Grafting is a technique to combine the shoot of a desirable fruit-producing cultivar (scion) onto a rootstock with a desirable production characteristic (e.g., soilborne disease resistance) from another cultivar or closely related species. Use of grafting is a very old technique to manipulate plant growth, and its use to increase the fruit size of gourd was mentioned in ancient books written in 5th century China and 17th century Korea (Lee and Oda Reference Lee and Oda2003). However, grafting was first introduced for disease control in Japan to increase yield and to manage fusarium wilt [Fusarium oxysporum Schlecht. (emend. Snyd. & Hans.) f. sp. niveum (E.F. Sm.)] in watermelon [Citrullus lanatus (Thunb.) Matsumura & Nakai var lanatus] in 1920 (Tateishi Reference Tateishi1927). Since then, vegetable grafting has become an important technique around the world for production of cucurbitaceous and solanaceous vegetables where intensive cultivation occurs using limited arable land (Bletsos Reference Bletsos2005; Lee Reference Lee2003). In 2008, more than 700 million grafted plants (cucurbits and solanaceous vegetables) were produced in Korea as well as in Japan (Lee et al. Reference Lee, Kubota, Tsao, Bie, Hoyos Echevarria, Morra and Oda2010). The use of grafted plants has increased in the United States because it is an environmentally safe alternative to methyl bromide fumigation (banned by Montreal Protocol) for managing soilborne diseases and pests (Louws et al. Reference Louws, Rivard and Kubota2010). More than 40 million grafted tomato seedlings are produced annually in North American greenhouses (Kubota et al. Reference Kubota, McClure, Kokalis-Burelle, Bausher and Rosskopf2008).
As the production of grafted plants increases worldwide (Kubota et al. Reference Kubota, McClure, Kokalis-Burelle, Bausher and Rosskopf2008; Lee et al. Reference Lee, Kubota, Tsao, Bie, Hoyos Echevarria, Morra and Oda2010), breeders and seed companies are incorporating desired traits into new rootstocks to improve yield, fruit quality, and tolerance to many biotic and abiotic stresses including insects, diseases, salt, drought, waterlogged soils, and temperature extremes (Barrett et al. Reference Barrett, Zhao and McSorley2012; Guan et al. Reference Guan, Zhao, Hassell and Thies2012; King et al. Reference King, Davis, Zhang and Crosby2010; Schwarz et al. Reference Schwarz, Rouphael, Colla and Venema2010). The use of interspecific (closely related species) and intraspecific (same species) rootstocks to achieve plants with the desired characteristics is very common in grafting. Eggplant accessions EG195 and EG203 are recommended for use as rootstocks for tomato production in flooded or waterlogged soils or in fields with soilborne problems such as bacterial wilt (caused by Ralstonia solanacearum), fusarium wilt [caused by Fusarium oxysporum f. sp. lycopersici (Sacc.) W.C. Snyder and H.N. Hans], and root-knot nematodes (Meloidogyne spp.) (Black et al. Reference Black, Wu, Wang, Kalb, Abbass and Chen2003).
Differential absorption, translocation, and metabolism play an important role in herbicide selectivity in plant species (Askew and Wilcut Reference Askew and Wilcut2002; Gallaher et al. Reference Gallaher, Mueller, Hayes, Schwartz and Barrett1999; Sidhu et al. Reference Sidhu, Yu and McCullough2014) because these processes affect the amount of the herbicide delivered to the site of action (Owen Reference Owen1989). Grafted plants are a combination of two different plants, either interspecific or intraspecific; therefore, it is important to know the herbicidal selectivity of the whole plant. Baker and Warren (Reference Baker and Warren1962) found that scion tolerance to amiben (3-amino-2,5-dichlorobenzoic acid) applied via a nutrient solution was dependent upon type of rootstock. Less amiben was absorbed by nongrafted squash (Cucurbita moschata Duchesne) plants, or by cucumber (Cucumis sativus L.) plants grafted onto squash rootstock, than was absorbed by nongrafted cucumber or squash grafted onto cucumber rootstock. Contrastingly, minor differences in translocation of absorbed atrazine and no difference in translocation of glyphosate based on rootstock were reported in grafted watermelon (Adkins Reference Adkins2011). However, the impact of grafting on herbicide absorption, translocation, and metabolism has not been studied in solanaceous crops.
