Introduction
The use of neuromuscular electrical stimulation as an adjunctive procedure to treat patients with pharyngeal dysphagia has become an increasingly common practice by speech and language pathologists.Reference Carnaby-Mann and Crary 1 A few studies also suggest the use of neuromuscular electrical stimulation as a treatment for voice difficulties.Reference Ptok and Strack 2 , Reference LaGorio, Carnaby-Mann and Crary 3 Ptok and Strack reported that electrical stimulation improved vibratory closure and stability in patients with vocal fold paresis.Reference Ptok and Strack 2 These authors concluded that voice therapy treatments completed while using neuromuscular electrical stimulation were more effective than those completed without neuromuscular electrical stimulation. Similarly, LaGorio et al. found improved glottal closure and reduced supraglottic compression following a three-week structured therapy programme utilising neuromuscular electrical stimulation in conjunction with other treatment modalities.Reference LaGorio, Carnaby-Mann and Crary 3 Likewise, Guzman et al. reported improved vocal function in two women with suspected superior laryngeal nerve weakness when neuromuscular electrical stimulation was used as an adjunctive therapy method.Reference Guzman, Rubin, Cox, Landini and Jackson-Menaldi 4 One patient demonstrated a reduction in voice breaks during passaggio, while the second patient exhibited reduced breathiness and a greater vocal range.
However, Ludlow and colleagues did not find evidence of changes in vocal fold movement among vocally normal participants as a result of neuromuscular electrical stimulation.Reference Humbert, Poletto, Saxon, Kearney and Ludlow 5 , Reference Ludlow, Humbert, Saxon, Poletto, Sonies and Crujido 6 Given the effective depth and pattern of neuromuscular electrical stimulation, one could conclude that laryngeal strap muscle stimulationReference Ptok and Strack 2 – Reference Guzman, Rubin, Cox, Landini and Jackson-Menaldi 4 or the cricothyroidReference Guzman, Rubin, Cox, Landini and Jackson-Menaldi 4 was the source of the effects noted. Previous studies have indicated that laryngeal strap muscle stimulation can affect glottal opening and the fundamental frequency of the voice.Reference Erickson, Baer, Harris, Bless and Abbs 7 – Reference Loucks, Poletto, Saxon and Ludlow 9
Although it has been used as an adjunctive voice treatment modality, research on the efficacy of neuromuscular electrical stimulation for treating voice is in its infancy, and, to date, there are no widely accepted protocols concerning when and how it may be applied. Prior research has examined the effect of electrical stimulation applied to the laryngeal area in non-dysphonic speakers.Reference Fowler, Gorham-Rowan and Hapner 10 – Reference Fowler, Awan, Gorham-Rowan and Morris 12 In these studies, the term ‘transcutaneous electrical stimulation’ was used to reflect passage of the electrical current through the skin to induce muscle contraction for rehabilitative needs. The use of transcutaneous electrical stimulation in these studies, therefore, was similar to that of neuromuscular electrical stimulation, which is the more appropriate term when discussing the use of electrical stimulation for rehabilitative purposes and muscle strengthening goals.Reference Cramp, Scott and Watson 13 This term is used differentially from ‘transcutaneous electrical nerve stimulation’, which refers to the application of low amplitude electrical pulses through the skin for pain management.Reference Mannheimer, Lampe, Mannheimer and Lampe 14
In the previously mentioned studies conducted by Fowler et al.Reference Fowler, Gorham-Rowan and Hapner 10 , Reference Fowler, Awan, Gorham-Rowan and Morris 12 and Gorham-Rowan et al.,Reference Gorham-Rowan, Fowler and Hapner 11 changes in vocal function were found in non-dysphonic speakers following 30–60 minutes of a single session of electrical stimulation applied to the laryngeal area. However, some of these individuals exhibited either hyperfunctional or hypofunctional phonation following this single session. Fowler et al.Reference Fowler, Gorham-Rowan and Hapner 10 and Gorham-Rowan et al.Reference Gorham-Rowan, Fowler and Hapner 11 noted measurable changes in both fundamental frequency (F0) and vocal loudness (sound pressure level (SPL)) following a 1-hour session of neuromuscular electrical stimulation. The observed acoustic changes in the participants' voices varied both in magnitude and direction of change (e.g. some individuals exhibited an increase in fundamental frequency and SPL, while others showed a decrease in these parameters).
