Introduction
Scaphoideus titanus Ball (Hemiptera: Cicadellidae: Deltocephalinae) is a leafhopper native to the Great Lakes region of the United States and Canada, accidentally introduced to Europe (Weintraub & Beanland, Reference Weintraub and Beanland2006). Its presence was first noticed in the 1950s in southwest France (Bonfils & Schvester, Reference Bonfils and Schvester1960). It is established now also in Italy, Switzerland, Slovenia, Croatia, Spain, Portugal and Serbia (Mazzoni et al., Reference Mazzoni, Alma, Lucchi, Bertaccini and Braccini2005) and was recorded recently also from Austria (Steffek et al., Reference Steffek, Reisenzein and Zeisner2007), Hungary (Dér et al., Reference Dér, Koczor, Zsolnai, Ember, Kölber, Bertaccini and Alma2007) and Bosnia and Herzegovina (Delić et al., Reference Delić, Seljak, Martini, Ermacora, Carraro, Myrta and Đurić2007).
Although in its native region S. titanus is found on herbaceous vegetation and different shrubs and trees (i.e. Crataegus spp., Polygonium spp., Salix spp., Juniperus virginiana, Ulmus spp., Fraxinus spp.) (Barnett, Reference Barnett1976; Hill & Sinclair, Reference Hill and Sinclair2000), it is also widespread and abundant on the wild vine Vitis riparia (Maixner et al., Reference Maixner, Pearson, Boudon-Padieu and Caudwell1993). In Europe, S. titanus is considered to be a strictly ampelophagous species, mainly associated with cultivated Vitis vinifera (Bonfils & Schvester, Reference Bonfils and Schvester1960; Vidano, Reference Vidano1964). While the leafhopper itself does not cause any major damage to grapevine plants, it is the vector of a phytoplasma that causes Flavescence dorée, one of the most damaging grapevine diseases in Europe (e.g. Bressan et al., Reference Bressan, Larrue and Boudon Padieu2006). Nymphs acquire phytoplasma while feeding on the phloem of infected plants and transmit it to healthy plants after a latency period of 28–35 days. Adults are effective vectors of Flavescence dorée throughout their life (Schvester et al., Reference Schvester, Carle and Moutous1969).
Since Flavescence dorée is recognized as a quarantine disease, compulsory control measures include the large-scale treatments of vineyards with insecticides, including neurotoxic compounds and chitin synthesis inhibitors (Posenato et al., Reference Posenato, Mori, Bressan, Girolami and Sancassani2001; Bressan et al., Reference Bressan, Larrue and Boudon Padieu2006). However, sustainable management strategies for insect vectors should also include methods that aim to disrupt interactions such as reproductive behaviour (Redak et al., Reference Redak, Purcell, Lopes, Blua, Mizell and Andersen2004; Almeida et al., Reference Almeida, Blua, Lopes and Purcell2005; Weintraub & Beanland, Reference Weintraub and Beanland2006). Although there is information available on some aspects of biology and behaviour of S. titanus, such as the general biology, disease transmission (e.g. Schvester et al., Reference Schvester, Carle and Moutous1969; Mori et al., Reference Mori, Bressan, Martini, Guadagnini, Girolami and Bertaccini2002; Bressan et al., Reference Bressan, Spiazzi, Girolami and Boudon-Padieu2005, Reference Bressan, Larrue and Boudon Padieu2006; Marzorati et al., Reference Marzorati, Alma, Sacchi, Pajoro, Palermo, Brusetti, Raddadi, Balloi, Tedeschi, Clementi, Corona, Quaglino, Bianco, Beninati, Bandi and Daffonchio2006), spread across Europe (Bertin et al., Reference Bertin, Guglielmino, Karam, Gomulski, Malacrida and Gasperi2007) and general patterns of dispersal and activity (Bosco et al., Reference Bosco, Alma and Arzone1997; Lessio & Alma, Reference Lessio and Alma2004a,Reference Lessio and Almab; Beanland et al., Reference Beanland, Noble and Wolf2006), the mating behaviour of this pest species has not yet been investigated.
The main objective of this study was to provide essential information on the reproductive behaviour of S. titanus on which the direction of future, more environmentally friendly, control practices could be developed. Integrated pest management tactics usually rely on pheromone dispensers to disrupt mating behaviour of pest species (e.g. Witzgall et al., Reference Witzgall, Stelinski, Gut and Thomson2008); however, up to now there is no evidence that chemical communication plays a role in the reproductive behaviour of leafhoppers. Mate recognition and localization in ‘Auchenorrhyncha’ (with the exception of most cicadas) are mediated via vibrational signals transmitted through the substrate (reviewed in Claridge, Reference Claridge1985a,Reference Claridge, Nault and Rodriguezb; Čokl & Virant-Doberlet, Reference Čokl and Virant-Doberlet2003; Virant-Doberlet & Čokl, Reference Virant-Doberlet and Čokl2004; Virant-Doberlet et al., Reference Virant-Doberlet, Čokl, Zorović, Drosopoulos and Claridge2006). In this study, we focused on the vibrational communication of S. titanus in order to increase our understanding of its mating strategy.
