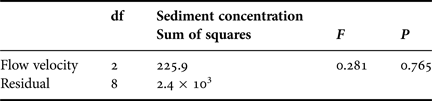INTRODUCTION
Scleractinian corals are important components of many marine ecosystems in the Mediterranean and elsewhere. Depending on species, scleractinians either form extensive branching frameworks as colonies develop, with individual polyps branching off from previous generations, secreting with growth a calcium carbonate skeleton directly onto previously deposited skeletal material (Wilson, Reference Wilson1979; Roberts et al., Reference Roberts, Wheeler, Freiwald and Cairns2009) or they can occur as individual cup-corals attached to previous coral structures or more commonly as isolated individuals on the seafloor (Bell & Turner, Reference Bell and Turner2000). Aside from the large-scale reef structures which may be built up by framework-constructing tropical, temperate and cold-water coral species such as Madracis mirabilis, Montastrea annularis, Lophelia pertusa, Solenosmilia variabilis (Dunstan, Reference Dunstan1975; Kaandorp et al., Reference Kaandorp, Sloot, Merks, Bak, Vermeij and Maier2005; Roberts et al., Reference Roberts, Wheeler, Freiwald and Cairns2009; Tracey et al., Reference Tracey, Rowden, Mackay and Compton2011), individuals of cup coral species (or small clusters of individuals) can increase the hard substrate surface of an area of seafloor, as well as influencing local flow dynamics and sediment transport/deposition (Bell & Turner, Reference Bell and Turner2000; Bell, Reference Bell2002; Cairns, Reference Cairns2007), thereby further increasing habitat complexity (Monismith, Reference Monismith2007; Buhl-Mortensen et al., Reference Buhl-Mortensen, Vanreusel, Gooday, Levin, Priede, Buhl-Mortensen, Gheerardyn, King and Raes2010).
Environmental parameters (e.g. temperature, salinity, pressure, illumination, suitable substrate availability) and biological characteristics (e.g. reproduction strategies and larval dispersal pathways) have been shown to influence distributions of various scleractinian species (Wilson & Harrison, Reference Wilson and Harrison1998; Kleypas et al., Reference Kleypas, Mcmanus and Menez1999; Randall & Szmant, Reference Randall and Szmant2009; Connolly & Baird, Reference Connolly and Baird2010; Caroselli et al., Reference Caroselli, Zaccanti, Mattioli, Falini, Levy, Dubinsky and Goffredo2012). A suitable food supply is also required for successful growth and colonization. The availability of a suitable food supply in suspension does not necessarily correlate with the ability of a particular species to successfully capture the food. Flow velocity is an important consideration here—high flow velocities can deliver a high flux of food to the vicinity of an organism, but can also deform the feeding tentacles of suspension feeding organisms (Labarbera, Reference Labarbera1984; Shimeta, 1993; Wildish & Kristmanson, Reference Wildish and Kristmanson2005; Purser et al., Reference Purser, Larsson, Thomsen and van Oevelen2010). Such deformation about tentacles reduces the exposed feeding surface area and may also cause detachment of prey following contact (Leversee, Reference Leversee1976; Wijgerde et al., Reference Wijgerde, Spijkers, Karruppannan, Verreth and Osinga2012).
Prey capture by suspension feeding organisms may also be less efficient when exposed to very turbid waters containing high amounts of refractory material, with feeding mechanisms becoming blocked or with energy expended on gathering unsuitable food material (i.e. refractory particles), though for most species the decision as to whether or not it is worth investing energy in the capture and digestion of suspended material is determined rapidly by the individual at onset of delivery (Genovese & Witman, Reference Genovese and Witman1999). High particulate abundance can also reduce the efficiency of photosynthetic zooxanthellae (Weber et al., Reference Weber, Lott and Fabricius2006). The utilization of refractory material does seem to be a feeding strategy for some coral species in certain turbid environmental niches, such as within areas of the Great Barrier Reef (Anthony, Reference Anthony2000), the Jamaican coast (Mills & Sebens, Reference Mills and Sebens2004) or the Rockall Bank (Van Oevelen et al., Reference Van Oevelen, Duineveld, Lavaleye, Mienis, Soetaert and Heip2009). In the Gulf of Lions in the Mediterranean Sea, the high energy hydrodynamics of the region, (characterized by high current speeds and high suspended sediment load) renders passive suspension feeders exposed to high particulate fluxes and sedimentation rates (Palanques et al., Reference Palanques, de Madron, Puig, Fabres, Guillén, Calafat, Canals, Heussner and Bonnin2006; Curran et al., Reference Curran, Hill, Milligan, Mikkelsen, Law, Madron and Bourrin2007; Puig et al., Reference Puig, Palanques, Orange, Lastras and Canals2008). Further, the resuspension of seabed sediments during trawling, an activity carried out extensively through the Gulf (Durrieu de Madron et al., Reference Durrieu de Madron, Ferré, Corre, Grenz, Conan, Pujo-Pay, Buscail and Bodiot2005; Ferré et al., Reference Ferré, Madron, Estournel, Ulses and Corre2008), can further increase the suspended load of refractory material (Yahel et al., Reference Yahel, Yahel and Genin2002).
In this paper we investigate the net prey capture efficiency of Balanophyllia europaea, an endemic Mediterranean sublittoral zooxanthellate solitary coral (Zibrowius, Reference Zibrowius1980; Aleem & Aleem, Reference Aleem and Aleem1992; Goffredo et al., Reference Goffredo, Mezzomonaco and Zaccanti2004). Although distributed widely around the Mediterranean ocean, the preferred ecological niche of the species is not well known. By examining net prey capture in this species under a range of experimental flow and suspended particulate concentration conditions (such as under elevated particle concentrations as may result from trawling activity) we aim to assess whether either or both of these two environmental factors may potentially be important controls on species distribution.
MATERIALS AND METHODS
Coral and sediment sampling
Balanophyllia europaea specimens were collected on the 15, 17 and 21 June 2011 by the dive team of the Banyuls-sur-Mer Oceanological Observatory, France. In total, 70 live coral cups were collected intact for use in the experimental work. Corals were collected from Cape Oullestrell (42°30′001″N 3°8′000″E 10–25 m depth, seven corals) and Cape d'Osne (42°28′000″N 3°8′500″E, 10–25 m depth, 63 corals). The region is commonly subjected to storm events (Guillén et al., Reference Guillén, Bouriin, Palanques, Durrieu de Madron, Puig and Buscail2006; Ulses et al., Reference Ulses, Estourmel, Puig, de Durrieu and Marsaleix2008) and occasionally high particle transport events, where material is swept westward along the upper continental shelf of the Gulf of Lions as a whole and into the western Mediterranean canyons systems (Palanques et al., Reference Palanques, de Madron, Puig, Fabres, Guillén, Calafat, Canals, Heussner and Bonnin2006), with Ferré et al. (Reference Ferré, Guizien, Madron, Palanques, Guillén and Grémare2005, Reference Ferré, Madron, Estournel, Ulses and Corre2008) reporting that the shallow regions of the Gulf of Lions can be highly effected by these storms, with large volumes of material resuspended.
