INTRODUCTION
Increasing evidence from neuropsychological (Verdejo-García et al., 2004) and neuroimaging studies (Garavan & Stout, 2005) support the notion that substance dependence is associated with dysfunctional neural circuits in which the prefrontal cortex (PFC) is a key component. The PFC plays a major role in the formulation and monitoring of goal-directed actions (Stuss & Knight, 2002; Roberts et al., 1998), and it is also involved in emotional regulation (Bechara et al., 2000; Davidson, 2002). Regional specialization within the human PFC is as diverse as its functions, and three different functional circuits relevant to executive control and emotional regulation have been described: the dorsolateral prefrontal cortex (DLPC), orbitofrontal cortex (OFC), and anterior cingulate cortex (ACC) circuits (Cummings, 1993; Tekin & Cummings, 2002). Dysfunction within each PFC circuit is associated with different deficits. The DLPC is mainly associated with executive control, and patients with DLPC lesions usually perform poorly on tests of working memory and mental flexibility (Bechara et al., 2000). The OFC is associated with emotional regulation, stimulus-reinforcement learning, and decision-making. Patients with damage to the OFC usually perform poorly on tests that involve emotional processing, reversal learning, and decision-making (Bechara et al., 2000; Clark et al., 2004; Rolls, 2004). Lesions to the ACC are mainly associated with lack of motivation and initiative. One neuropsychological correlate of ACC lesions is defective performance on tests of response inhibition, including go/no go tasks (Garavan et al., 2002; Tekin & Cummings, 2002).
Several studies have shown that substance dependence is associated with cognitive dysfunctions in which the PFC is critically involved. For example, poorer performance on tests of working memory and cognitive flexibility, linked to the functioning of the DLPC, has been reported in users of alcohol (Errico et al., 2002) and polysubstance users of amphetamines (Ornstein et al., 2000) and cocaine (Klüber et al., 2005). Similarly, deficits in inhibitory control, linked to the functioning of the ACC, have been detected in users of alcohol (Fillmore & Weafer, 2004) and polysubstance users of cocaine (Fillmore & Rush, 2002) and methamphetamine (Monterosso et al., 2005). A growing body of evidence also reveals that substance-dependent individuals (SDI) present with impairments in emotional processing and decision-making, which have been linked to the OFC. For instance, poor decision-making has been observed in polysubstance users of different substances (Grant et al., 2000; Stout et al., 2004; Whitlow et al., 2004). Deficits in emotional processing were also reported in SDI, including inaccurate perception of facial expressions (Hoshi et al., 2004; Townshend & Duka, 2003) and abnormal responses to affective images (Aguilar de Arcos et al., 2005; Gerra et al., 2003).
Studies using neuroimaging techniques have demonstrated that substance dependence is associated with abnormalities in different key components of a PFC–striatal neural circuit (Franklin et al., 2002; Lim et al., 2002; Matochik et al., 2003). Furthermore, several functional imaging studies have shown abnormal activation of PFC systems in response to cognitive and emotional tasks in users of multiple drugs (Bolla et al., 2003, 2004; Ersche et al., 2005; Fishbein et al., 2005; Garavan et al., 2000).