Materials and methods
Insects
Eggs and juveniles of all stages of S. titanus were collected at La Spezia and Massa Carrara vineyards (Central Italy) in 2006 and 2007. Each year in February, two-year-old grapevine canes were collected from organic farms and stored in a cold room at 4°C. Eggs were hatched in a controlled environment chamber (25±1°C, L16:D8, RH: 75±5%), and vine leaves were provided as a food source. Vine leaves were checked daily for the presence of newly hatched individuals and hatchlings were moved to plexiglas cages (see below) on the day of emergence. Nymphs of all stages were collected from vineyards in May and June each year by direct collection from vines by a pooter and reared to adults in the laboratory. All nymphs were kept on grapevine shoots in plexiglas cages (25×5×5 cm) at 25±1°C, 65±5% relative humidity, and 16:8 (L:D) photoperiod. Rearing cages were checked every day and adult males and females were removed from the nymphal culture on the day of eclosion and kept separated by gender. Adults were housed in glass vials (length 20 cm; diameter 3 cm) and fed with single vine leaves. To ensure that leafhoppers were sexually mature and receptive, all tests were done with virgin males and females that were at least six and ten days old, respectively.
Recording vibrational signals and behaviour
Recordings were conducted in June–August 2006 and 2007 at the National Institute of Biology (Ljubljana, Slovenia) in an anechoic and sound insulated chamber (Amplifon Fa., Amplaid, Italy) at temperatures of 22–25°C and relative humidity between 70% and 75%. Vibrational signals were detected on the leaf lamina by the use of a laser vibrometer (PDV 100, Polytec GmbH, Waldbronn, Germany). Signals were digitized with 48 kHz sample rate and 16-bit depth and stored directly onto a hard drive of a computer using Sound Blaster Audigy 4 sound card (Creative Labs Inc.) and Cool Edit Pro 2 (Syntrilium Software). Signal recordings were analyzed using the computer software program Raven 1.2.1 (Charif et al., Reference Charif, Clark and Fristup2004). The behaviour of S. titanus was recorded with a Canon MV1 miniDV camera together with vibrational signals. This enabled us to identify individuals emitting the signals and to associate vibrational signals with particular behaviour. Video recordings were transferred into the computer with Windows Movie Maker 2.0.
Leafhoppers were placed on a cut grapevine stem with a leaf. The bottom of the stem was put into a vial filled with water to prevent withering and placed upright in a jar filled with moist artificial substrate. To prevent insects escaping during the recordings, a plexiglas cylinder (height 50 cm; diameter 30 cm) with a small opening for a laser beam was put over the jar.
To study the daily pattern of male calling activity, we made preliminary 24 h observations of mating activity and, accordingly, divided a day into six time periods for subsequent tests: night (01:00–05:00 h), early morning (06:00–08:00 h), late morning (10:00–12:00 h), afternoon (14:00–16:00 h), early evening (18:00–20:00 h) and late evening (22:00–24:00 h). We chose these periods in order to avoid time overlap. The following tests were done in all six time periods:
1 Single males (n=20) were placed on a vine leaf and vibrational signals and behaviour have been recorded for 20 min.
2 Single pairs (male and female, n=20) were put on a leaf, and vibrational signals and behaviour were recorded either until the male reached the pre-copula position and attempted to copulate or for 20 min in cases where leafhoppers did not show any mating behaviour.
Two additional tests were carried out only during two time periods (summertime), afternoon (14:00–16:00 h) and early evening (18:00–20:00):
1 Single females (n=20) were placed on a vine leaf, and vibrational signals and behaviour were recorded for 20 min.
2 Trios (two males and a female, n=15) were placed on a leaf, and their behaviour was recorded for 20 min after the onset of vibrational communication.
Mating frequency
To determine the frequency of mating, behavioural observations were also made without recording vibrational signals. Ten pairs of virgin males and females (at least six and ten days old, respectively) were placed into separate plexiglas cages with grapevine cuttings (see above). Cages were monitored continuously, and mated females were removed from the cage within 30 min after the end of copulation and substituted with new virgin females. The trials were conducted for eight hours each day (11:00–19:00) until the death of the males. Between consecutive daily tests, males were kept in isolation. We noted the number of copulations for each male.
