmental history was reported to be unremarkable. Family history included cardiovascular disease, stroke, and osteoarthritis. More importantly, Mr. X had a history of paternal neurodegeneration. It was reported that his father had been diagnosed with the cerebellar subtype of multiple system atrophy, beginning in the fifth decade of life and with progressive dysphagia, gait instability, and cognitive decline until death at the age of 62. Mr. X had a younger brother with a similar biochemical profile to a CTX diagnosis. There was also unspecified late-life cognitive decline in a paternal grandfather. Family psychiatric history was denied.
In early 2012, Mr. X presented to a community neurologist with complaints of increased fatigue, dizziness, imbalance, and dull aching in his lower extremities. He also reported a vague mental “fogginess” but no other subjective cognitive changes. Results were suspicious for neurodegeneration, particularly in light of reported paternal mortality due to multiple system atrophy.
RESULTS
Neurological Examination
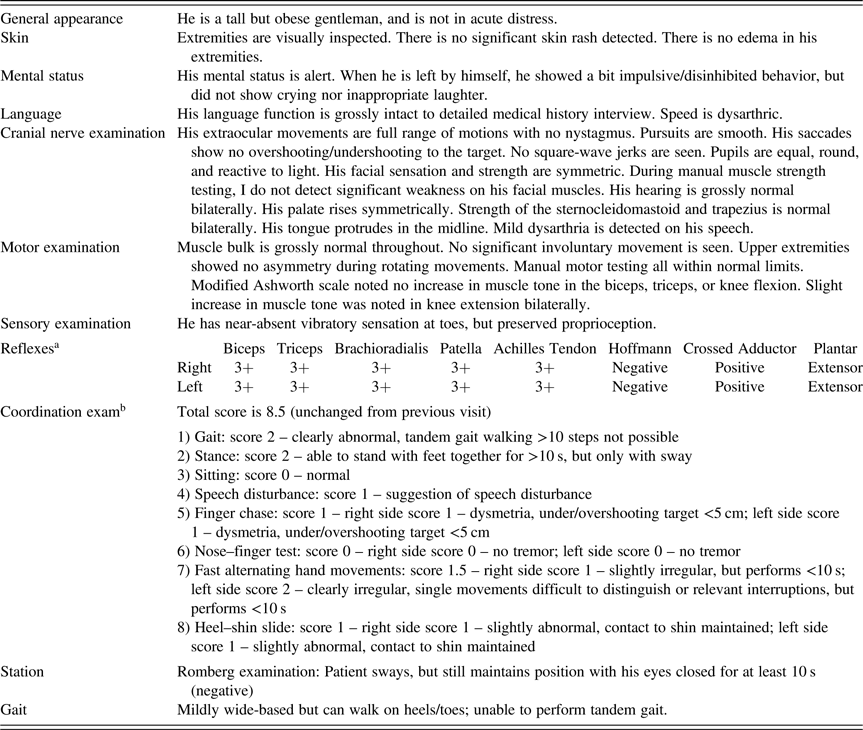
The attending neurologist conducted a neurological exam prior to our evaluation (See Table 1). Findings indicated cerebellar signs, including mild dysmetria, dysarthric speech, and difficulties with tandem gait. Bilateral increase in muscle tone in knee extensions and near-absent vibratory sensation in both toes was also reported. Pyramidal signs were noted, including equivocal deep tendon reflexes for biceps, triceps, brachioradialis, patellar, and Achilles tendons as well as abnormal crossed adductor response and extensor response of the plantar reflex (i.e., positive Babinski response).
Table 1. Neurological exam

a Deep tendon reflexes: 3+ = brisk response, may or may not be normal. Positive crossed adductor and extensor plantar findings are abnormal.
b Scale for the Assessment and Rating of Ataxia: score ranges from 0 (no ataxia) to 40 (severe ataxia).
Neurobehavioral Examination
Mr. X arrived on time to his evaluation and was accompanied by his mother. He ambulated independently, with mildly ataxic gait. His spatial judgment was questionable, as on several occasions he nearly bumped into the examiner or portions of the clinic (e.g., a hall column). There was no visible tremor. Formal neurobehavioral examination conducted by the attending neuropsychologist indicated mild slowing, mild dysmetria, and mild dysdiadochokinesia (i.e., an inability to perform coordinated smooth rapid alternating movements of the hands). Cognitively, Mr. X was alert and fully oriented. He was highly distractible and required significant encouragement to stay on task. While comprehension was intact, his speech was mildly dysarthric, bradyphrenic, and with minimal prosody. Emotionally, Mr. X’s mood appeared irritable and apathetic with flat affect. Mr. X’s insight appeared poor. Stimulus-bound behaviors were observed during testing. For example, on a line bisection task, he had a tendency to be pulled toward the previous bisection and drifted toward one side of the page. He also exhibited impulsivity on the Wisconsin Card Sorting Test, reaching for the next card before feedback was provided on the card previously laid. Mr. X’s scores on embedded and standalone measures of effort were variable with a majority of his scores falling below the acceptable cutoff. Non-credible responding and cognitive impairment can be difficult to disentangle (e.g., Erdodi & Lichtenstein, Reference Erdodi and Lichtenstein2017); Mr. X’s performance was deemed to reflect the extent and severity of his cognitive impairment and not frank malingering.