SDI present with a wide array of behavioral problems which are similar to those observed in patients with damage to different functional components of the PFC, especially in real-life settings. These problems include apathy, lack of initiative, and low motivation for natural reinforcers (linked to ACC; Kalechstein et al., 2002); poor emotional regulation, poor judgment, and impulsivity (linked to OFC; Bechara et al., 2001); and goal-neglect, disorganized behavior (linked to DLPC; Verdejo-García et al., 2004). Nonetheless, these accounts are mainly based on clinical observations, and empirical evidence based on tests that can detect and measure these PFC-related problems in SDI remain elusive. Therefore, the primary aim of this study was to examine the behavioral problems associated with dysfunction in different components of the PFC, as measured by the Frontal Systems Behavioral Scale (FrSBe; Grace & Malloy, 2001), in a clinical sample of SDI. Because some studies have described partial recovery of cognitive–executive deficits during abstinence, a related aim of this study was to contrast the PFC behavioral deficits of SDI exhibited during drug consumption with those exhibited during abstinence using the FrSBe. To address the relevance of PFC systems on several aspects of substance-dependence rehabilitation, we also analyzed the relationship between the FrSBe scores and several indices of the severity of substance dependence. Finally, a fourth aim of our study was to examine whether the PFC behavioral problems were associated with measures of executive functioning and emotional processing in SDI. We hypothesized that SDI, despite partial improvement during abstinence, will show greater behavioral problems than healthy participants across different domains, including apathy, disinhibition, and executive dysfunction. We also hypothesized that PFC behavioral deficits will be associated with abnormalities in several real-life domains, in which SDI typically have problems, and with specific measures of cognitive functions and emotion processing. Specifically, neurological models and empirical studies have suggested that one neuropsychological correlate of apathy is poorer performance on tests of response inhibition (Castellon et al., 2000; Tekin & Cummings, 2002). Furthermore, apathy is associated with blunted emotional expression and experience. Thus, we hypothesized that apathy scores would be related to poor performance on measures of response inhibition, and to blunted affective responses on emotion test measures. Accordingly, studies have shown that disinhibited behavior is associated with poorer ability to suppress pre-potent responses (Fillmore & Weafer, 2004) and with abnormal regulation of arousal (Graham, 2004). Thus, we predicted that scores reflecting disinhibition would correlate with cognitive measures associated with poor inhibitory control and with emotion test measures reflecting abnormal regulation of arousal. Furthermore, signs of executive dysfunction, which include poor planning, problem solving, and perseverations have been consistently linked to neuropsychological measures of working memory and flexibility (Stuss & Knight, 2002). Thus, we hypothesized that executive dysfunction scores would correlate with poor performance on cognitive measures that tax working memory and mental flexibility.
METHODS
Participants
A total of 35 SDI and 36 healthy comparison participants (HCP) volunteered for this study. The demographic and addiction severity data of the two groups are presented in Table 1. HCP were recruited through local advertisement and were paid for their participation. The selection criteria of HCP included the absence of a history of mental retardation, learning disability, psychiatric disorder, substance abuse, neurological disorder, or systemic disease that might affect the central nervous system (CNS). SDI were recruited from the Mid-Eastern Center for Chemical Abuse (MECCA), a local detoxification and treatment center, and they were paid for their participation in the study in gift certificates an hourly rate identical to that earned by HCP. The selection criteria for SDI were (1) meeting the DSM-IV criteria for substance dependence; (2) absence of psychosis; (3) no documented head injury or seizure disorder; and (4) absence of current diagnosis or a history of ADHD. Substance dependence was assessed using the Structured Clinical Interview for DSM-IV (SCID-IV). The complete screening and psychological testing procedures are described elsewhere in more detail (Bechara et al., 2001). Each SDI was tested at the end-stage of their treatment, shortly before their discharge. The duration of abstinence from substance use was known in these participants based on their length of stay at MECCA. The time varied among individuals, but the minimum period of abstinence from any substance use was 15 days. Thus, at the time of their testing, the SDI were no longer in acute withdrawal or taking any medications to control withdrawal. Urine toxicology screening (for opiates, stimulants, marijuana) and breathalyzer tests were conducted on these SDI routinely, allowing us to rule out recent substance abuse, as well as the use of substances during the entire period of abstinence. SDI were asked to report their drug of choice, and they were able to select more than one substance. In instances where SDI selected more than one substance, the drug of choice was defined as the substance that was used 80% of the time or more during the year before their admission for treatment. Based on these criteria, the drug of choice was alcohol in 15 of the participating SDI, methamphetamine in 13, and cocaine in 7 of the SDI participants. Because each participant was a polysubstance abuser, we have recorded the substances that were co-abused with the drug of choice, as well as the duration of abuse of each substance (see Table 2). All participants provided informed consent that was approved by the appropriate human subject committees at the University of Iowa.
Demographics and Addiction Severity Index (ASI) scores of the substance-dependent individuals (SDI) and healthy comparison participants (HCP)a

Substance-dependent individuals (SDI) subgroups divided according to their drug of choice, along with a list of co-abused substancesa

Instruments
Background information
Addiction Severity Index (ASI). This instrument was aimed to measure a variety of real-life domains in which SDI typically have problems. It is composed of seven subscales measuring severity of medical, employment, alcohol and drug use, legal, family/social, and psychiatric problems. These measures of real-life functioning were used as predictive variables of behavioral performance.