Terminology and analysis procedures
Vibrational signals were labelled according to their behavioural context. Calling signals (calls) were defined as signals that were emitted spontaneously by isolated insects (Booij, Reference Booij1982). Pulse was defined as a unitary homogenous parcel of sound of finite duration (Broughton, Reference Broughton and Busnel1963). Pulses arranged into repeatable and temporally distinct groups were termed pulse trains. The sequences of more or less regularly repeated pulse trains, with distinct time and amplitude pattern, were termed phrases.
We measured the following parameters: fundamental frequency of ‘buzz’ (see below), dominant frequency of pulse, ‘rumble’ and ‘noise’, duration of the pulse train, duration of the phrase, duration of the sections in the phrase (see below), number of pulses in the sections, pulse repetition time, male signalling latency (time from the ‘arrival’ at the leaf to the emission of the first vibrational signal), female response latency (time between male pulse and female response pulse), female response rate (ratio between male and female pulses) and search time (time between the first recorded female reply and arrival of the male at the female). Results are presented as means, ranges and standard deviations (SD) together with the number of signals analysed for each individual (N) and number of leafhoppers (n) from which signals were obtained. The recordings of 20 single males, 25 pairs and ten trios were used for detailed analysis of temporal and spectral properties of male and female vibrational signals, while 48 and 64 males were used for analysis of call latency in single male and pair treatments, respectively, and 59 males were used for analysis of search time.
The number of signalling males in each period of the day was taken as a measure of signalling activity and G-tests for contingency tables (using log-likelihood ratios) after Williams' correction (Zar, Reference Zar1999) were used to test the differences in male calling activity in different periods of the day and in the presence or absence of a female. Wilcoxon-Mann-Whitney test (Zar, Reference Zar1999) was used to test the differences in call latency in single male and pair treatments. Chi-squared (with Yates' correction) (Zar, Reference Zar1999) was used to compare mating behaviour in pair and trio trials. From pair trials, only those from afternoon and early evening time periods, in which male and female established a duet, were used (n=24). We analysed the following parameters: number of males locating the female, number of males that attempted copulation and number of females maintaining a duet until the end of the trial. Data were analysed using KyPlot 5.0 (KyPlot, KyensLab Inc., Tokio, Japan).
Results
General description of mating behaviour
Observations of mating behaviour showed that S. titanus males emitted vibrational signals spontaneously within ten minutes of being placed on a plant. Virgin females readily responded to male signals; however, they were never observed to initiate a duet. In the absence of a female response, males either remained stationary or they jumped off the plant (see below). A female response resulted in searching behaviour by males even when they were positioned on the opposite sides of the leaf. Duetting females remained stationary and never searched for a male. Males did not immediately turn towards the female, and they did not approach her in a direct path, and they made many turns. Males searching for a female on a grapevine leaf were stopping and emitting vibrational signals and waiting for a female response before starting to move again. After locating the female, the male positioned himself closely (less than 1 mm) behind the female at a slight angle towards the female body axis and continued emitting vibrational signals. No physical contact was observed prior to a copulation attempt, which starts when the male spins around and tries to join his genitalia with those of the female. In most of the observed matings, males attempted copulation several times before they succeeded. After unsuccessful attempts, males returned to the original position behind the female and continued with courting.
Pairs stayed in copula from 40–70 minutes, and a series of pulses was recorded during the first 2–3 min of copulation. Males started to emit calling signals a few seconds after the end of copulation. Behavioural observations showed that males can mate for a second time as early as one hour after a previous copulation and can mate with three different females in the course of eight hours.
Vibrational signals
Vibrational signals of S. titanus are composed of four different sound elements: pulse with a dominant frequency around 150 Hz and broad-band frequency characteristics and three elements onomatopoetically termed ‘rumble’, ‘buzz’ and ‘noise’. The ‘rumble’ and ‘noise’ are composed from a series of short pulses and have a dominant frequency around 570 Hz and 230 Hz, respectively, whereas the ‘buzz’ is characterized by a continuous sound with fundamental frequency around 280 Hz and with clear harmonic structure (tables 1, 2 and 3; figs 1, 2 and 4).
Table 1. Temporal and spectral properties of the male calling signal of Scaphoideus titanus.
Means with standard deviation (SD), together with maximal and minimal measured values, are shown. n, number of animals; N, number of signals analysed for each individual; MP1, male pulse 1.
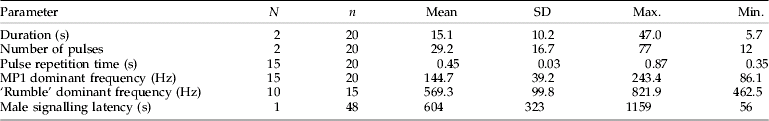
Table 2. Temporal and spectral properties of the male courtship phrase, female pulse and male-female duet of Scaphoideus titanus.