Neuropsychological Test Findings
Mr. X’s neuropsychological profile was consistent with major neurocognitive disorder given the breadth and depth of impairments in global cognition, spatial and constructional function, attention and executive functioning, visuomotor speed and dexterity/coordination, learning and memory, and expressive language (see Table 2). Interestingly, Mr. X and his mother reported few cognitive concerns during clinical interview, which was striking in the context of his performance on objective testing. Mr. X reported significant levels of prospective and retrospective memory concerns on a questionnaire (Table 3). His mother noted that Mr. X was independent in all instrumental activities of daily living and physical self-maintenance skills. No collateral-report questionnaires were given as his mother adamantly denied all concerns at the start of evaluation. In contrast, during a phone interview with Mr. X’s wife, she indicated decreased ability to care for himself and their two children. She also reported temper outburst in the home and harassment at work prior to his termination.
Table 2. Comprehensive neuropsychological assessment results organized by domain

a Norms from publisher.
b Heaton et al. norms.
c Mitrushina et al. norms.
Table 3. Self-reported impairment

a Norms from publisher.
Recommendations and Follow-up
Referral to our in-house social worker was made where Mr. X engaged in services for six sessions before care was transferred to a provider closer to his home. Targets of treatment included education on cognitive impairment and behavioral modification. The therapist reported that it was necessary to use direct statements and utilized visual aids to help with comprehension and organization. The social worker acted more as an educator, in lieu of a traditional CBT approach. She provided Mr. X additional information on his current functioning and corrected inappropriate behaviors as they occurred in session. Mr. X’s mother participated in some aspects of treatment, which increased her awareness to the extent of Mr. X’s cognitive decline. Overall, Mr. X demonstrated variable insight across therapy sessions.
Unfortunately, Mr. X’s cognitive profile and neuroimaging demonstrated extensive decline and nearly unchanged after pharmacotherapy. For example, a quick assessment of Mr. X’s ability to draw a clock to command demonstrated increased difficulty over a period of just 1 year (Figure 2). He was at the maximally tolerable dose of CDCA with cholestanol levels in the normal range; however, the progression of neurodegeneration was irreversible. A later neurology note indicated worsening upper motor neuron deficits, including spasticity and weakness affecting gait (i.e., he required a four-wheeled walker due to cerebellar decline). Tragically, Mr. X passed away at the age of 41 due to progression of his disease. His neurology team noted that his liver had difficulty tolerating the dose of CDCA needed to keep his serum levels close to normal. Mr. X was increasingly reliant on his mother for all activities. Prior to his death, Mr. X’s mother reported he was experiencing significant disorientation, increased falls, nausea with weight loss, spasticity of most extremities, urinary and bowel incontinence, and insomnia. Symptomatic therapy became the goal of Mr. X’s transdisciplinary treatment team (e.g., psychotropic medications for mood and Botox injections for spasticity). Perhaps encouragingly, Mr. X continued to report his mood was good even with variable insight throughout his final year.

Figure 2. Clock drawing to command by Mr. X over a 1-year span. (a) Mr. X was asked to draw a clock, put in all the numbers, and set the time to 1:45 (Royall, Cordes, & Polk, Reference Royall, Cordes and Polk1998). (b) Mr. X was asked to draw a clock, put in all the numbers, and set the time to 10 past 11.
DISCUSSION
CTX is characterized by a gene mutation that prevents the biosynthesis of cholesterol, which results in deposits of cholestanol throughout the central and peripheral nervous systems. The diffuse nature of these fatty deposits determines the multifarious clinical presentations; previous investigations of neurocognitive functioning in individuals with CTX are limited. While the prevalence of CTX is relatively low, if left untreated, CTX results in progressive neurological symptoms, including irreversible dementia. In the present paper, we sought to increase awareness of the neurocognitive profile of individuals with this rare genetic disease via a detailed case report of a gentleman who presented to our academic medical center with a recent diagnosis of CTX.