An understanding of herbicide absorption, translocation, and metabolism may further explain the differences and similarities in herbicide sensitivity for grafted and nongrafted solanaceous crops reported by Ghosheh et al. (Reference Ghosheh, Al-Kawamleh and Makhadmeh2010) and Chaudhari et al. (Reference Chaudhari, Jennings, Monks, Jordan, Gunter and Louws2015, Reference Chaudhari, Jennings, Monks, Jordan, Gunter, Basinger and Louws2016, Reference Chaudhari, Jennings, Monks, Jordan, Gunter and Louws2017), respectively. Ghosheh et al. (Reference Ghosheh, Al-Kawamleh and Makhadmeh2010) reported that grafted tomato under greenhouse conditions had higher sensitivity to a mixture of metribuzin and sethoxydim applied post-transplant than did nongrafted tomato. Chaudhari et al. (Reference Chaudhari, Jennings, Monks, Jordan, Gunter and Louws2015, Reference Chaudhari, Jennings, Monks, Jordan, Gunter, Basinger and Louws2016) found similar effects with regard to injury caused by herbicide (fomesafen, halosulfuron, metribuzin, napropamide, S-metolachlor, and trifluralin) application (pre- and post-transplant) in nongrafted and grafted tomato and eggplant. However, halosulfuron (39 and 52 g ha−1) and metribuzin (280 and 550 g ha−1) caused yield reduction in eggplant scion grafted onto tomato rootstock but no effect on yield when tomato scion was grafted onto tomato rootstock. Therefore, the objective of this research was to compare the absorption, translocation, and metabolism of foliar-applied radiolabeled halosulfuron in grafted and nongrafted tomato and eggplant.
Materials and Methods
Plant Materials
Eggplant cultivar ‘Santana’ (Siegers Seed Company, 13031 Reflections Dr., Holland, MI) and tomato cultivar ‘Amelia’ (Harris Moran, P.O. Box 4938, Modesto, CA) were used as nongrafted plants and as the scion in grafted plants. Commercially available tomato rootstock ‘Maxifort’ (DeRuiter, 800 North Lindbergh Blvd, St. Louis, MO) was used as the rootstock for grafted plants. Maxifort is an interspecific hybrid of cultivated tomato and a wild-type, short-lived, perennial tomato plant (Solanum habrochaites S. Knapp & D.M. Spooner). This rootstock was used because it enhances the vigor of the grafted plant and confers resistance to tomato mosaic virus, fusarium wilt, corky root, verticillium wilt, and root-knot nematodes (Rivard and Louws Reference Rivard and Louws2006). Transplant types included nongrafted Amelia, nongrafted Santana, Amelia scion grafted onto Maxifort tomato rootstock (A-Maxifort; intraspecific grafted plant), and Santana scion grafted onto Maxifort rootstock (S-Maxifort; interspecific grafted plant. Grafted plants were produced by the Japanese top-grafting or tube-grafting method (Rivard and Louws Reference Rivard and Louws2006). Six-week-old plants were transplanted into pots (750 ml volume) with commercial potting mix (Fafard 4P, Conrad Fafard Inc., Agawam, MA). Plants were fertilized with 30 ml per pot of a 4 g L−1 fertilizer solution [Miracle-Gro® Fertilizer (24-8-16), The Scotts Company LLC, Marysville, OH] immediately after transplanting to ensure optimum plant growth. Plants were hand-watered as needed with overhead irrigation. The greenhouse was maintained at 29±3 C under natural sunlight.