Means with standard deviation (SD) together with maximal and minimal measured values are shown. n, number of animals; N, number of signals analysed for each individual; S1–S4, sections 1–4 of the male courtship phrase; MP1, male pulse 1; MP2, male pulse 2; FP, female pulse.

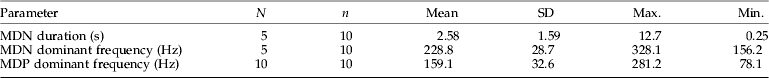
Table 3. Temporal and spectral properties of the male disturbance signals of Scaphoideus titanus.
Means with standard deviation (SD) together with maximal and minimal measured values are shown. n, number of animals; N, number of signals analysed for each individual; MDN, male disturbance noise; MDP, male disturbance pulse.
The male repertoire consisted of three different types of vibrational signal: the calling signal, the courtship phrase and the disturbance signal. The only recorded signals emitted by females were single pulses emitted in response to male pulses.
Male Calling Signal
Almost all of the vibrational signals recorded from a single male could be classified as calling signals (MCS) (table 4, fig. 1). During MCS emission, the male adopted a stereotyped posture with hind legs lifted from the ground and positioned parallel to the abdomen. The male calling signal consists of a series of single pulses associated with slow dorso-ventral movements of the abdomen (male pulse 1, MP1). The repetition time of pulses was rather constant while the amplitude of pulses increased over the signal (table 1, fig. 1a). MP1 was preceded by a shorter pre-pulse of lower amplitude and higher frequency (fig. 1b). The male calling signal is always preceded by the ‘rumble’. A few seconds before the first MP1, the repetition time of pulses in the rumble increased, and then the rumble gradually disappeared (fig. 1a).

Fig. 1. Male calling signal (MCS) of Scaphoideus titanus. (a) a sonagram (above) and oscillogram (below) of a representative signal, (b) expanded detail of the signal shown in (a).
Table 4. Differences in vibrational activity of Scaphoideus titanus males at different times of the day. Number of males emitting a particular type of vibrational signal in each time period is shown. Each active male has been assigned only to one behavioural category. Values for single males and pairs are shown.

n, number of tested males; MCS-s, male calling signal-single; MCS-m, male calling signal-multiple; MCrP, male courtship phrase.
1 total number of active males in each time period.
2 call-fly behaviour is a sequence of 1–2 consecutive male calling signals followed by a jump from a plant.
3 duet is formed by female pulses inserted into a period between pulses in the male courtship phrase. Asterisks indicate significant difference in signalling activity between single males and pairs in each time period. G test, df=1.
** P<0.01; * P<0.05; ns, non significant (P>0.25).
Male courtship phrase
In the presence of a female, almost all the vibrational signals emitted by males could be classified as the courtship phrase (MCrP) (table 4, fig. 2). The MCrP was a sequence of regularly repeated sound elements and could be divided into four sections.

Fig. 2. Male courtship phrase (MCrP) of Scaphoideus titanus. (a) a representative signal, (b) expanded detail of section 1 and 2 shown in (a). In (a) and (b) sonagram (above) and oscillogram (below) are shown. S1–S4, sections 1–4 of the male courtship phrase. In (a) S2–S4 of the preceding phrase in a series are also shown.
Section 1 (S1) was a pulse train composed of male pulses 1 (MP1) with a regular repetition time and a ‘buzz’ in the intervals between pulses. The ‘buzz’ and pulse were never produced simultaneously, and there was a short period of around 30 ms between the end of a ‘buzz’ and onset of a pulse. Section 2 (S2) differed from the previous one in having a longer pulse repetition time and the introduction of a second high amplitude pulse (male pulse 2, MP2) coupled with male pulse 1 (fig. 2). MP2 was associated with a pronounced dorso-ventral abdominal swing. Section 3 (S3) consisted exclusively of ‘buzz’ elements that progressively decreased in duration. During these first three sections, the male kept a stationary position on the leaf.
Section 4 (S4) was characterized by short pulses that were associated with a fast shaking of the abdomen. When the female replied during S1 and S2, the male searched for her during S4, while in the absence of a female reply the male performed a stationary S4. This section always ended with a single pulse of high amplitude (fig. 2a) that was associated with a strong dorso-ventral abdominal swing and wing-flicking movement. Occasionally, S4 was repeated several times before the beginning of the next courthship phrase, especially when the phrase was disrupted by a disturbance signal from another male (see below).
As in the male calling signal, the first phrase in a series was preceded by a ‘rumble’ that gradually turned into a ‘buzz’, throughout the S1 and S2. After the first courtship phrase, the ‘rumble’ was completely replaced by a ‘buzz’.