Behavioral and cognitive measures
The Frontal Systems Behavioral Scale (FrSBe). The FrSBe is a rating scale aimed at assessing distinctive behavioral problems associated with damage to the different PFC systems of the brain. The FrSBe is composed of three independent subscales that assess apathy, disinhibition, and executive dysfunction. Each subscale is designed to measure behavioral problems associated with the functioning of three different PFC circuits: the ACC (apathy subscale), OFC (disinhibition subscale), and DLPC (executive dysfunction subscale). Factor analyses of the FrSBe in several neurological populations have supported the validity of these subscales (Stout et al., 2003). Thus, the FrSBe has been shown to have good construct validity for the assessment of these different clinical syndromes. Furthermore, there is evidence in support of the reliability and utility of the FrSBe in the detection of frontal behavioral symptoms in neuropsychiatric populations such as schizophrenia (Velligan et al., 2002), and in substance abusers (Spinella, 2003). The FrSBe has also been shown to be sensitive to the degree of behavioral changes due to frontal lobe lesions (Grace & Malloy, 2001). In the case of SDI, we were interested in quantifying and contrasting both the behavioral problems during drug consumption and after drug abuse has ceased. Therefore, we have obtained SDI ratings from both conditions, that is, “During Drug Use” and “During Abstinence” (present time). In the case of HCP, we have only obtained present time ratings. For both groups, we only administered the self-rating version of the FrSBe.
Go/No Go Task. This task was aimed to measure mechanisms of response initiation and inhibitory control. In the task, one of two possible drawings (differing in identity or color) was presented at one time on the computer screen. Participants were instructed to press any button on the keyboard, as quickly and accurately as possible, when the target drawing was presented (Go trials) and to withhold the response when the nontarget drawing was presented (No Go trials). Each participant made 100 decision trials, which consisted of 20 initial practice trials, followed by 4 blocks of 20 trials. In odd blocks (first and third) participants were instructed to respond to one of the drawings (target drawing), creating a predisposition to press the button in response to target stimuli. By contrast, in even blocks (second and fourth) participants were instructed to respond to the nontarget drawing, so that they had to inhibit or control any behavioral tendency acquired from previous trials. Each block consisted of 10 Go trials and 10 No Go trials presented in a counterbalanced order. Inter-stimulus interval was set at 900 ms. We included total “number of hits” (response to target), “false alarms” (response to no-target), and “correct rejections” (no response to no-target) as dependent variables. Number of hits can be regarded as a measure of behavioral initiation, whereas false alarms and correct rejections can be considered measures of inhibitory control.
Wisconsin Card Sorting Test (WCST). This task was aimed to measure cognitive flexibility and set-shifting processes. We administered a computerized version of this task. We included “number of categories” and “percentage of perseverative errors” as dependent variables.
N-back Task. This task was aimed to measure working memory. A continuous row of letters appeared on the computer screen one at a time, and participants were instructed to press different buttons on the keyboard to indicate if each letter presented was (Y) or not (N) repeated with regard to the preceding letter (1-back block), two preceding letters (2-back block), or three preceding letters (3-back block). Each block (1-, 2-, and 3-back) started with a practice row of 10 letters, followed by a continuous row of 100 letters. ISI for the n-back trials was 2 seconds. This task requires participants to temporarily maintain in memory and continuously update information about the identity and order of the letters appearing on the screen, providing a measure of working memory. We used the accuracy index of each block (Hit Rate − False Alarm Rate) as a dependent measure for this task.
Emotional Testing
We used two sets of affective images extracted from the International Affective Picture System (IAPS; Lang et al., 2001). These images were used to assess emotional processing in response to affective images on two relevant dimensions of emotion: valence and arousal. Images were classified according to their valence normative values (pleasant vs. unpleasant) and were matched in their arousal normative values (all highly arousing). The Self-Assessment Manikin (SAM) was used to collect self-rating evaluation of the images in the dimensions of valence and arousal.