Absorption and Translocation
Ten days after transplanting, plants were at the desired stage (five to six leaves) for herbicide treatment. Prior to treatment with radiolabeled halosulfuron, plants were sprayed with halosulfuron (Sandea®, Gowan Company, Yuma, AZ) at 39 g ai ha−1 plus 0.25% (v/v) nonionic surfactant (Scanner®, Loveland Products Inc., P.O. Box 1286, Greeley, CO) in a spray chamber with a CO2-pressurized sprayer calibrated to deliver 187 L ha−1 with an 8002 EVS nozzle (Teejet Technologies, Springfield, IL) at 275 kPa. Radiolabeled (14C-pyrazole) halosulfuron, with specific activity 40 mCi mmol−1 and 98.2% radioactive purity, was foliar-applied as described by McElroy et al. (Reference McElroy, Yelverton, Burke and Wilcut2004). Immediately following the commercial halosulfuron application, ten 1-μl droplets (Hamilton® Microliter Pipetter, Hamilton Co., Reno, NV) of 14C-halosulfuron were applied to the adaxial surface of a fully expanded second-youngest leaf of each plant. Radioactive spotting solution was a 1:1 (v/v) mixture of deionized water and acetone, 0.25% nonionic surfactant (NIS; Induce surfactant, Helena Chemical Co., Memphis, TN), and 363 Bq μl−1 (first run) and 340 Bq μl−1 (second run) radioactivity from 14C-halosulfuron. Acetone was added as the primary solvent to facilitate the stability of 14C-halosulfuron (McElroy et al. Reference McElroy, Yelverton, Burke and Wilcut2004). From this point, plants were subirrigated to prevent washing of the herbicide from the leaf surface.
Plants were harvested at 6, 12, 24, 48, and 98 h after treatment (HAT). Soil from plant roots was hand-removed by gentle shaking, and plants were divided into 1) treated leaf, 2) shoot, and 3) root. In grafted plants, shoots were divided into scion-shoot (scion shoot above graft union) and rootstock-shoot (only rootstock shoot below graft union). Plants were also spotted with a radiolabeled herbicide solution and harvested after 10 s to test the efficacy of the leaf wash technique. Treated leaves were rinsed with 20 ml of a 1:1 (v/v) mixture of methanol and deionized water and 0.25% (v/v) NIS to remove unabsorbed halosulfuron. A 1-ml aliquot of the leaf rinsate was added to 15 ml of scintillation fluid (ScintiVerse® SX1 8-4 Universal Liquid Scintillation Cocktail, Fisher Scientific, Fair Lawn, NJ) and radioactivity was quantified by liquid scintillation spectrometry (Packard TRI-CARB 2100TR Liquid Scintillation Spectrometer, Packard Instrument Company, Downers Grove, IL). All plant parts were placed in paper bags and oven-dried at 50 C for 4 d and then combusted for 2 min in a biological sample oxidizer (Model OX-500, R.J. Harvey Instrument Corp., Hillsdale, NJ). Radioactivity trapped as 14CO2 was quantified by liquid scintillation spectrometry.
Metabolism
The metabolism study was performed concurrently with the absorption and translocation study. Plants were grown and treated similarly as in the absorption and translocation study. In the metabolism study, plants were only harvested at 12 and 48 HAT. After plant harvest, all the plant parts were immediately placed in plastic bags and stored at −20 C until future analysis was performed. The results from the absorption and translocation study showed that the majority of radioactivity was located in the treated leaves. Therefore, treated leaves were used for metabolism analysis. Treated leaf samples were blended using a high speed homogenizer for 5 to 10 min with 7 ml of ice-cold 40% aqueous acetonitrile followed by filtration through a Whatman glass filter paper. The filtered cakes were re-extracted by adding 2 to 3 ml solvent. The filtrate was concentrated to 1 ml by evaporation at 30±2 C under a stream of air and filtered again through 0.45 µm nylon disposable syringe filters to transfer into high-performance liquid chromatography (HPLC) vials. Metabolites were separated using an Agilent 1260 Infinity Quaternary LC series HPLC system (Chromtech - Analytical Instruments, Buchwiese 3, Idstein, Deutschland) controlled by Laura (4.2.6) software. The column was eluted with water containing 0.1% trifluoroacetic acid and methanol at a flow rate of 1.0 ml min−1. A MAC-MOD Halo C-18 (3.0 by 100 mm, 2.7 µm particle size) column was used, and column temperature was held at 40 C. The peaks were detected using a variable wavelength ultraviolet light detector (Beta Ram Model 5) operated at 254 nm. The run time was 35 min and retention time for halosulfuron was 18 min.