Despite its complexity, the male courtship phrase was relatively constant in total duration (table 2).
Female signals
The only recorded female vibrational signals were single pulses (female pulse, FP) inserted between male pulses in sections 1 and 2 of the male courtship phrase (fig. 3). The female pulse had similar frequency characteristics as the male pulses (table 2) and emission was associated with dorso-ventral movements of the abdomen. The amplitude of female pulses varied greatly and was correlated with the observed amplitude of abdominal movements. Low amplitude female pulses were usually emitted in response to the male pulse 1, whereas the ones with the highest amplitude mainly in response to the male pulse 2.

Fig. 3. Vibrational signal of Scaphoideus titanus female. Male courtship phrase together with a female response are shown. (a) a representative male signal and female replies, (b) expanded section of the signals shown in (a). In (a) and (b) sonagram (above) and oscillogram (below) are shown. Asterisks indicate pulses emitted by female. S1–S4, sections 1–4 of the male courtship phrase. In (a) S2–S4 of the preceding phrase in a series are also shown.
Male disturbance signals
In response to a duet between male and female, a rival male emitted male disturbance pulses (MDP) and male disturbance noise (MDN) (fig. 4). The male disturbance pulse had the same frequency characteristics as the male pulse 1 (tables 1, 2 and 3); however, it differed in the absence of a pre-pulse. The male disturbance noise was formed by a train of short, quickly repeated MDPs that were associated with weak shaking of the abdomen and was characterized by a higher dominant frequency (table 3). Towards the end of the disturbance noise, the pulse rate progressively decreased; and discrete pulses were observed at the end (fig. 4). The duration of male disturbance noise had a large variance (table 3).

Fig. 4. Male disturbance signals of Scaphoideus titanus. Section 1 (S1) of male courtship phrase and male disturbance noise and male disturbance pulses are shown. (a) sonagram (above) and oscillogram (below) of representative signals, (b) expanded detail of male disturbance pulses shown in (a).
Behaviour
The first activity recorded after a leafhopper (male or female) was placed on the plant was grooming, usually performed within five minutes. Both genders groomed their antennae, wings and abdomen with stereotyped legs movements. Another activity, brochosomes anointing, was most frequently recorded during the night. It consists of collecting droplets after they are released from the anal opening with the tarsi of the hind legs and spreading them over all six legs.
Single males
In our experiments, 40% of tested males spontaneously emitted vibrational signals during the given time (20 min) (table 4). The male signalling latency was highly variable (table 1). Males often emitted calling signals (MCS) as a part of a ‘call and fly’ behaviour, while few males produced courtship phrases (MCrP). ‘Call and fly’ behaviour consisted of a sequence of 1–2 consecutive calling signals followed by a jump from a plant. The highest observed number of ‘call-fly’ sequences in one male was 20.
There was a significant difference in male calling activity between different parts of the day (G test; G=33.8, df=5, P<0.001). The highest signalling activity and the highest number of males expressing ‘call-fly’ behaviour were observed in the early evening period (18:00–20:00), when 85% of the tested males emitted vibrational signals (table 4). In the following late evening period (22:00–24:00), the male activity was lower; but, nevertheless, 65% of all active males were recorded in these two time periods, whereas only a few of observed ‘call-fly’ sequences were performed at other times of the day. The highest number of emitted courtship phrases was observed during the late evening and night periods.
Pairs
Vibrational signals were recorded from 54% of tested males (table 4). Although the male signalling latency was highly variable (table 2), it was significantly shorter than in single male tests (Mann-Whitney test; m=48, n=64, U=1900.5, P=0.028). Most males that emitted vibrational signals established a duet with a female (table 4). While the latency of the female reply had low variance, the female response rate was highly variable (table 2); and the female could reply only once during the male courtship phrase. A female response always triggered searching behaviour in the males. When searching for a female on a grapevine leaf, the male emitted courtship phrases and waited for a female response before starting to move again. The mean search time was 220 s (table 2). The females did not reply to all male signals, sometimes ignoring whole series of courtship phrases. In the absence of a female's reply, the male continued emitting phrases with stationary S4. Four unreceptive females interrupted male signalling with emission of few (2–4) high amplitude pulses during the S1 section of the courtship phrase. In this case, males did not resume singing within the time of the trial.
The presence of a female significantly increased male overall signalling activity (G test; G=4.3, df=1, P=0.038). In contrast to tests with a single male, there was no significant difference in male signalling activity between different parts of the day (G test; G=10.9, df=5, P=0.054); and the increase in overall activity resulted from the higher signalling activity in the first part of the day. There was no significant difference in male signalling activity between single male and pair tests during the evening and night periods (table 4).