Statistical Analyses
One-way analysis of variance (ANOVA) was used to explore group differences in age, years of education, and verbal IQ. HCP had a higher average years of education and verbal IQ. Therefore, we included these variables as covariates (when appropriate) in subsequent analyses. Group differences regarding gender were examined using a χ2 analysis. We conducted repeated measures ANOVAs to examine possible differences between FrSBe subscales scores during drug use and during abstinence. Group differences on the FrSBe subscales scores were examined using a multivariate analysis of covariance (MANCOVA), including years of education and verbal IQ as covariates. Group differences on the WCST and the IAPS were analyzed using univariate ANOVAs. Go/No Go scores were not normally distributed (as assessed by Kolgomorov–Smirnoff tests) and were analyzed using Mann–Whitney nonparametric tests. A 2 (Group) × 3 (memory load) ANOVA was conducted on the N-back scores, and post hoc t tests were used to examine group differences on the three conditions. In all these comparisons, we conducted post hoc one-way ANOVAs or Mann–Whitney tests to examine possible differences between subgroups of SDI classified according to their primary drug of choice (3 factors: alcohol vs. cocaine vs. amphetamines) and according to the main effect of the primary drug of choice on the CNS (2 subgroups: alcohol vs. stimulants).
We conducted three multiple regression analyses to examine the predictive effects of severity of addiction (as measured by the ASI subscales) on FrSBe subscales scores. The relationship between FrSBe scores and selected cognitive and emotional measures was analyzed using planned Spearman rank order correlations. We hypothesized that apathy scores should be related to Go/No Go number of hits and valence ratings of positive affective images; that disinhibition scores should be related to Go/No Go false alarms and arousal ratings of positive affective images; and that executive dysfunction scores should be related to WCST percent of perseverative errors and N-back accuracy indices.
We established an alpha level below .05 for statistical significance in all comparisons. Planned correlations between FrSBe scores and cognitive–executive and emotional measures were hypothesis driven. Thus, the α level was not corrected for multiple comparisons.
RESULTS
Behavioral and Cognitive Measures
Internal consistency of the FrSBe
The α coefficient for the FrSBe scale was .92. FrSBe subscales also exhibited adequate internal consistency: α coefficients were .84, .81, and .80 for Apathy, Disinhibition, and Executive Dysfunction, respectively.
SDI frontal behavioral problems during drug abuse versus during abstinence
We conducted three repeated-measures ANOVAs on the self-rating scores of the three FrSBe subscales (Apathy, Disinhibition, and Executive) during Drug Abuse and During Abstinence in 35 SDI. Results showed significant differences between Drug Abuse and Abstinence scores on the Apathy (F = 52.18; df = 1,34; p < .001), Disinhibition (F = 121.97; df = 1,34, p < .001), and Executive (F = 150.58; df = 1,34; p < .001) subscales. SDI scores were lower in the Abstinence condition across the three subscales (see Table 3).
Comparison of substance-dependent individuals (SDI) Frontal Systems Behavioural Scale (FrSBe) subscales scores during the period of Drug Abuse versus that During Abstinence

Contrasting frontal behavioral problems in SDI versus healthy comparison participants
A total of 35 SDI and 35 HCP completed this scale. Self-rating scores of the three FrSBe's subscales were submitted to a MANCOVA, including three dependent variables (Apathy, Disinhibition, and Executive), and group as factor (SDI vs. HCP). We used Years of Education and Verbal IQ as covariates in these analyses. Results showed a main effect for Group (Wilks' λ = 5.30; df = 3,64; p < .001). Planned comparisons revealed significant differences between SDI and HCP on Disinhibition (F = 11.83; df = 3,66; p < .001) and Executive subscales (F = 4.49; df = 3,66; p < .01), as well as marginally significant differences on Apathy (F = 2.71; df = 3,66; p = .05). SDI's Abstinence scores were higher than the scores of HCP across the three subscales (see Table 4).