The Gulf of Lions is also highly impacted by trawling activity, especially the shelf and slope areas, with the impact of these activities (seafloor perturbation and particulate resuspension (Rosenberg et al., Reference Rosenberg, Nilsson, Grémare and Amorouroux2003) felt across the continental shelf, slope and within the canyon systems (Puig et al., 2012). Though fishing activity is reported as likely having an impact also in the shallow coastal areas (Rosenberg et al., Reference Rosenberg, Nilsson, Grémare and Amorouroux2003) we are unaware of any scientific studies on the magnitude and consequences of this activity published to date for the area of coral sampling.
After collection the corals were maintained in a temperature controlled laboratory (maintained throughout the experimental period at 18–20°C, to match temperatures recorded at sites of collection) in flow-through aquaria, supplied with temperature controlled surface seawater.
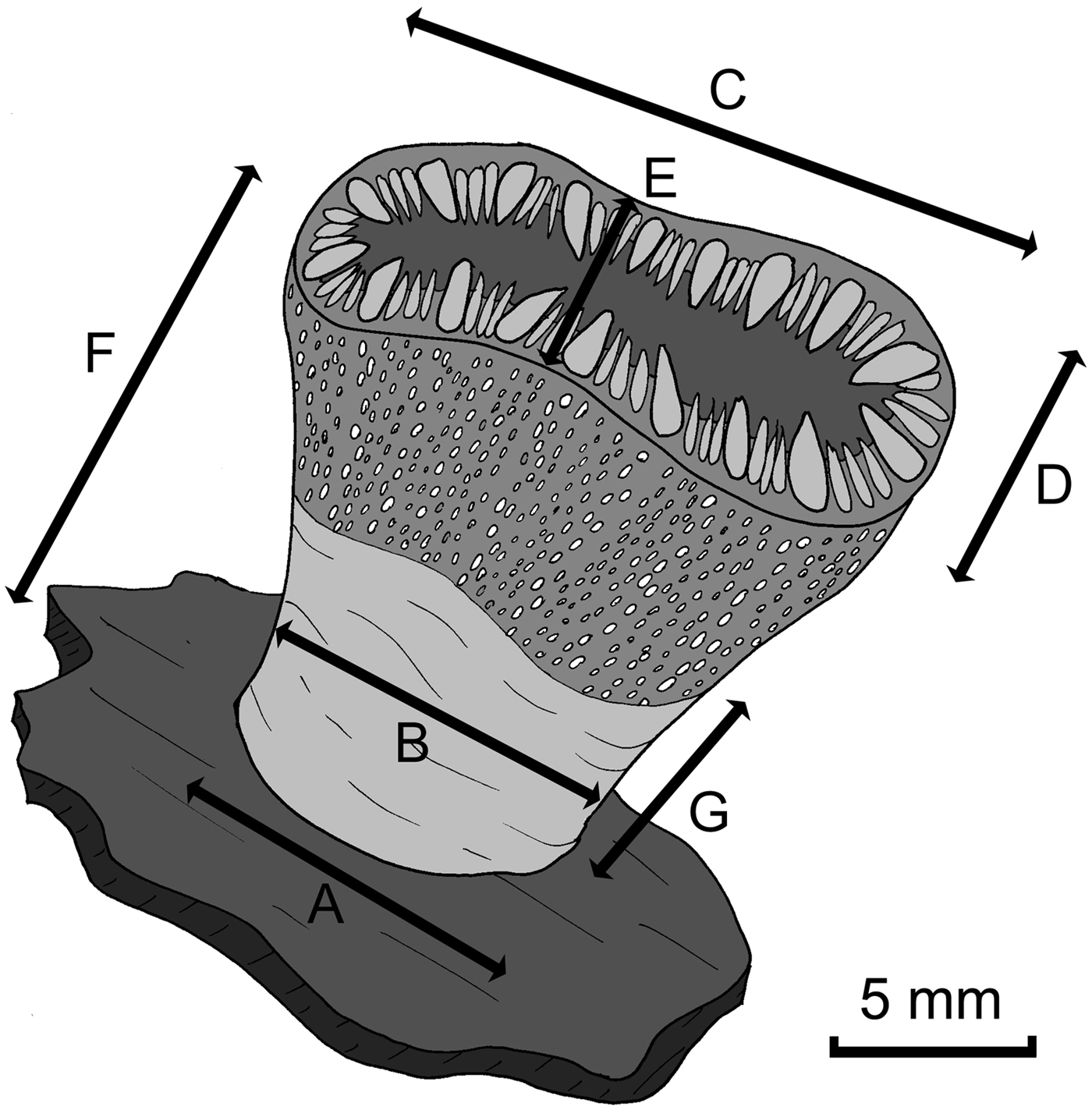
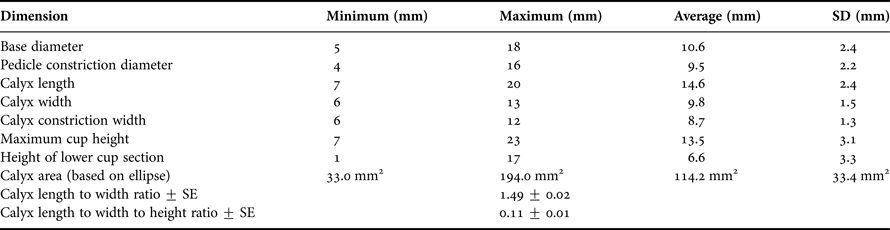
Physical dimensions of each coral cup were measured, after the protocol used for the cup coral Caryophyllia smithii by Bell & Turner (Reference Bell and Turner2000) with mean averages, maximum, minimum and standard deviations for each of the measured dimensions computed (Figure 1). Additionally, the volume of each polyp was estimated using the formula
(Table 1) after Goffredo et al. (Reference Goffredo, Arnone and Zaccanti2002). The majority of the corals collected were still attached to small pieces of the rocky or calcareous substratum, these were cleaned of epifauna then left attached throughout the experimental runs.