Trios
Our results show a strong rivalry between males, based on the emission of male disturbance signals. One way to interrupt the male-female duet was to emit disturbance pulses (MDP) and either alternate them with male pulses in the male courtship phrase (in an a-b-a-b sequence) or overlap them. Disturbance pulses were usually emitted during S1 section and only rarely in S2 section of the male courtship phrase. The other way of interruption was to emit disturbance noise (MDN) after a female pulse in S1 or after the first male pulse 2 in S2 section of the male courtship phrase. The interruptions to a mating duet were only temporary and the courting male restarted vibrational signalling either by S1 or by stationary S4 after a few seconds. When a duet was interrupted for a longer time (up to several minutes), the courting male started a phrase from the beginning. When disturbance pulses were emitted in S1, this section rarely progressed into S2 and never developed into a proper courtship phrase. In only two tests, the courting and rival male kept their role for the entire duration of the test; in others, multiple role reversals were observed.
In all trials, the rival males adopted another tactic, in addition to emission of disturbance signals, and silently approached a female that was duetting with a courting male. When the rival male stopped emitting disturbance signals, the courting male shortened a duet by emitting only a few male pulses 1 instead of a complete courtship phrase, presumably to reduce the time needed for localization.
When a rival male reached a courting male that was already in pre-copula position, he positioned himself behind the courting male. This initiated aggressive behaviour between males, including kicking and hitting with strong abdominal swings.
The presence of a rival significantly affected the male-female duet and, consequently, also female localization and copulation (table 5). Eighty-seven percent of males that at the start initiated a duet with a female were not able to maintain it. This resulted either because the female stopped responding and moved away (even jumped off the leaf) or because they were displaced by a rival. Some males that established a duet even failed to locate the female. In two out of three observed copulations, it was the silent rival that mated with the female.
Table 5. Effect of male rivalry on mating behaviour. Percentage of males localizing the female and attempting a copulation and percentage of females duetting at the end of the trial are shown. Comparison between pairs and trios is shown.

n, number of pairs and trios tested. Chi-square test (with Yates' correction) was used to compare results between pairs and trios.
Discussion
Results of the present study showed that mate recognition and location in S. titanus is mediated by vibrational signals. Among ‘Auchenorrhyncha’, vibrational communication has been particularly extensively studied in leafhoppers (Cicadellidae) (e.g. Claridge, Reference Claridge1985a,Reference Claridge, Nault and Rodriguezb; Tishechkin, Reference Tishechkin, Drosopoulos and Claridge2006), delphacid planthoppers (e.g. Claridge & de Vrijer, Reference Claridge, de Vrijer, Denno and Perfect1994) and treehoppers (Membracidae) (e.g. Cocroft & McNett, Reference Cocroft, McNett, Drosopoulos and Claridge2006). The general pattern, found also in S. titanus, is that: (i) pair formation begins with the emission of male vibrational signals; (ii) male signals are more complex than female signals; and (iii) after establishing a vibrational duet with a receptive female, the male searches for a replying stationary female. However, the unique features of vibrational communication in S. titanus are: (i) crepuscular signalling activity; (ii) that female response is reduced to only one pulse that closely resembles the male pulses; and (iii) well-developed intrasexual competition in the form of alternative tactics, such as male disturbance signals and silent approach to a duetting female (satellite behaviour).