Comparison of substance-dependent individuals (SDI) and healthy comparison participants (HCP) on different subscales of the Frontal Systems Behavioural Scale

Relationship between severity of ASI problem domains and FrSBe subscales scores
To examine the effects of the severity of several problem domains related to substance dependence on frontal behavioral problems, we conducted three multiple regression analyses. As predictor variables, we included the scores of participants in the seven subscales of the ASI. As dependent variables, we included the scores of participants in the FrSBe subscales. Results showed ASI scores significantly predicted Apathy (R2 = .274; n = 70; p < .01), Disinhibition (R2 = .400; n = 70; p < .001), and Executive (R2 = .306; n = 70; p < .001) scores. Severity of medical problems was the best predictor of Apathy problems, both variables being directly correlated. Severity of drug and psychiatric problems were the best predictors of Disinhibition problems, both variables being directly correlated. Finally, severity of employment, psychiatric, legal, and medical problems were the best predictors of Executive problems, although none of them reached significance. All variables were directly correlated (see Table 5).
Regression analyses of the relationship between Addiction Severity Index (ASI) composite scores and Frontal Systems Behavioural Scale (FrSBe) subscales

N-back task
Thirty SDI and 30 HCP completed the 1-back and 2-back conditions of this task. Only 27 of 30 SDI were able to complete the 3-back task. A two (group) × three (load) ANOVA was conducted on the accuracy measure of the N-back task. Results showed a main effects of “group” F = 4.52; df = 1,55; p < .05, and memory “load” F = 95.94; df = 2,54; p < .001, but no effects of “group × memory load” interaction, F = .37; df = 2,54; p = .69 (see Table 6). Planned post hoc t tests showed that SDI had significantly lower scores on the 2-back condition, t = −2.12; n = 60; p < .05, but we found no differences between groups on the 1-back or 3-back conditions.
Contrasts between substance-dependent individuals (SDI) and healthy comparison participants (HCP) groups on the cognitive-executive and emotion test measures

Go/No Go task
A total of 35 SDI and 35 HCP completed this task. Mann–Whitney tests were conducted on each dependent measure of the Go/No Go task as a function of group (SDI vs. HCP). Results showed no significant differences between groups on “number of hits” and “false alarms”. There were significant differences between groups on “correct rejections” (U = .445; p < .05), with SDI scoring lower than HCP (see Table 6).
WCST
There were 35 SDI and 36 HCP who completed this task. Univariate ANOVAs were conducted on the WCST number of categories and percentage of perseverative errors as a function of group (SDI vs. HCP). Results showed no significant differences on number of categories, and marginally significant differences on the percentage of perseverative errors (F = 3.848; df = 1,67; p = .05), with SDI showing a higher percentage (see Table 6).
Emotional Tests
Pleasant pictures
A total of 24 SDI and 24 HCP completed this task. Univariate ANOVAs were carried out on the “valence” and “arousal” SAM ratings. Results showed no significant differences between groups on these measures.
Unpleasant pictures
Univariate ANOVAs were carried out on the “valence” and “arousal” SAM ratings. Results showed significant differences between groups in the “arousal” dimension (F = 3.572, df = 1,46; p < .05), with SDI scoring higher than HCP (see Table 6).
Correlations between behavioral, cognitive, and emotional measures
We analyzed the relationship between the FrSBe subscales scores and selected cognitive and emotional measures in SDI according to our initial hypothesis. As predicted, apathy scores were significantly inversely associated with Go/No Go number of hits and IAPS valence ratings of positive images. Disinhibition scores were significantly correlated with IAPS arousal ratings of positive images. Finally, executive dysfunction scores were significantly correlated with 3-back task accuracy index (see Table 7). As shown in Table 7, only predicted correlations were found to be significant.
Correlations between Frontal Systems Behavioural Scale (FrSBe) subscales and their hypothesized cognitive-executive or emotional correlatesa
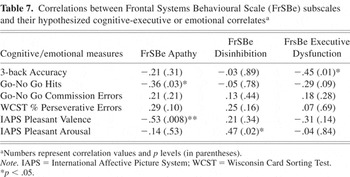
Post Hoc Analyses as a Function of Primary Drug of Choice
We conducted two series of post hoc analyses to examine the effects of (1) primary drug of choice (alcohol, n = 15 vs. amphetamine, n = 13 vs. cocaine, n = 7), and (2) principal effect of primary drug of choice on CNS (alcohol, n = 15 vs. stimulants, n = 20) on the different dependent measures. None of these comparisons were statistically significant, with the exception of principal effect of drug of choice on IAPS positive valence evaluation; stimulant polysubstance abusers showed higher evaluation of pleasant images than alcohol abusers, F(1,22) = 4.46; p < .05.