Fig. 1. Dimensions of each collected coral cup measured (see Table 1): (A) base diameter; (B) pedicle constriction diameter; (C) calyx length; (D) calyx width; (E) calyx constriction width; (F) maximum cup height; (G) height of lower cup section.
Table 1. Mean dimensions (with standard deviations) of the 70 coral cups used in the present study.

Sediment for use in experimental runs was collected from a sublittoral soft bottom close to the site of coral collection (42°28′250″N, 3°9′500″E, 40 m depth). Collection was made using the RV ‘Norppa’, operated by Jacobs University Bremen on 22 June 2011. The sediment was sieved to remove the coarser >0.5 mm fraction. The finer fraction was then kept in the laboratory at 17°C until used in experimental runs. This finer fraction was quantified by taking three subsamples and determining the dry weight (DW) of these on 0.45 cellulose acetate filters. From these sub-sample weights, the DW associated with a particular volume of fine fraction material was calculated.
Flume set-up
Three replicate recirculating flumes were set up in an 18–20°C thermo constant room at the Banyuls-sur-Mer Oceanographic Observatory, France. The flumes used followed the design described in Berntsson et al. (Reference Berntsson, Jonsson, Larsson and Holdt2004) and Purser et al. (Reference Purser, Larsson, Thomsen and van Oevelen2010). For this investigation, the motors used to drive the recirculation propellers were capable of maintaining a constant flow of between 2.5 and 15 cm s−1. In all experimental runs, a 50 l volume of freshly pumped, temperature controlled seawater (18–20°C) was used in each flume.
Prey and sediment characterization
The prey used in the experimental runs was freshly hatched Artemia salina nauplii (Great Lakes, Sanders brand). Artemia salina nauplii, approximately 400–500 μm length when freshly hatched (Naceur et al., Reference Naceur, Jenhani, El cafsi and Romdhane2008), are commonly used as a food supply for scleractinian (Coles, Reference Coles1969; Hii et al., Reference Hii, Soo and Liew2009; Purser et al., Reference Purser, Larsson, Thomsen and van Oevelen2010; Tsounis et al., Reference Tsounis, Orejas, Reynaud, Gili, Allemand and Ferrier-Pages2010) and gorgonian (Dai & Lin, Reference Dai and Lin1993) corals in feeding experiments.
Throughout the experimental study, fresh batches of A. salina nauplii were prepared daily. Following hatching the daily concentrations of A. salina available were quantified by taking five 0.5 ml sub-samples from the incubation aquaria (Eppendorf pipette used). These sub-samples were then filtered onto 0.45 µm filter papers. The number of nauplii on each filter paper was then counted under a stereomicroscope (20× magnification). From these five counts, the mean average A. salina filter−1 was calculated and the concentration of hatched A. salina nauplii within the incubation aquaria then determined. During all of the experimental runs conducted, a starting prey density of ~500 A. salina l−1 was used. Given that the flumes were filled for each experimental run with 50 l seawater, ~25,000 A. salina were therefore required for each flume for each experimental run. The volume of water required from the incubation aquaria to achieve this total was calculated from the hatched A. salina concentration. A 5–10% variability in this starting density was apparent from subsample analysis.
To determine the carbon (C) and nitrogen (N) content of the A. salina nauplii used in this study, three 0.5 ml subsamples of freshly hatched nauplii were filtered onto Whatman GF/F filters. Samples were analysed in a EURO EA Elemental Analyser after the method in Pike & Moran (Reference Pike and Moran1997).
The percentage concentrations of total particulate nitrogen (TPN), total particulate carbon (TPC) and particulate organic carbon (POC) within the sediment used in the experimental runs was determined using a EURO EA Elemental Analyser (Wagner et al., Reference Wagner, Purser, Thomsen, Jesus and Lundälv2011).
Experimental runs
To investigate the relationship that flow velocity and/or suspended particulate concentration may have with the net prey capture rates of Balanophyllia europaea corals, eight experimental runs were conducted in three replicate recirculation flumes. Experimental work commenced 2 d after collection of the last corals, with one experimental run conducted daily from then on. In all cases, experimental runs were conducted in darkness from 10:00–14:00 CET, and in the temperature controlled laboratory described above. The order of experimental runs was randomized. The full list of runs with flow velocities and sediment concentrations investigated is given in Table 2. Flow velocities (2.5, 5, 7.5 and 15 cm s−1) were selected to represent those experienced in the western regions of the Mediterranean shelf ecosystems (Lapouyade & Durrieu De Madron, Reference Lapouyade and Durrieu de Madron2001; Curran et al., Reference Curran, Hill, Milligan, Mikkelsen, Law, Madron and Bourrin2007; Ulses et al., Reference Ulses, Estourmel, Puig, de Durrieu and Marsaleix2008). Sediment concentrations (0, 7.3 and 170 mg l−1) were chosen to reflect both those levels regularly experienced in periodic turbidity events in the westerly Gulf of Lions, as well as the concentrations experienced more irregularly, as a result of the occasional high concentration particulate transport events reported in the region (Durrieu De Madron et al., Reference Durrieu de Madron, Nyffeler and Godet1990; Lapouyade & Durrieu De Madron, Reference Lapouyade and Durrieu de Madron2001; Ferré et al., Reference Ferré, Guizien, Madron, Palanques, Guillén and Grémare2005; Palanques et al., Reference Palanques, de Madron, Puig, Fabres, Guillén, Calafat, Canals, Heussner and Bonnin2006).
Table 2. ANOVA table showing the comparison of calyx area of the corals used in the 18 experimental runs.