An increase in fundamental frequency and/or SPL following prolonged voice use, such as would occur with increased vocal fold adduction, may be viewed as a compensatory response to loading of the laryngeal mechanism.Reference Laukkanen, Järvinen, Artkosi, Waaramaa-Mäki-Kulmala, Kankare and Sipolla 15 , Reference Laukkanen, Ilomäki, Leppänen and Vilkman 16 A reduction in fundamental frequency could occur with the application of neuromuscular electrical stimulation to the laryngeal area that results in stimulation of the infrahyoid muscles, thereby lowering the laryngeal complex.Reference Ludlow, Humbert, Saxon, Poletto, Sonies and Crujido 6 In response to this laryngeal lowering, some participants may utilise the suprahyoid musculature to counteract the downward pull of the larynx, which would effectively result in vocal loading.Reference Fowler, Gorham-Rowan and Hapner 10 The attempt to counteract laryngeal descent may be reflected further in increased phonatory instability, as evidenced by elevated shimmer levels following a 1-hour session of neuromuscular electrical stimulation.Reference Gorham-Rowan, Fowler and Hapner 11
Fowler and colleagues used cepstral analysis to examine possible changes in vocal function associated with neuromuscular electrical stimulation, and noted a decrease in the low/high spectral ratio following 30 minutes of stimulation.Reference Fowler, Awan, Gorham-Rowan and Morris 12 The low/high spectral ratio is a measure of spectral tilt and is calculated as the ratio of low frequency energy compared to high frequency energy in the voice. A reduction in the low/high spectral ratio would suggest an increase in high frequency noise, and may be correlated with breathiness and hypofunctional voice production.Reference Watts and Awan 17 Hypofunctional phonation may occur as a result of vocal fatigue in response to the vocal loading effect of neuromuscular electrical stimulation.Reference Stemple, Stanley and Lee 18
Fowler et al. divided participants into three groups based on subjective reports of non-acoustic changes that occurred following neuromuscular electrical stimulation.Reference Fowler, Gorham-Rowan and Hapner 10 One group reported either a feeling of vocal warm-up and improved voice output or no noticeable effects, while a second group reported symptoms of vocal fatigue. The third group of participants reported sensations of vocal fatigue and delayed-onset muscle soreness. Delayed-onset muscle soreness is defined as the sensation of pain and stiffness in the muscles that occurs from 1 to 5 days following unaccustomed or eccentric exercise.Reference Armstrong 19 , Reference Connolly, Sayers and McHugh 20 These findings were confirmed in subsequent studies.Reference Gorham-Rowan, Fowler and Hapner 11 , Reference Fowler, Awan, Gorham-Rowan and Morris 12 Thus, the 30- and 60-minute neuromuscular electrical stimulation sessions were too long for some of these participants with normal voices.
Given that individuals with normal voices may experience voice changes, vocal fatigue and delayed-onset muscle soreness after 30–60 minutes of neuromuscular electrical stimulation, it is plausible that individuals with dysphonia would also experience these symptoms following stimulation. Individuals with hyperfunctional dysphonia frequently complain of vocal fatigue and/or muscle soreness following prolonged periods of voice use due to increased activity of the laryngeal muscles,Reference Stemple, Stanley and Lee 18 while those with hypofunctional dysphonia may report similar sensations as a result of compensatory efforts. Although neuromuscular electrical stimulation may be beneficial as an adjunctive therapy treatment, it is possible that a session of 30–60 minutes may be too long for some individuals. A brief neuromuscular electrical stimulation session, however, may still provide therapeutic benefit (e.g. reducing excessive muscular tension), but without the aforementioned fatigue and/or delayed-onset muscle soreness. Hence, the purpose of the following experiments was to determine the duration of neuromuscular electrical stimulation that provides a therapeutic effect with minimal vocal fatigue and/or delayed-onset muscle soreness. A therapeutic effect would be indicated by a perceivable or measurable change in voice production.