Both absorption and translocation and metabolism studies, replicated in time, were conducted in a completely randomized design with four single-plant replicates per treatment. Percent absorption was calculated from the fraction of total applied 14C-halosulfuron not recovered in the leaf wash. The percent translocated to each plant part was calculated by dividing the radioactivity in that plant part by the total amount applied. Data from both experiments were analyzed with PROC MIXED (SAS 9.3 Institute Inc., Cary, NC). Translocation data for scion-shoot, rootstock-shoot, and root were subjected to arcsine square-root transformation. However, to facilitate the interpretation of results, back-transformed means are presented. Data from both runs were combined because run by transplant type by harvest interval interaction was not significant. Harvest interval and transplant type were considered fixed effects in the model. Replication, experimental run, and their interaction were considered random effects. Where appropriate, treatment means were separated using Fisher’s protected LSD test at a significance level of 0.05. Nonlinear regression analysis using SigmaPlot 10 (Systat Software Inc., San Jose, CA) was used to determine the effect of harvest intervals on absorption and leaf wash data.
Results and Discussion
Absorption and Translocation
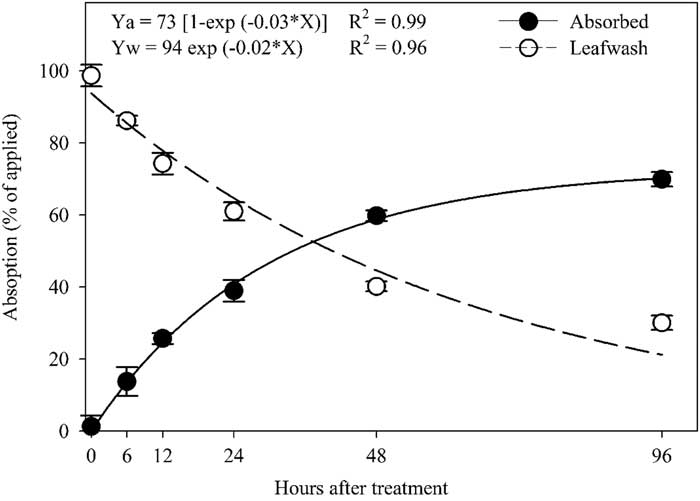
Total absorption in a single plant was the sum of the 14C-halosulfuron in the treated leaf, shoot (scion-shoot and rootstock-shoot in grafted plants), and root. No significant difference was observed between transplant types for absorption of 14C-halosulfuron, therefore data were pooled over transplant type. Plant absorption of 14C-halosulfuron increased exponentially over time, while the amount of 14C-halosulfuron in leaf wash decreased exponentially over time (Figure 1). Absorption was rapid initially and reached 42% by 24 HAT, and then it gradually increased to 60% and 70% at 48 and 96 HAT, respectively. Likewise, Buker (Reference Buker2002) reported 40% and 60% absorption of 14C-halosulfuron in tomato and pepper at 24 and 72 HAT, respectively.
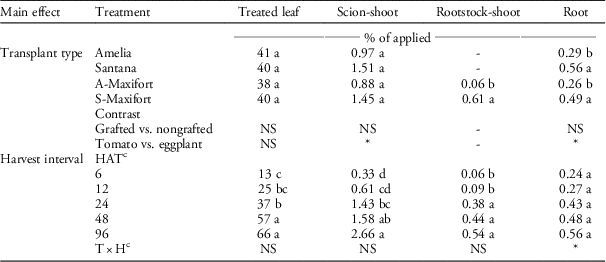
The interaction between transplant type and harvest interval was not significant, but the effect of transplant type on percent translocation (measured as percent of applied) was significant in rootstock-shoot and root, and harvest interval was significant in all plant parts but root (Table 1). Most of the 14C-halosulfuron applied to the leaf stayed in the leaf, and only a limited amount (<4%) was translocated to other plant parts. Translocation increased in shoot (from 0.33% to 2.66%) and rootstock-shoot (0.06% to 0.54%) with time (Table 1). In rootstock-shoot, grafted eggplant, S-Maxifort, had higher translocation than grafted tomato, A-Maxifort. Similarly, contrast statements showed that eggplant had greater translocation in both shoot and root than did tomato (Table 1). Subtle differences in translocation were observed between eggplant and tomato, but these differences represented less than of 2% of the applied herbicide, and thus were not physiologically significant in terms of crop tolerance.