The peak in male signalling activity was shown to be associated with twilight and early night. Our observation that, during these periods, single males left the plant when there was no response to their vibrational signals also indicates that, under natural conditions, males probably move from plant to plant and call to determine the presence of a female. This is in agreement with previous field observations that showed that flight activity of male and female of S. titanus is much increased between late afternoon and early morning (Lessio & Alma, Reference Lessio and Alma2004b). Taken together, our results and previous observations suggest that, under field conditions, most mating activity would also occur during twilight or at night. However, when pairs were tested, time of day had no significant affect on mating activity (signalling and copulation). Similarly, although crepuscular flight activity has been shown for leafhoppers of the genus Dalbulus (Taylor et al., Reference Taylor, Nault and Styer1993), it was noted that these leafhoppers mated any time during the day or night (Heady et al., Reference Heady, Nault, Shambaugh and Fairchild1986). Although the daily flight activity of S. titanus depends mainly on the photoperiod, it was also partially influenced by temperature (Lessio & Alma, Reference Lessio and Alma2004b). It is possible that extended signalling and mating activity, as found in the present study, could result from tests done in laboratory conditions under constant temperature. However, such conditions apparently had no effect on the activity of single males. Our results indicate that it might be more likely that the crucial factor for such extended mating periods could be an increase in overall male signalling activity that results from the male's ability to detect the presence of a female on the same plant. Under our experimental conditions, the presence of a female significantly decreased male signalling latency and increased signalling activity. Furthermore, while a majority of single males emitted only calling signals, a majority of males tested, in pairs, emitted only courtship phrases, apparently even before the first female signal was recorded. One possible explanation for this could be that distances limited to one vine leaf enable leafhoppers to perceive incidental vibrational cues originating from grooming, brochosome anointing or walking of conspecifics. Such signals could provide information about the presence nearby of another leafhopper. Under such conditions, more males might start signalling and with shorter latency. They might also omit long-range calling signals that are usually associated with the initial stages of mating behaviour, when individuals advertise their presence. The ‘call-fly’ behaviour, as observed in S. titanus, appears to be common among ‘Auchenorrhyncha’ (e.g. de Vrijer, Reference de Vrijer1986; Gwynne, Reference Gwynne1987; Hunt & Nault, Reference Hunt and Nault1991). It is conceivable that, when under natural conditions males and females are moving from plant to plant, they are unlikely to land on the same leaf and the most reliable way to test the plant for the presence of a sexually receptive female is to emit vibrational signals. Since, in S. titanus, the male calling signal is a shorter and less complex signal than the courtship phrase, less energy is needed for its production and, therefore, such signals are better suited for a quick test that, in most cases, will fail to elicit any response. In this respect, it is surprising that, while in play-back experiments females replied to a male calling signal (Mazzoni et al., unpublished results), in the work reported here, the female reply was never recorded in response to a male vibrational signal that could be classified as a calling signal, only to a courtship phrase. However, a male-female duet is a dynamic interaction, and both individuals can modify their signals and behaviour according to the partners' reply. It is possible that, when a female replied during the male calling signal, the male immediately extended his signal into a courtship phrase by adding other sections and different elements. There are some indications that the courtship phrase could be an extended and modified form of a calling signal. The first phrase in a series is characterized by the presence of a ‘rumble’ that is, during the first phrase, replaced by the ‘buzz’. The calling signal, and S1 section of a courtship phrase, are also composed of the same type of pulse (male pulse 1) and have the same pulse repetition time. It is interesting to note that the presence of courtship signals that consist of the basic calling signal with some additional components also raises some questions about the taxonomic position of S. titanus. Such courtship signals are characteristic of Cicadulini, Platymetopiini and Fieberiellini (Tishechkin, Reference Tishechkin, Drosopoulos and Claridge2006). This species is currently included in the Athysanini tribe, which is characterized by a single type of call that is produced during all stages of courtship behaviour.
Other possible explanations for decreased male signalling latency, and increased signalling activity and qualitative change in the type of male signal emitted, could be that males use visual cues and/or chemical signals to determine the presence of a female. However, although the role of chemical signals can not be excluded, there are, up to now, no reports confirming the role of chemical signals in the reproductive behaviour of leafhoppers. While visual cues could be used at close range, after males located the females, there was no indication that vision played a role in the initial stage. Males started to emit vibrational signals also when they faced away from the female or when they were on the opposite leaf surface.
In S. titanus, the female response is reduced to only one pulse that closely resembles the male pulses and is placed between pulses in the male signal. In leafhoppers and planthoppers, female signals usually consist of a series of single pulses (clicks) that are easily distinguished from male vibrational signals (e.g. Claridge, Reference Claridge1985a,Reference Claridge, Nault and Rodriguezb; de Vrijer, Reference de Vrijer1986; Heady et al., Reference Heady, Nault, Shambaugh and Fairchild1986; Gillham & de Vrijer, Reference Gillham and de Vrijer1995; Nuhardiyati & Bailey, Reference Nuhardiyati and Bailey2005). In S. titanus, the female signal has no sex-specific characteristics and, in the absence of any time pattern, on its own probably also carries only minimal species-specific information. However, female response latency is constant, and males can probably recognize a conspecific female signal because it is coupled with his own pulse. A constant latency of female reply has also been described in the duet of the deltocephaline leafhopper Balclutha incisa (Matsumura), in which females respond immediately after the main call component (Nuhardiyati & Bailey, Reference Nuhardiyati and Bailey2005). The fact that S. titanus females emit a varying number of pulses that alternate with those of the male signal indicates that females do not produce their signals with regular rhythm (i.e. generated by an endogenous oscillator) but, instead, wait and listen out for a male signal (i.e. pulse) and then reply to it. High amplitude pulses were used by some females to silence the signalling males. Rearing in the laboratory indicates that males typically eclose a week earlier than females, and they also need less time to reach sexual maturity than females (V. Mazzoni, unpublished results). In the field, interactions between sexually mature male leafhoppers and immature and unreceptive females are certainly very common; however, to our knowledge, there are no studies on the vibrational signals used in such interactions. Vibrational signals used by unreceptive females to silence a calling or courting male were, however, described in the southern green stink bug Nezara viridula (L.) (Čokl et al., Reference Čokl, Virant-Doberlet and Stritih2000), and it has been observed that females of the treehopper Ennya chrysura Fairmaire use vibrational signals to reject a courting male (Miranda, Reference Miranda2006). Further studies of male-female interactions, using younger and mated females, should reveal how frequent and important such repelling behaviour is.