Experiment one
The first experiment was completed to assess the vocal and physical effects of neuromuscular electrical stimulation applied to the laryngeal area of individuals with normal voices for 15 minutes. This study was an expansion of previous studies completed by Fowler et al.Reference Fowler, Gorham-Rowan and Hapner 10 , Reference Fowler, Awan, Gorham-Rowan and Morris 12 and Gorham-Rowan et al.Reference Gorham-Rowan, Fowler and Hapner 11 It was hypothesised that the use of neuromuscular electrical stimulation for this short period of time would result in minimal to no alterations in muscular fatigue or pain, with or without measurable changes in vocal performance. Thus, these data should help determine the minimum time necessary for neuromuscular electrical stimulation to provide therapeutic benefit.
Materials and methods
Participants
Twelve women, aged 20–61 years, served as participants. Approval from the Valdosta State University Institutional Review Board was obtained prior to enrolment of these participants into the study. This study focused on women, as prior research has demonstrated that women are more likely to report sensations of vocal fatigue and/or delayed-onset muscle soreness following neuromuscular electrical stimulation.Reference Gorham-Rowan, Fowler and Hapner 11 All participants were in good general health, with no history of respiratory, cardiovascular or neurological disease. All participants exhibited vocal quality within normal limits, and had no history of voice or speech difficulties. None of the participants were trained singers. Body mass index (BMI) and neck fat data are presented in Table I. These data were obtained as they may be related to the presence of vocal fatigue and/or delayed-onset muscle soreness following neuromuscular electrical stimulation.Reference Fowler, Gorham-Rowan and Hapner 10 , Reference Gorham-Rowan, Fowler and Hapner 11
Table I Participant information
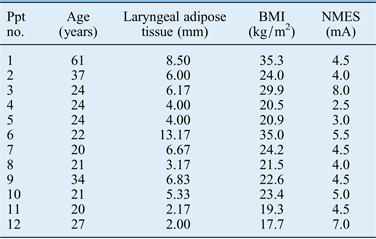
Ppt no. = participant number; BMI = body mass index; NMES = neuromuscular electrical stimulation intensity
Neuromuscular electrical stimulation
Each participant attended two 15-minute sessions, which were scheduled 48 hours apart. One session was completed with neuromuscular electrical stimulation and the other without neuromuscular electrical stimulation. The order of neuromuscular electrical stimulation versus no neuromuscular electrical stimulation was counterbalanced across participants.
Neuromuscular electrical stimulation was applied according to procedures previously described by Fowler et al.Reference Fowler, Gorham-Rowan and Hapner 10 Two pairs of bipolar surface electrodes were applied to the laryngeal area. For this study, one pair of electrodes was applied lateral to the thyroid notch, on the thyroid lamina; the second pair was applied superior to the first pair, at the level of the thyrohyoid space. Neuromuscular electrical stimulation was applied via a VitalStim® electrotherapy unit (DJO Global, Vista, CA). This unit provides electrical stimulation via a biphasic pulsed waveform at a frequency of 80 Hz with a phase duration of 300 ms. A continuous pulse is provided for 57 seconds. To complete a 1-minute cycle, there is a 1-second duration of signal intensity decrease, a 1-second duration of no stimulation and a 1-second duration of signal intensity increase to return to the established intensity level.Reference Witjing and Freed 21
The initial signal intensity level was established according to participant reports of a ‘grabbing’ sensation, indicative of perceivable muscle contraction (Table I). The participants were instructed to increase the intensity level when they no longer felt the grabbing sensation, as an adaptation effect to neuromuscular electrical stimulation may occur.Reference McDonough and Watson 22 , Reference Cramp, Scott and Watson 23 None of the participants chose to increase the intensity level. This would be expected given the brief duration of their neuromuscular electrical stimulation exposure.