Table 1 The percentage of 14C-halosulfuron translocated in nongrafted Amelia, nongrafted Santana, Amelia scion grafted onto Maxifort tomato rootstock (A-Maxifort), and Santana scion grafted onto Maxifort rootstock (S-Maxifort) plant parts.Footnote a , Footnote b
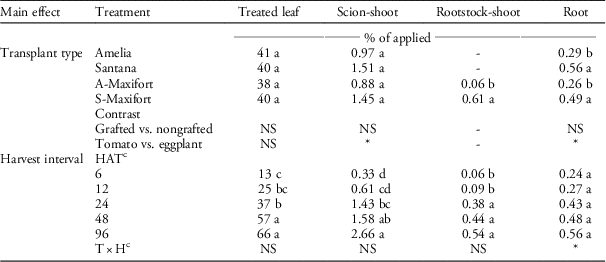
a Pooled analysis over two experimental runs.
b Means within column for main effects (transplant type or harvest interval) with the same letter are not significantly different according to Fisher’s protected LSD (α=0.05). * or NS represents significant and nonsignificant at α=0.05, respectively.
c Abbreviations: H, harvest interval; HAT, h after treatment; T, transplant type.
Metabolism
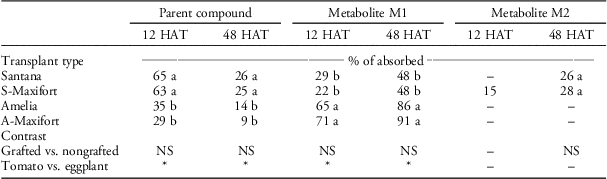
Despite similarity in uptake levels, metabolism of halosulfuron was significantly different in tomato and eggplant regardless of grafting. One unknown metabolite (M1) at retention time 10 min was detected in both nongrafted Amelia and grafted A-Maxifort tomato at both 12 and 48 HAT. Two unknown metabolites (M1 at retention time 10 min and M2 at retention time 13 min) were detected in grafted S-Maxifort and nongrafted Santana eggplant at 12 and 48 HAT. However, only metabolite M1 was detected at 12 HAT in nongrafted Santana eggplant (Table 2). The amount of metabolites was significantly higher in tomato than it was in eggplant at both 12 and 48 HAT; however, this difference was not significant when grafted and nongrafted plants were compared within either tomato or eggplant (Table 2). As a result, regardless of grafting, the amount of nonmetabolized parent halosulfuron was significantly greater in eggplant than it was in tomato at both 12 and 48 HAT (Table 2).
Table 2 Metabolism of foliar-applied 14C-halosulfuron in treated leaf extracts of nongrafted Amelia, nongrafted Santana, Amelia scion grafted onto Maxifort tomato rootstock (A-Maxifort) and Santana scion grafted onto Maxifort rootstock (S-Maxifort) at 12 and 48 hours after treatment (HAT).Footnote a , Footnote b , Footnote c
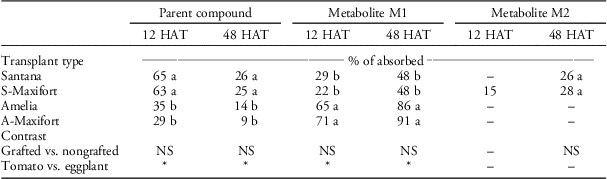
a Pooled analysis over two experimental runs.
b Means within column with the same letter are not significantly different according to Fisher’s protected LSD (α=0.05). * or NS represents significant and nonsignificant at α=0.05, respectively.
c Abbreviations: HAT, h after treatment.