DISCUSSION
Results showed a generalized pattern of abnormal scores in SDI across the three PFC domains assessed by the FrSBe. Group differences were more marked on the disinhibition and executive dysfunction domains, although the apathy subscale also showed marginally significant effects. Therefore, behavioral results are consistent with several previous neuropsychological studies indicating that SDI deficits extend to several executive and emotional processes relying on the functioning of different PFC systems, including the DLPC, OFC, and ACC (see Rogers & Robbins, 2001, and Verdejo-García et al., 2004, for reviews). Neuroimaging studies also have provided support for this generalized pattern of behavioral problems in SDI that are linked to abnormalities in different functional systems of the PFC (see Garavan & Stout, 2005, for a review).
However, interpretation of these results is partially constrained by the fact that SDI included in our sample differed in the profiles of their primary drug of choice and other co-abused substances. The variety of abused drugs in this sample may have a differential impact on the CNS and on different behavioral domains associated with PFC systems, as inferred from animal (Robinson & Kolb, 2004) and human (Bolla et al., 2000) neuropsychological studies. This methodological limitation is due to the fact that it is extremely difficult to find SDI who have used only one particular substance. A close inspection of drug use characteristics in the SDI participants included in our study reveals that most of them were polysubstance abusers. Analyses showed no differences between duration of use of different drugs across subgroups of SDI classified according to their primary drug of choice. Additionally, post hoc tests on behavioral measures showed no significant differences between abusers of different substances. Therefore, our results should be regarded as more related to polysubstance abuse than to the specific effects of any particular substance. Although this remains a limitation of our study, several behavioral and neuroimaging studies, as outlined earlier, have shown common alterations in the PFC functions among users of several substances. Additionally, similar PFC deficits have been observed in relatively pure and in polysubstance users (Bechara et al., 2001; Di Sclafani et al., 2002; Gonzalez et al., 2004; Grant et al., 2000). Furthermore, it is important to note that the PFC–striatal neural circuit constitutes a general neural system that may be impacted by the effects of a variety of substances, as opposed to one specific substance (Bechara, 2005; Rogers & Robbins, 2001).
Previous neuropsychological and imaging studies have proposed that PFC-related deficits tend to recover during protracted abstinence (Selby & Azrin, 1998; Wang et al., 2004). Our study reveals a discrepancy between the degree of frontal behavioral problems during acute drug consumption and during abstinence, by showing a significant decrease of behavioral problems during abstinence. These results support the hypothesis of partial recovery of PFC behavioral problems during abstinence. However, these results also highlight that this functional recovery is incomplete, because their frontal behavioral scores were still significantly higher than those of HCP. On the one hand, the high scores reported by SDI during drug consumption may explain why these patients become frequently involved in social and legal problems during drug use, as they frequently show aggressive and violent behaviors (Gerra et al., 2000; Graham et al., 2000). On the other hand, the persistent high scores during abstinence suggest that these PFC abnormalities persist and may render SDI vulnerable to relapse. One limitation of the current study is that the time of abstinence in our study sample was too short, and recovery could have been complete had we allowed a longer period of abstinence. Although this remains a possibility, some studies suggest that these frontal lobe signs may persist even in SDI who have been successfully abstinent for more than 6 months (Di Sclafani et al., 2002; Fein et al., 2004). Another possible limitation is that SDI ratings may have been influenced by social desirability, in an attempt to overestimate the improvements achieved during treatment. However, we consider this possibility as unlikely, because SDI were tested at a different institution, in a context that was unrelated to their treatment outcome, and after signing a “Certificate of Confidentiality” document, which ensures the confidentiality of the obtained information and protects participants from any obligation to release such information to a third party.