Procedures
During both sessions, the participants followed a standard speaking protocol, consisting of a reading task or picture description every 5 minutes.Reference Fowler, Awan, Gorham-Rowan and Morris 12 The reading tasks involved reading the Rainbow PassageReference Fairbanks 24 divided into three paragraphs, or the Grandfather Passage.Reference Darley, Aronson and Brown 25 The picture description tasks involved describing the Cookie Theft picture from the Boston Diagnostic Aphasia ExaminationReference Goodglass, Kaplan and Barresi 26 or the picnic picture from the Western Aphasia Battery.Reference Kertesz 27 The order of presentation of the reading passages and the pictures were randomised among the participants and according to the experimental session.
Acoustic data
Voice recordings, consisting of three trials of the first two sentences of the Rainbow Passage,Reference Fairbanks 24 were obtained prior to and immediately following the experimental sessions. The recordings were completed in a sound-attenuating booth using an Audio-Technica™ AT3032 omnidirectional condenser microphone connected to an M-Audio™ MobilePre amplifier. The recordings were digitised directly into a desktop computer using a Computerized Speech Lab hardware and software system (model 4500; KayPentax, Lincoln Park, New Jersey, USA). A constant mouth-to-microphone distance of 30 cm was maintained for all recordings.Reference Titze and Winholtz 28
Measurements of speaking fundamental frequency (‘pitch’) and relative loudness level were obtained using the Computerized Speech Lab program. Measures of cepstral peak prominence and the ratio of average amplitude of the low frequencies in comparison to the high frequencies in a long-term spectrum (low/high spectral ratio) were obtained using the Analysis of Dysphonia in Speech and Voice (‘ADSV’) program (Pentax Medical, Montvale, New Jersey, USA). Cepstral peak prominence refers to the height of the cepstral peak in relation to other frequencies in the vocal tract; normal voice production is associated with a well-defined and prominent peak. Low/high spectral ratio is an index of the level of harmonic energy compared to noise in the voice; normal voice production is associated with a greater amount of harmonic energy.Reference Fowler, Awan, Gorham-Rowan and Morris 12
Individual means for each participant were obtained from the three trials and were used to determine group means and standard deviations. Data were analysed to determine pre- versus post-differences for both sessions using a repeated measures analysis of variance (ANOVA).
Perceptual ratings
The participants were asked to rate the extent of vocal fatigue and muscle soreness in the neck area at the following time points: prior to and after reading, after 15 minutes of neuromuscular electrical stimulation or rest, and 24 hours following neuromuscular electrical stimulation or rest. The ratings were completed using a 100 mm visual analogue scale, with 0 representing no vocal fatigue or muscle soreness and 100 representing severe vocal fatigue and/or muscle soreness.
Results
Acoustic data
The average magnitudes of change for the acoustic and spectral data are presented in Table II. The repeated measures ANOVA revealed no significant difference in speaking fundamental frequency (F (1, 9) = 1.00, p = 0.34) or relative loudness level (F (1, 9) = 1.07, p = 0.327) following the experimental session without neuromuscular electrical stimulation. Likewise, no significant difference in speaking fundamental frequency (F (1, 9) = 0.001, p = 0.982) or relative loudness level (F (1, 9) = 0.007, p = 0.934) was found following the experimental session completed with the administration of neuromuscular electrical stimulation. Similarly, no difference in cepstral peak prominence occurred following the non-neuromuscular electrical stimulation session (F (1, 9) = 0.503, p = 0.50) or the neuromuscular electrical stimulation session (F (1, 9) = 4.66, p = 0.06). In addition, the low/high spectral ratio was not significantly different after the non-neuromuscular electrical stimulation session (F (1) = 2.31, p = 0.16) or the neuromuscular electrical stimulation session (F (1, 9) = 0.128, p = 0.73).