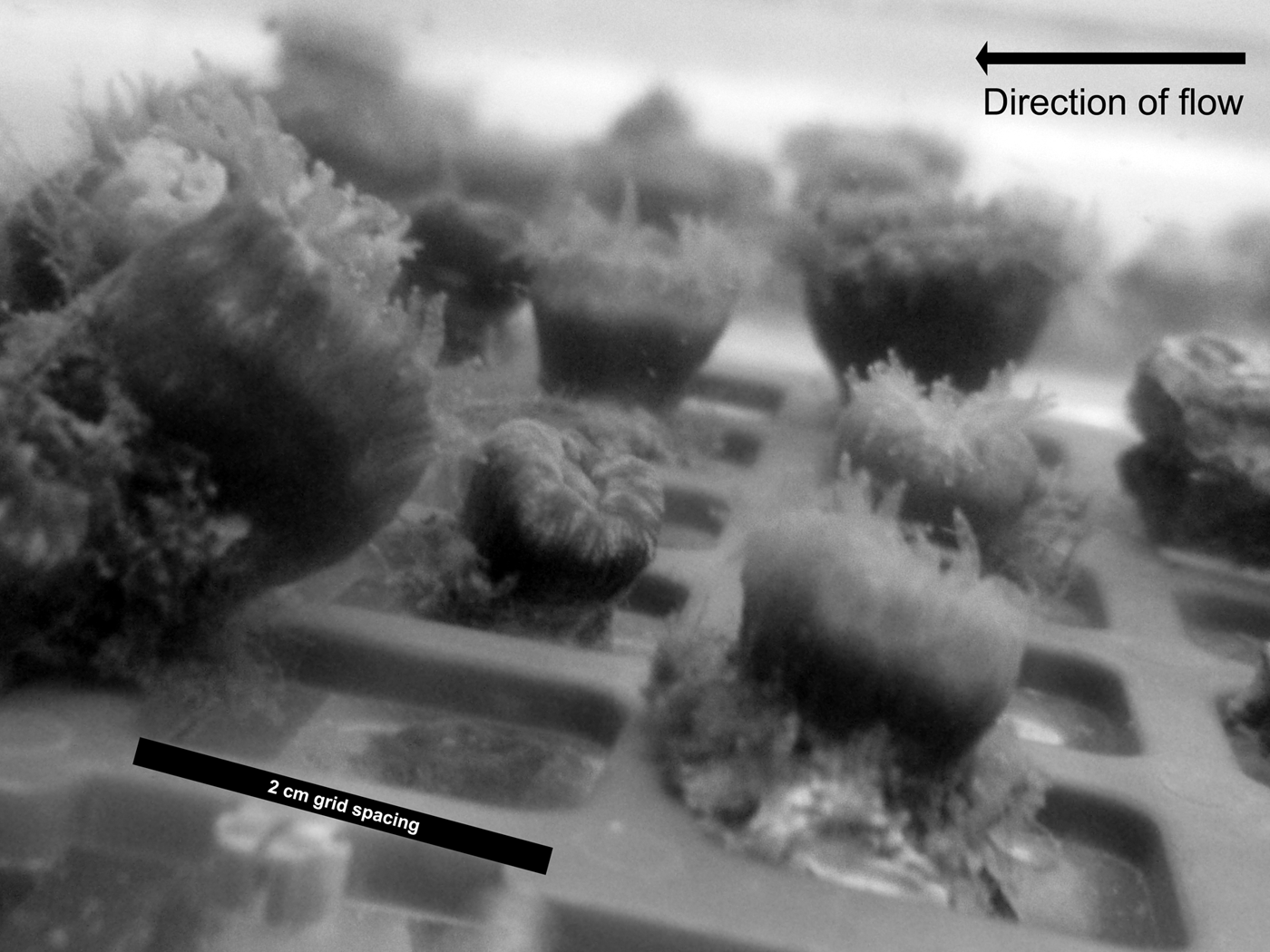
For each run, 23 corals were randomly assigned to each flume and arranged in a grid pattern in the flume test section (Figure 2) under the experimental flow velocity conditions selected for the particular run (see above). The corals used in each flume were randomly selected from the 70 collected individuals for each run. The corals were then allowed to acclimatize to the flume environment for 2 h.

Fig. 2. Coral specimens placed within the test section of a recirculation flume. The majority of polyp tentacles can clearly be seen extended from the calyx, as was observed throughout all experimental runs.
Following the acclimatization period, the particular concentration of sediment required for the experimental run was delivered to each flume in a controlled fashion with a 60 ml syringe. The concentrations of material required to reach the desired suspended sediment concentrations for each run were calculated from the DW determination made for the collected fine fraction of sediment.
After delivery of the sediment (if sediment required by the parameters of a particular experimental run) the Artemia salina nauplii were added at a steady rate to each flume to achieve the intended density of ~500 A. salina nauplii l−1, following the method described in Purser et al. (Reference Purser, Larsson, Thomsen and van Oevelen2010). In summary, a quantity of freshly hatched nauplii is added to each experimental flume from a freshly hatched batch of A. salina, to achieve an approximately equal and known starting density of A. salina in each flume (~500 A. salina nauplii l−1). This initial concentration of A. salina represented the time zero concentration for each experimental run. After A. salina delivery, triplicate sub-samples of ~150 ml volume were taken from each flume hourly, for a 4 h period. These sub-samples were filtered onto 0.45 µm filter papers and the numbers of A. salina nauplii on each paper counted under a dissection microscope. From these counts the hourly and average hourly net prey capture rates over the 4 h duration of each experimental run was determined for each flume. The hourly average capture rates were determined from the triplicate subsamples extracted at 1, 2, 3 and 4 h after A. salina delivery to the flumes, with the decrease in concentration between each time step (i.e. between 1 and 2 h sub-samples, 2 and 3 h sub-samples, etc.) being determined. The average net capture rate for the 4 h experimental period was made by comparing the initial concentration (ca500 A. salina nauplii l−1) with the concentrations in the subsamples extracted after 4 h. Given the known number of corals within each flume, the net prey capture rate coral−1 was then computed from the flume total capture rates.
Triplicate flume control runs were conducted in which the concentration of Artemia salina in suspension was monitored over the 4 h experimental period in flume runs without corals present. Further 3 × 4 h control runs were carried out with no corals in flumes, but with suspended sediments present, to determine whether sediment in suspension alone would result in a reduction of suspended A. salina over time.
Following each experimental run, the corals were removed and returned to the temperature controlled flow-through aquaria for 24 h. During this 24 h period the experimental flumes were flushed with flow-through piped seawater and filtered to ensure the removal of any remaining A. salina nauplii, waste products or coral mucus from each.
To ensure that coral randomizations for each experimental run resulted in coral populations within each flume being comparable, the calyx areas of the corals in the 18 experimental groups (the flume populations for each experimental run using live corals, rather than controls—three flumes and six experimental treatment runs, so 18 experimental groups) was compared with a one way ANOVA (Table 2).
Coral activity was checked hourly to ensure the polyps were expanded during the individual experimental runs.
Statistical analysis
For each experimental run, the average hourly net prey capture rates coral−1 was determined from the reduction in suspended A. salina concentration between the start of the experimental run and that measured after 4 h. Given the randomized assignment of corals to flumes for each run and the randomization of run order, these triplicate hourly net prey capture rate data were suitable for ANOVA statistical tests.