The absence of differences in 14C-herbicide translocation in grafted and nongrafted plants regardless of species (tomato or eggplant) indicates that grafting does not inhibit movement of halosulfuron to different parts of the plant. Similar to these results, Adkins (Reference Adkins2011) reported no difference in glyphosate and atrazine movement in grafted and nongrafted watermelon plants. Our experiment also confirms the results observed by Buker (Reference Buker2002) in which less than 0.8% of absorbed 14C-halosulfuron translocated above and below the treated leaf and root in both pepper (Capsicum annuum L.) and tomato. Isaacs et al. (Reference Isaacs, Hatzios, Wilson and Toler2006) also reported <4% translocation of 14C-halosulfuron plus 2,4-D in common lambsquarters (Chenopodium album L.) at 72 HAT. Similarly, Askew and Wilcut (Reference Askew and Wilcut2002) showed that the majority of foliar-applied 14C-trifloxysulfuron remained in the treated leaves of peanut (Arachis hypogaea L.), cotton (Gossypium hirsutum L.), and sicklepod [Senna obtusifolia (L.) H.S. Irwin and Barnaby], and <2% translocated to other plant parts.
Contrast statements indicate that there was no difference in 14C-halosulfuron parent and metabolites in grafted and nongrafted plants, whereas the difference was significant between tomato and eggplant. This suggests that grafting did not impact halosulfuron metabolism, and tomato tolerance to halosulfuron might be due to tomato’s ability to rapidly detoxify halosulfuron to nonherbicidal metabolites. Previous research on other sulfonylurea herbicides has identified the rate of metabolism inactivation as the basis for crop tolerance (Askew and Wilcut Reference Askew and Wilcut2002; Buker Reference Buker2002; Richardson et al. Reference Richardson, Hatzios and Wilson2003). Buker (Reference Buker2002) reported that bell pepper is less susceptible to rimsulfuron than is tomato, a tolerant species, because of differences in metabolism.
In conclusion, grafting does not appear to impact absorption, translocation, and metabolism of foliar-applied halosulfuron in grafted (interspecific and intraspecific) tomato and eggplant. This could explain the similar halosulfuron tolerance level of grafted and nongrafted tomato and eggplant in field and greenhouse experiments reported by Chaudhari et al. (Reference Chaudhari, Jennings, Monks, Jordan, Gunter and Louws2015, Reference Chaudhari, Jennings, Monks, Jordan, Gunter, Basinger and Louws2016). Future research should investigate the impact of grafting on absorption, translocation, and metabolism of commonly used PRE and POST herbicides using different types of tomato rootstocks.
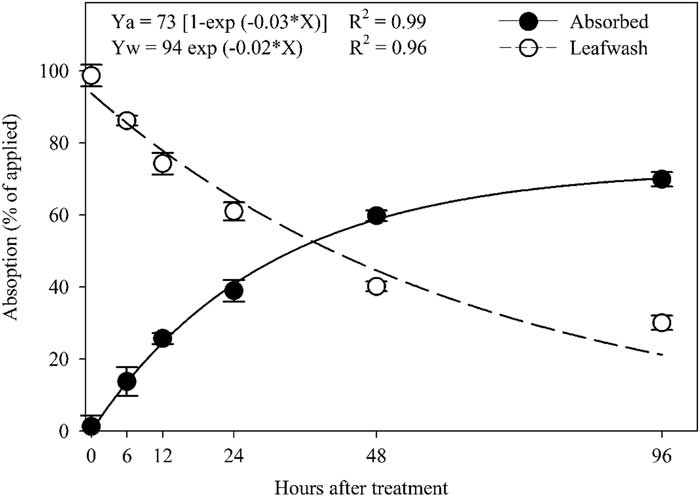
Figure 1 Percentage of 14C-halosulfuron absorbed in plants and remaining in the leaf wash during 96 h period. Points are means±SE. Means are average from two runs across all transplant types.
Acknowledgments
The authors thank Dr. Khalied Ahmed, Shawn Beam, and Samuel McGowen for their assistance with conducting this research, and Drs. Rob Richardson and Travis Gannon for providing space and equipment in their laboratory for conducting the experiment. The authors thank the US Department of Agriculture for providing funding for proposals (USDA-NIFA-2009-2376 and USDA-SCRI-2011-51181-30963). Furthermore, the authors thank Dr. Cavell Brownie for her assistance with data analysis.