Male-male interactions have been extensively studied in insects that rely on airborne sound communication (e.g Greenfield, Reference Greenfield1994, Reference Greenfield2005); however, much less is known about rivalry in insects communicating via substrate-borne vibrations. Substrate vibrations have been traditionally regarded as an inherently private channel that is free of potential competitors (e.g. Henry, Reference Henry1994), and only recently has it been argued that this is a less private communication channel than previously thought (Cocroft & Rodríguez, Reference Cocroft and Rodríguez2005). Male-male vibrational interactions, as a part of a vibrational repertoire, have been described in delphacid planthoppers (Ichikawa, Reference Ichikawa1982; Claridge & de Vrijer, Reference Claridge, de Vrijer, Denno and Perfect1994; Ott, Reference Ott, Denno and Perfect1994), flatid planthoppers (Virant-Doberlet & Žežlina, Reference Virant-Doberlet and Žežlina2007), leafhoppers (Heady et al., Reference Heady, Nault, Shambaugh and Fairchild1986; Hunt & Morton, Reference Hunt and Morton2001; Nuhardiyati & Bailey, Reference Nuhardiyati and Bailey2005), treehoppers (Cocroft & McNett Reference Cocroft, McNett, Drosopoulos and Claridge2006; Miranda Reference Miranda2006) and stink bugs (Čokl et al., Reference Čokl, Virant-Doberlet and Stritih2000). However, very little is known about the function of these male rivalry signals. In the leafhopper Graminella nigrifrons (Forbes), male chorusing has been described as a competitive strategy in courtship disruption (Hunt & Morton, Reference Hunt and Morton2001), and a similar function has been proposed for the planthopper Metcalfa pruinosa (Say) (Virant-Doberlet & Žežlina, Reference Virant-Doberlet and Žežlina2007). It has been proposed that males of the treehopper E. chrysura use vibrational signals to interfere, or jam, the courtship song of another male (Miranda, Reference Miranda2006). Our observations of S. titanus indicate the existence of complex male-male interactions, including disruption of an existing duet by masking the courtship phrase with a disturbance noise and satellite behaviour (e.g. eavesdropping – silent males approaching duetting female). Our results indicate that such alternative male tactics might be successful in preventing the males that initiated a duet from copulating with a female.
Despite its relatively simple form, the mating behaviour described in S. titanus suggests complex interactions, including alternative mating tactics (e.g. reviewed in Bailey, Reference Bailey2003; Bailey et al., Reference Bailey, Macleay and Gordon2006). Further studies should provide more insight into mechanisms that might be involved in the evolution of such a reproductive strategy. Despite considerable knowledge about the role of vibrational signals in species recognition in ‘Auchenorrhyncha’, we still know very little about mating systems and reproductive strategies under field conditions. The mating systems may also be shaped in part by ecological aspects of the environment, including population density (Ott, Reference Ott, Denno and Perfect1994; Cocroft, Reference Cocroft2003; Cocroft & Rodriguez, Reference Cocroft and Rodríguez2005). We do not know under what ecological conditions the mating behaviour of S. titanus evolved in its area of origin (US) since our knowledge derives almost exclusively from agricultural ecosystems (vineyards) in Europe.
The present study has provided the first information about the mating behaviour of S. titanus, which should serve as a foundation for more detailed studies. Our results show that it is worthwhile testing whether reproductive behaviour in S. titanus can be disrupted by play-back of vibrational signals. It has been shown that, in some leafhoppers, production of the male calling song is inhibited by the playback of random noise (Hunt & Morton, Reference Hunt and Morton2001) and that mating may be interrupted by external sounds of certain frequencies (Saxena & Kumar, Reference Saxena and Kumar1980). Further experiments might, therefore, suggest techniques for exploiting this phenomenon to design effective and low environmental impact control practices for these insects.
Acknowledgements
We wish to thank Drs Mike Claridge, Andrej Čokl and Mike Wilson for critically reading the earlier version of the manuscript, as well as Bill Symondson for improving the revised version. The work has been supported by research program P1-0255 (Slovenian National Research Agency), by Fondi di Ateneo of Pisa University and by a Safecrop grant.