To determine whether coral presence/absence or sediment presence/absence had an impact on the net prey capture rates by Balanophyllia europaea (or the rate of depletion of suspended A. salina per hour in the case of flumes containing no corals), a two-way ANOVA was conducted. For this two-way ANOVA the independent factors used were coral presence/absence (levels set as either ‘live corals present’ or ‘live corals absent’) and sediment present/absent (levels set as either ‘170 mg l−1 sediment in suspension’ or ‘0 mg l−1 sediment in suspension). For this test, only results from experimental runs at 5 cm s−1 were used, to remove the confounding factor of flow speed in the analysis.
To assess whether or not flow velocity has an effect on the net prey capture rates achieved, a one-way ANOVA was conducted. For this test four levels of flow velocity were set (2.5, 5, 7.5 and 15 cm s−1), which represent the investigated flow velocities.
The possible influence of suspended sediment concentrations on net prey capture rates was tested with a further one-way ANOVA. For this test three levels of suspended sediment concentration were set (0, 7.3 and 170 mg l−1). For this test, a 5 cm s−1 flow velocity was maintained across sediment concentration treatments.
For all ANOVA tests, the data were checked for normality (and found to be normal in all cases). Levene's test was used to check homogeneity of variance across all the tests. In all cases, Levene's test indicated that there was a significant deviation of homogeneity in the data. To allow the use of the ANOVA test in light of this the Welch's F statistic was reported for the ANOVA tests throughout (Field, Reference Field2013). In situations where significant differences in A. salina uptake between treatments were indicated by the ANOVA test, Fisher's LSD post hoc tests were used to determine between which levels these differences were significant.
RESULTS
The morphological measurements of the corals used in this study are summarized in Table 1. The calyx area measurements for the corals used in each experimental run were compared with an ANOVA test to ensure that the experimental populations comprised populations with a roughly similar variation in feeding area size. The outputs of this ANOVA are given in Table 2, showing that the coral calyx areas were not statistically different across experimental runs.
Artemia salina nauplii and sediment carbon and nitrogen characterization
The percentage of TPN within the sediment used in the experimental runs was 0.15% (SD = 0.005), percentage of TPC was 3.3% (SD = 0.09) and percentage POC was 0.89% (SD = 0.04).
Within each A. salina nauplius total N concentration was 0.27 µg N A. salina nauplius−1 (SD = 0.01), concentration of total C was 1.2 µg C A. salina nauplius−1 (SD = 0.05) and total organic C was 1.13 µg C A. salina nauplius−1 (SD = 0.06).
Flow velocity and net capture rates
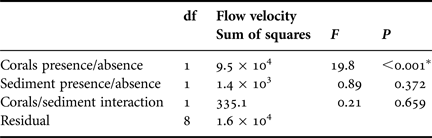
Given a flow velocity of 5 cm s−1, mean decreases in suspended nauplii in flumes containing live corals (204 A. salina polyp−1 h−1 (SD = 5.5)) were at least 4× those recorded from flumes containing no corals (4.5 A. salina polyp−1 h−1 (SD = 19.6)) (Table 3, Figure 3), with this difference being statistically significant (ANOVA, F = 19.8, P < 0.001, Table 4). The presence or absence of 170 mg l−1 suspended sediments within the flumes did not statistically impact on suspended nauplii concentrations within flumes containing no corals (ANOVA, F = 0.89, P > 0.05, Table 4). No significant interaction effect between corals presence/absence with suspended sediment presence/absence was indicated (ANOVA, F = 0.21, P > 0.05, Table 4).
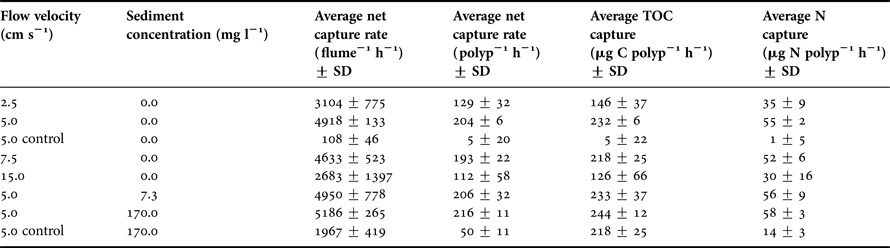
Table 3. Average Artemia salina net capture rates polyp−1 h−1, average TPC polyp−1 h−1 and average POC polyp−1 h−1 from the experimental and control runs. Control runs represent those with dead Balanophyllia europaea present in the experimental flumes, with hourly capture rates based on a hypothetical 24 polyp density.

Table 4. ANOVA table for the effects of presence/absence of corals, and sediments and the interaction between these two parameters on net prey capture rates.

*, indicates significant differences in the net prey capture rates at the 0.001 threshold.