Mr. X’s neuroimaging findings are consistent with a CTX diagnosis and importantly notable in neuropsychological testing and on neurobehavioral and neurological examinations. The cerebellum in particular is a major target of involvement in CTX both structurally and functionally. Patients with CTX have extensive and symmetrical grey matter loss in the cerebellar hemispheres and vermis as well as changes in the dentate nuclei and surrounding white matter (Chang et al., Reference Chang, Lui, Wang, Huang, Lu, Chen, Chen, Tu, Huang and Chang2010). Individuals with prominent cerebellar and/or pyramidal signs (e.g., dysdiadochokinesia, dysmetria, slurred speech, spastic paraparesis, hyperreflexia, and/or spasticity) also show corticospinal tract degeneration (Mignarri et al., Reference Mignarri, Rossi, Ballerini, Gallus, Del Puppo, Galluzzi, Federico and Dotti2011).
In the case of Mr. X, his cerebellum was largely compromised as demonstrated on neuroimaging and on behavioral performance (e.g., mild dysmetria and dysdiadochokinesia). Mr. X’s poor dexterity and general slowness was demonstrated by his impaired performance on the Grooved Pegboard task. Unfortunately, due to constraints of this clinical evaluation, additional motor measures were not administered. Mr. X’s neuroimaging revealed signal abnormalities of deep corticospinal tracts, which was corroborated by extensor plantar response (i.e., positive Babinski response) as well as crossed adductor response. As the disease progressed, Mr. X demonstrated increased cerebellar degeneration, including mild ataxic dysarthria (e.g., slurred speech and irregular stress patterns) and unsteady control of lower extremities. In the last months of his life, Mr. X was experiencing scanning speech and reduced output, consistent with MRI findings that showed involvement of the superior cerebellum (Spencer & Slocomb, Reference Spencer and Slocomb2007).
Mr. X’s reduced integrity of major associative fiber tracts, significant cerebellar volume loss, markedly involving the dentate nucleus and cerebellar peduncles, and xanthoma noted throughout the cerebrum may partially explain reduced cognitive functioning. Research suggests that the dentate nucleus of the cerebellum has connections to prefrontal and posterior parietal cortices via the thalamus (Allen et al., Reference Allen, McColl, Barnard, Ringe, Fleckenstein and Cullum2005; Dum & Strick, Reference Dum and Strick2003). Dysfunction of the fronto-cerebellar pathways in patients with degenerative cerebellar disease results in executive impairments on objective testing that appear very similar to individuals with prefrontal cortex lesions (Heyder, Suchan, & Daum, Reference Heyder, Suchan and Daum2004). It is difficult to determine the degree of executive dysfunction given the breadth of impairment across all domains; however, it is clear that Mr. X had frontal dysfunction accompanied by notable disinhibition/apathy. This is further muddled by the fact that patients with CTX also show variable psychiatric manifestations as the disease progresses (Fraidakis, Reference Fraidakis2013). In the case of Mr. X, his wife and mother reported he became more disinhibited and unconcerned regarding his ongoing medical disease up until his death.
Early treatment of CTX is paramount and has been shown to ameliorate some of the neurophysiological symptoms associated with elevated cholestanol levels. Pharmacotherapy treatments of CTX are easily administered to aide in the body’s breakdown of cholesterol and has been shown to increase white matter tract density (Catarino et al., Reference Catarino, Vollmar, Küpper, Seelos, Gallenmüller, Bartkiewicz, Biskup, Hörtnagel and Klopstock2017). There is also limited research to indicate that CDCA can reduce pyramidal signs in some patients with CTX (Mignarri et al., Reference Mignarri, Rossi, Ballerini, Gallus, Del Puppo, Galluzzi, Federico and Dotti2011). Unfortunately, there remains a significant delay in diagnosis, which limits the efficacy of pharmacotherapy. Medications delivered after protracted exposure to unregulated cholestanol levels may not slow or reverse progressive peri-dentate white matter changes (Chang et al., Reference Chang, Lui, Wang, Huang, Lu, Chen, Chen, Tu, Huang and Chang2010), which appeared to be the case with Mr. X. Regrettably, a diagnosis of CTX and requisite intervention was significantly delayed in the present case study.
CONCLUSION
Neuropsychology is a growing specialty that continues to find its place as part of transdisciplinary teams in the coordination of patient care. As such, our knowledge base and awareness of disorders and diseases should be ever expanding. The authors hope presentation of Mr. X will increase awareness of CTX. In the current case, CTX was previously diagnosed by his neurologist, and our assessment provided valuable treatment recommendations and educational information for the family. However, we hope that if a clinician suspects a genetic disorder, an appropriate referral to neurogenetics might be made for the purposes of coordinated care. While the neuropsychological profile of this individual is indistinguishable from major neurocognitive disorder of various etiologies, with the help of neurogenetics and a talented neurology department, we are able to present these findings with a degree of certainty that the etiology of this individual’s impairment is CTX.
ACKNOWLEDGMENTS
The authors have no funding sources to declare.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to declare.