The significant impact of several real-life functioning problems typically associated with substance dependence on the FrSBe subscales, and the differences revealed between SDI and HCP across domains, clearly highlight the validity of using the FrSBe in this population. One potential limitation of our study is that SDI may have poor insight about the extent of their problems, given the behavioral similarities they show to patients with orbitofrontal lobe damage (Bechara et al., 2001). Therefore, it would be useful to include a collateral-rating version of the FrSBe in future studies. Although we did not obtain ratings from collaterals in our study, due to the fact that the relatives of our SDI were not reachable, the consistent pattern of higher SDI scores suggest that the FrSBe instrument was still valid and capable of detecting deficits, despite this possible lack of awareness about their problems.
Results from cognitive tests were consistent with behavioral results and with previous studies in SDI. Poorer accuracy of SDI on the 2-back task, relative to controls, indicates poorer working memory. Overall, SDI seem to be less accurate in updating and filtering information during working memory tasks, a function that has been linked to the DLPC. This finding is consistent with several other studies revealing working memory deficits in SDI (Bechara & Martin, 2004; Martin et al., 2003; Mintzer & Stitzer, 2002). Results from the go/no go task revealed subtle deficits in the ability of SDI to suppress pre-potent motor responses. This deficit was observed previously under acute doses of alcohol or cocaine (Fillmore et al., 2002; Finn et al., 1999) and in chronic drug abusers (Bolla et al., 2000; Fillmore & Rush, 2002). Results from the WCST revealed marginally significant differences between SDI and HCP in relation to the percentage of perseverative responses but not the number of completed categories. Although these results may be limited by the ceiling of HCP scores, the observed differences are consistent with previous reports showing differences in perseveration errors but not number of completed categories between groups of SDI and HCP (Bechara et al., 2001; Errico et al., 2002). The WCST is a relatively complex task that taxes several processes, including working memory, inhibitory control, and set-shifting. However, it has been suggested that the underlying cause of the poor performance of SDI on this task is linked to its set-shifting component (critically requiring inhibition), as opposed to its concept formation or working memory components and that this function may be dependent on the integrity of the lateral orbitofrontal/inferior frontal gyrus sector of the PFC (Bechara, 2005).
An interesting aspect of our study was the evaluation of emotional experience. Results showed SDI in our sample tended to evaluate unpleasant images as significantly more arousing than HCP. These results suggest that abstinent SDI present with a sensitized emotional experience, which is consistent with previous research showing that polysubstance stimulant users evaluate negative affective images more extremely (Aguilar de Arcos et al., 2005).
A final finding of our study was the existence of modest but significant correlations between behavioral, cognitive, and emotional measures. The apathy subscale was associated with lower number of hits on the response inhibition task, which is consistent with previous neurological models and empirical studies (Castellon et al., 2000; Tekin & Cummings, 2002). Of interest, apathy scores were also inversely correlated with valence ratings of pleasant images in SDI, suggesting a relationship between the assignment of emotional value to naturally reinforcing stimuli and behavioral manifestations of poor motivation and initiative in abstinent SDI. Working memory performance was significantly associated with executive scores, supporting previous animal and human studies on the important role of the DLPC in working memory (Roberts et al., 1998; Stuss & Knight, 2002). Finally, disinhibition scores showed a significant correlation with the arousal evaluations of positive images, which suggests an important influence of emotional dysregulation on behavioral disinhibition (Graham, 2004).
In conclusion, SDI present with a wide array of behavioral problems associated with abnormalities of different PFC functional systems. Although these deficits cannot be attributed to one specific substance, they are nonetheless the manifestation of the abuse of multiple substances. These deficits seem to be associated with not only the dependence on such substances, but also with problems in real-life domains. It remains unclear at this point whether these deficits are the product of chronic drug use, or whether premorbid differences in education, IQ, socioeconomic status, temperament, personality, and psychopathology account for these observed differences in behavior. Specifically, the healthy control participants in our study had more years of education and higher verbal IQ, than the SDI group. Although we have controlled for these variables in our subsequent between-group analyses, this covariance analysis does not address the design problem entirely (Adams et al., 1985), considering that substance dependence may be a marker for a whole cluster of educational, occupational, and health factors that negatively impact neuropsychological performance.
ACKNOWLEDGMENTS
This research was supported by NIDA grant RO1 DA11779 to A. Bechara. We thank Laura Vanderploeg and Jill Arnold for their invaluable assistance in testing the participants of this study and collecting the data.