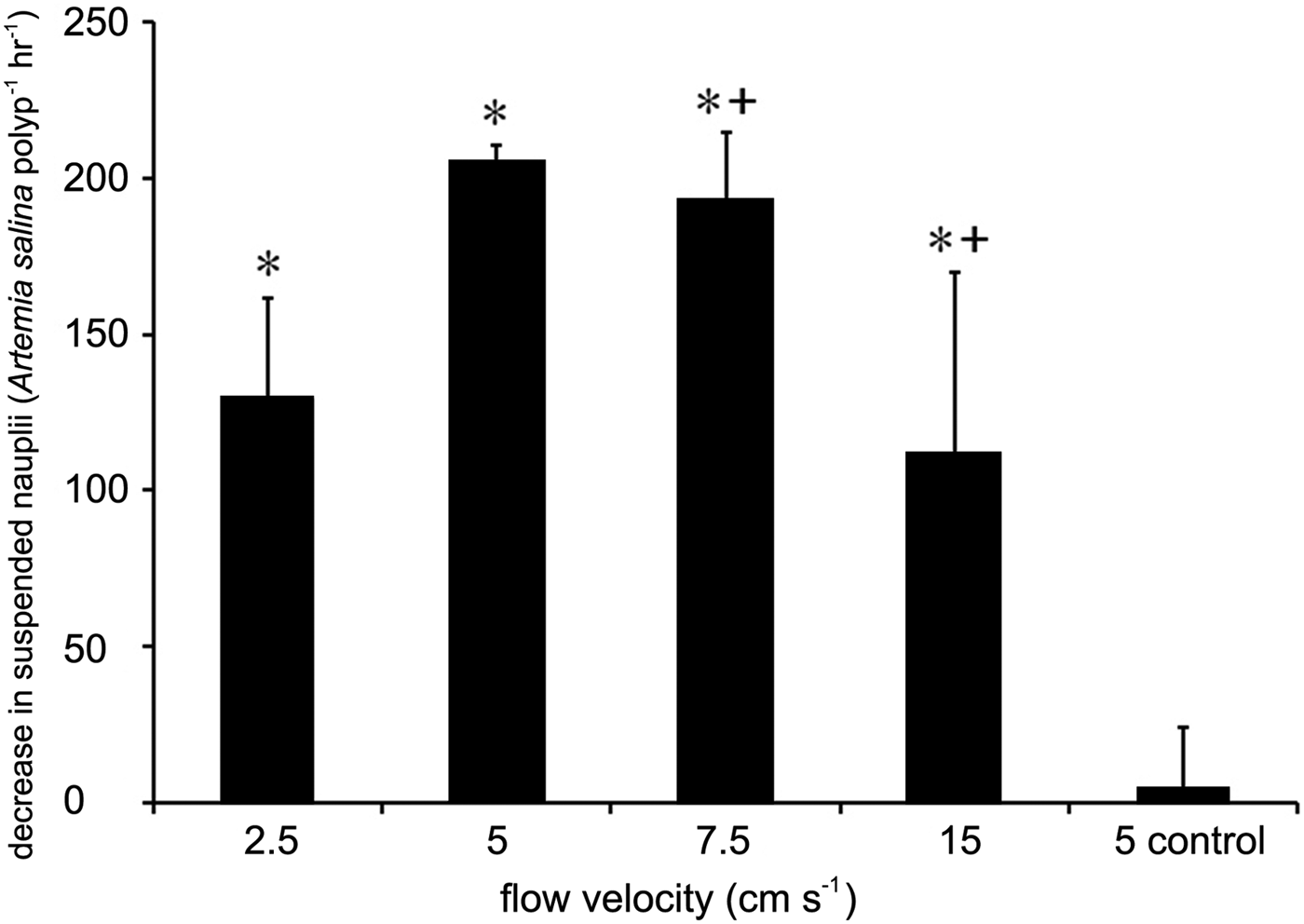
A range of mean net capture rates by polyps were recorded under the different flow velocities tested, with maximum net capture rates measured in corals under 5 cm s−1 flow (204 A. salina polyp−1 h−1 (SD = 5.5)) (Figure 3; Table 3). Net prey capture rates were significantly different between flow rates (ANOVA, F = 5.2, P < 0.05, Table 5). The post hoc Fisher's LSD test indicated that prey net capture rates under flow velocities of 2.5 cm s−1 (129.3 A. salina polyp−1 h−1 (SD = 32.3)) and 15 cm s−1 (111.8 A. salina polyp−1 h−1 (SD = 58.2)) were significantly lower (P < 0.05) than under flow velocities of 5 cm s−1. The test also indicated net capture rates were significantly lower under 15 cm s−1 flow than under 7.5 cm s−1 flow velocities (193.1 A. salina polyp−1 h−1 (SD = 21.8)) (Figure 3).

Fig. 3. Mean net capture rates of Artemia salina polyp−1 h−1 in freshly piped seawater under different flow velocities. Error bars represent 1 SD (N = 3 for each treatment). * indicates significant differences from the control runs at the 0.05 threshold. + indicates a significant difference in net capture rates between 7.5 and 15 cm s−1 at the 0.05 threshold.

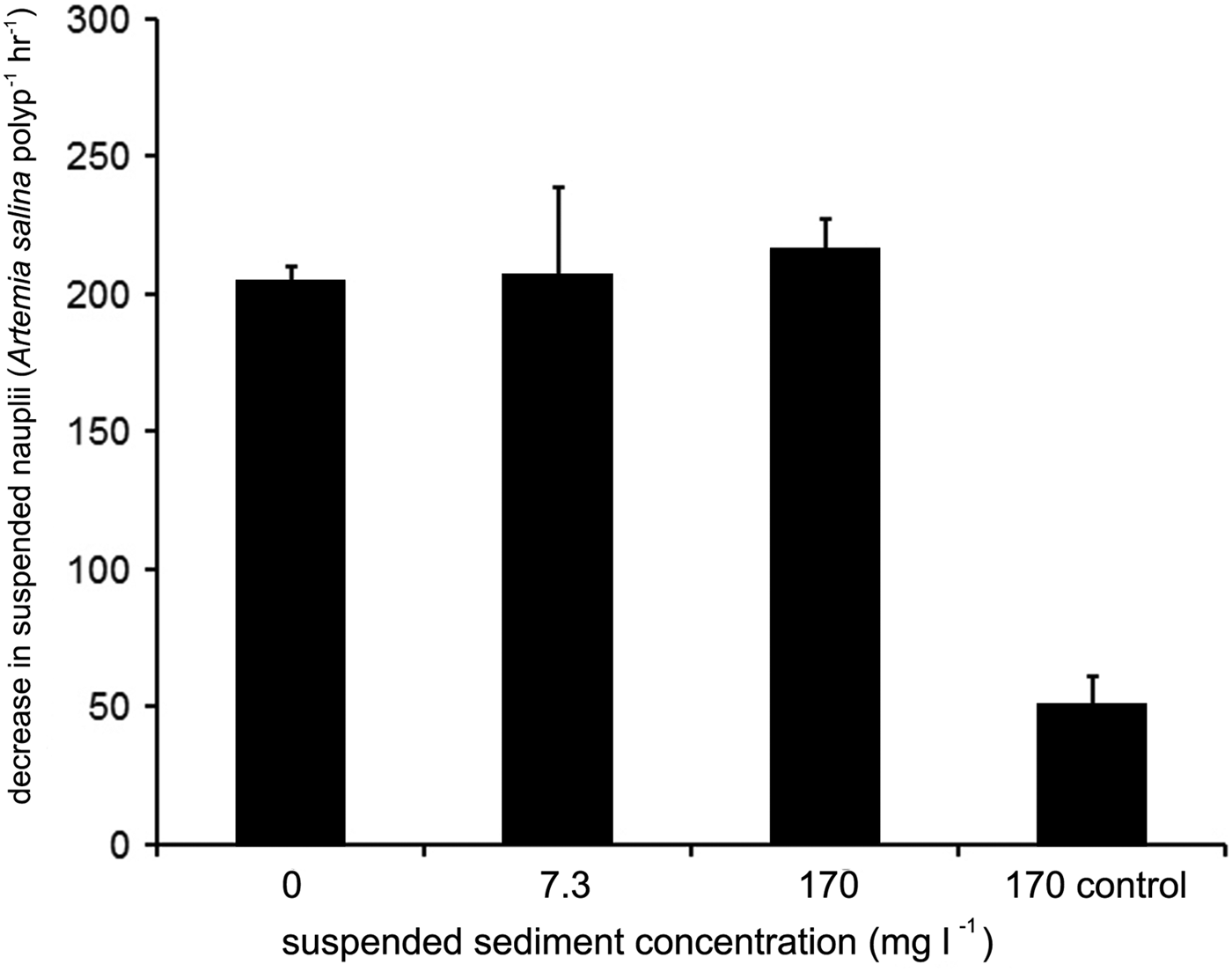
Fig. 4. Mean net capture rates of Artemia salina polyp−1 h−1 under 5 cm s−1 when exposed to various concentrations of suspended sediments. Error bars represent 1 SD (N = 3 for each treatment).
Table 5. ANOVA table for net prey capture rates by corals maintained in flumes under different flow velocities.

*, indicates significant differences in the net prey capture rates at the 0.001 threshold.
Suspended sediment concentrations and net capture rates
No significant increase in mean net capture rates by corals across flumes containing increasingly high suspended sediment concentrations was recorded (Table 3; Figure 4), (ANOVA, F = 0.281, P > 0.05, Table 6). Though there was some loss (1966 nauplii flume−1 h−1, SD = 419.1) of suspended nauplii in flumes containing 170 mg l−1 sediment and no corals, this rate was far lower than in flumes containing both corals and sediment at the same concentration (5187.5 nauplii flume−1 h−1, SD = 264.5). There was no initial retraction of tentacles apparent following delivery of suspended sediment (at any of the concentrations investigated) to the flumes. Further, during runs with or without suspended sediment addition, tentacles of the coral polyps were observed to be generally extended throughout the 4 h experimental period (data not shown).
Table 6. ANOVA table for net prey capture rates under exposures to various concentrations of suspended sediments.
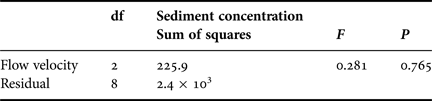
DISCUSSION
Flow speed and net capture rates by Balanophyllia europaea
Net capture rates of Artemia salina nauplii by Balanophyllia europaea polyps were higher under 5 cm s−1 flow velocity than the other velocities tested. Prey capture under such a velocity has been shown to be most efficient in other species, such as the shallow water (<40 m, depths comparable with the coral specimens used in the current study) tropical coral Meandrina mendrites (Sebens & Johnson, Reference Sebens and Johnson1991). Other scleractinian species occupying similar depths, however, have been found to capture prey most efficiently under higher flow velocities. Some morphotypes of the tropical coral Agaricia agaricites achieve highest net capture rates under flows of ~30 cm s−1, well above the 5 cm s−1 conditions, observed here for B. europaea (Helmuth & Sebens, Reference Helmuth and Sebens1993). Both M. mendrites and A. agaricites exhibit a more tabulate, colonial growth form than B. europaea, whereas cup corals, and framework building corals with a less continuous solid structure, seem to capture food under lower flow velocities most effectively (e.g. Lophelia pertusa, under ~2.5 cm s−1 flow in Purser et al. (Reference Purser, Larsson, Thomsen and van Oevelen2010)).
Net prey capture efficiency under flow is predominantly determined by the ability of an individual to maintain active extension of feeding tentacles within a particular flow regime and/or to keep attached any secreted mucus strands utilized in prey capture (Shimeta & Koehl, Reference Shimeta and Koehl1997; Mills & Sebens, Reference Mills and Sebens2004; Lewis & Price, Reference Lewis and Price2009; Riisgård & Larsen, Reference Riisgård and Larsen2010) in an energy efficient manner. The inability to maintain extended polyp tentacles reduces the feeding surface area and may render food capture mechanisms less efficient (Leversee, Reference Leversee1976; Labarbera, Reference Labarbera1984; Naumann et al., Reference Naumann, Richter, Zibdah and Wild2009). The loss of mucus material to suspension represents both a loss of the captured prey and the resources expended in the secretion of the mucus (Wild et al, Reference Wild, Rasheed, Werner, Franke, Johnstone and Huettel2004; Larsson et al., Reference Larsson, Van Oevelen, Purser and Thomsen2013). Though freshly hatched nauplii as reported here are commonly used in such experimental work (Purser et al., Reference Purser, Larsson, Thomsen and van Oevelen2010; Tsounis et al., Reference Tsounis, Orejas, Reynaud, Gili, Allemand and Ferrier-Pages2010; Wijgerde et al., Reference Wijgerde, Diantari, Lewaru, Verreth and Osinga2011), net capture or consumption rates of unhatched Artemia cysts (Sebens & Johnson, Reference Sebens and Johnson1991) or particulate material (Anthony, Reference Anthony2000) are also reported. The differences in both size and composition of these food types may trigger different feeding responses in different species, and therefore render strict comparisons of net prey capture results difficult.
Given that the corals used were collected from two distinct locations the possibility that there could be some genetic difference in the prey capture rates between populations cannot be wholly discounted. To date, however, the authors are unaware of any published empirical results indicating that genetic variation within coral species can lead to differences in prey capture ability. As opportunistic suspension feeders, it is possible that the two populations were exposed to different food types in the field, and that there may have been some adaptation toward different prey categories or sizes as a factor of differing environmental conditions. We consider it likely that any such differences between these two populations would be minor, and would have less of an impact on feeding ability than the variability between individuals within the species, and that calyx area is the most significant factor in prey capture rate determination (Hughes, Reference Hughes1980). The randomization of corals used in each experimental run ensured that there was no significant variation in the calyx area of the corals used in each run of the current study (Table 2).
The impact of particle exposure on Balanophyllia europaea net prey capture rates
The presence, absence or concentration of particulate material in suspension had no significant impact on the observed net capture rates by Balanophyllia europaea in this study. Though a general tolerance and even utilisation of refractory resuspended particulate material by scleractinians has been reported (Rosenfield et al., Reference Rosenfeld, Bresler and Abelson1999; Anthony, Reference Anthony2000; Van Oevelen, Reference Van Oevelen, Duineveld, Lavaleye, Mienis, Soetaert and Heip2009) high particle concentrations have been shown to inhibit feeding and stress corals to a variable degree, dependant on particulate composition and coral species (Pearson & Rosenberg, Reference Pearson and Rosenberg1978; Weber et al., Reference Weber, Lott and Fabricius2006; Flores et al., Reference Flores, Hoogenboom, Smith, Cooper, Abrego and Negri2012). In temperate and cold water corals the experimental data are less extensive than for tropical corals, and variable in detail according to species, but a reduced prey capture efficiency under suspended particle exposure is generally reported (Brooke et al., Reference Brooke, Holmes and Young2009; Larsson & Purser, Reference Larsson and Purser2011; Larsson et al., Reference Larsson and Purser2013).
Though the net prey capture rates of Artemia salina were not influenced by the presence or absence of suspended sediments, it is possible that direct ingestion of the captured nauplii could have been inhibited by particle presence. In a recent paper, Wijgerde et al. (Reference Wijgerde, Diantari, Lewaru, Verreth and Osinga2011) indicate that digestion of A. salina prey can occur externally to the scleractinian Galaxea fascicularis. This may indicate that the presence of refractory particulate material in addition to nauplii on the tentacles does not mechanically interfere with feeding; that is, the tentacles do not have to retract to transfer the nauplii to the coral cup prior to digestion, a mechanical process which may be inhibited by particulate presence (Shimeta & Jumars, Reference Shimeta and Jumars1991; Stafford-Smith & Ormond, Reference Stafford-Smith and Ormond1992).
Resilience to sediment exposure is not surprising in corals collected from the Gulf of Lions coastal sublittoral environment. The region is subjected to storm events (Guillén et al., Reference Guillén, Bouriin, Palanques, Durrieu de Madron, Puig and Buscail2006; Ulses et al., Reference Ulses, Estourmel, Puig, de Durrieu and Marsaleix2008) and occasional large scale flushing events (‘cascading’), where material is swept westward along the upper continental shelf prior to transport via the western Mediterranean canyons systems to the deep sea. Under the tested, environmentally reasonable, particulate concentrations a net capture of >230 µg C polyp−1 h−1 was achieved across treatments (as determined from nauplii analysis and net capture rates), though the amount of this carbon actually utilized by the coral was not quantified here. Material removed from suspension by incorporation within coral mucus strands, and potentially deposited ‘downstream’ of the coral within the flume following mucus breakage, would represent in the field an increased flux of carbon to the benthic ecosystem, potentially influencing community development.
Habitat selection by Balanophyllia europaea
The current study has demonstrated under laboratory conditions the ability of Balanophyllia europaea to capture Artemia salina nauplii under particle concentration and flow velocity conditions encountered during storm events in the western Mediterranean (Ulses et al., Reference Ulses, Estourmel, Puig, de Durrieu and Marsaleix2008). Within the geological record a more ubiquitous presence of the major scleractinian species across the Mediterranean in the past has been indicated (Taviani et al., Reference Taviani, Freiwald, Zibrowius, Freiwald and Roberts2005). The higher abundance of both shallow and deep water scleractinian corals in the western (in comparison with the eastern) Mediterranean today (Freiwald et al., Reference Freiwald, Beuck, Rüggeberg, Taviani and Hebbeln2009) may to some degree be the result of the delivery of large volumes of food via large scale circulation processes, processes possibly susceptible to climate change following increasing surface warming and the associated potential slowdown of ocean transport patterns in the area (Durrieu de Madron et al., Reference Durrieu de Madron, Guieu, Sempéré, Conan, Cossa, D'Ortenzio, Estournel, Gazeau, Rabouille, Stemmann, Bonnet, Diaz, Koubbi, Radakovitch, Babin, Baklouti, Bancon-Montigny, Belviso, Bensoussan, Bonsang, Bouloubassi, Brunet, Cadiou, Carlotti, Chami, Charmasson, Charrière, Dachs, Doxaran, Dutay, Elbaz-Poulichet, Eléaume, Eyrolles, Fernandez, Fowler, Francour, Gaertner, Galzin, Gasparini, Ghiglione, Gonzalez, Goyet, Guidi, Guizien, Heimbürger, Jacquet, Jeffrey, Joux, Le Hir, Leblanc, Lefèvre, Lejeusne, Lemé, Loÿe-Pilot, Mallet, Méjanelle, Mélin, Mellon, Mérigot, Merle, Migon, Miller, Mortier, Mostajir, Mousseau, Moutin, Para, Pérez, Petrenko, Poggiale, Prieur, Pujo-Pay, Pulido-Villena, Raimbault, Rees, Ridame, Rontani, Ruiz Pino, Sicre, Taillandier, Tamburini, Tanaka, Taupier-Letage, Tedetti, Testor, Thébault, Thouvenin, Touratier, Tronczynski, Ulses, Van Wambeke, Vantrepotte, Vaz and Verney2011). The last 35 years has seen a general increase in the temperatures of surface waters in the Mediterranean, with this increase generally faster in the eastern Mediterranean (Skliris et al., Reference Skliris, Sofianos, Gkanasos, Mantziafou, Vervatis, Axaopoulos and Lascaratos2012). In addition to the Gulf of Lions being a main area for formation of cold waters within the Mediterranean Sea, the proximity of the region to the cooling influence of the Atlantic during periods of global temperature rise may be a further reason for the higher densities of scleractinians observed in the western Mediterranean, given the high metabolic sensitivity to temperature often reported for species (Dodds et al., Reference Dodds, Roberts, Taylor and Marubini2007; Goffredo et al., Reference Goffredo, Caroselli, Pignotti, Mattioli and Zaccanti2007, Reference Goffredo, Caroselli, Mattioli, Pignotti and Zaccanti2008; Brooke et al., Reference Brooke, Ross and Young2012). Balanophyllia europaea, as such a temperature-sensitive species, may be under considerable risk (Goffredo et al., Reference Goffredo, Caroselli, Mattioli, Pignotti, Dubinsky and Zaccanti2009). It is likely that coral individuals living under the most suitable flow conditions for maximum prey capture efficiency may be the most suitably situated to counter the reduction in zooxanthellae efficiency following seawater temperature increase (Goffredo et al., Reference Goffredo, Caroselli, Pignotti, Mattioli and Zaccanti2007) by increasing heterotrophic feeding behaviour.
ACKNOWLEDGEMENTS
This work was carried out at the Banyuls-sur-Mer (OOB) ASSEMBLE infrastructure site, the staff of which are thanked for all of their assistance in laboratory set-up and material supply, particularly the dive and aquarium teams.
FINANCIAL SUPPORT
This project was funded through the European Community's Seventh Framework programme (FP7/2007-2013) under the HERMIONE project (grant agreement no. 226354) and the ASSEMBLE TA project (grant agreement no. 227799).