Table II Mean magnitudes of change for acoustic and cepstral data*

* Following 15 minutes of rest or 15 minutes of neuromuscular electrical stimulation. NMES = neuromuscular electrical stimulation; F0 = fundamental frequency
Perceptual ratings
Three participants reported mild vocal fatigue after 15 minutes of neuromuscular electrical stimulation (participants four, five and seven), while one participant reported moderate fatigue (participant number eight). None of the participants reported any symptoms of vocal fatigue 24 hours following the neuromuscular electrical stimulation session. Two participants (numbers 4 and 10) reported mild sensations of muscle soreness after 15 minutes of neuromuscular electrical stimulation, while one participant (number 8) reported moderate soreness. None of the participants reported symptoms associated with delayed-onset muscle soreness 24 hours following neuromuscular electrical stimulation.
Discussion
The results of the present study demonstrated no significant changes in acoustic measures of voice production following 15 minutes of neuromuscular electrical stimulation. These findings contrast with prior reports of marked change in these parameters following longer neuromuscular electrical stimulation exposure. For example, Fowler et al.Reference Fowler, Gorham-Rowan and Hapner 10 reported increased fundamental frequency and SPL values during reading of the Rainbow Passage following 1 hour of neuromuscular electrical stimulation, while Fowler et al.Reference Fowler, Awan, Gorham-Rowan and Morris 12 reported a decrease in SPL and increased high frequency noise for the Rainbow Passage following only 30 minutes of neuromuscular electrical stimulation. These changes were attributed to activation of the extrinsic laryngeal musculature, which contributes to an increase in muscular tension and possible vocal fatigue. The combined results of these studies, including the current findings, suggest that the application of neuromuscular electrical stimulation needs to be longer than 15 minutes in order to be associated with measurable changes in voice.
The 15-minute neuromuscular electrical stimulation session did have an effect on the laryngeal muscles for some of the participants. Three participants (25 per cent) reported improved vocal output or a feeling of relaxation following neuromuscular electrical stimulation. These reports, combined with the non-significant differences in acoustic and cepstral measures, suggest that neuromuscular electrical stimulation may reduce muscular tension. The minimal change in phonation, combined with the temporary and mild effects of fatigue and/or muscle soreness, indicate that a short session of neuromuscular electrical stimulation may be an appropriate starting point for some individuals. This session could serve as an initial dose for these individuals, and be used to mitigate excessive fatigue and/or muscular soreness associated with neuromuscular electrical stimulation.
Four participants (33 per cent) reported mild to moderate degrees of vocal fatigue and muscular soreness, and three participants (25 per cent) reported mild to moderate muscle soreness following 15 minutes of neuromuscular electrical stimulation. However, these effects were short-lived, as none of the participants experienced vocal difficulties or delayed-onset muscle soreness 24 hours later. These reports of fatigue and soreness were somewhat surprising, as it was initially hypothesised that little to no fatigue or soreness would occur following this relatively brief application of neuromuscular electrical stimulation.
These reactions could be related to a number of factors. Delitto et al. observed that some of the negative sensations associated with neuromuscular electrical stimulation are a direct result of electrical stimulation characteristics (i.e. direct stimulation of afferent nerves and high muscle contractile properties).Reference Delitto, Strube, Shulman and Minor 29 The mere inclusion of an electrically induced muscle contraction, in contrast to an afferent input, also resulted in higher indices of pain perception. This is likely due to selective recruitment of type II muscle fibres, which have an intermediate resistance to fatigue, and thus may not be able to tolerate neuromuscular electrical stimulation for extended periods of time.Reference Fowler, Gorham-Rowan and Hapner 10 , Reference Gersh 30
Additional factors, however, also appear to enhance or minimise the perception of pain; for example, the cognitive moderation of pain. This includes the manner in which individuals cope with adverse experiences, which thus affects their perception of that experience.Reference Delitto, Strube, Shulman and Minor 29
Gender also appears to play a role. Alon and Smith found that women exhibited a reduced tolerance level to electrical stimulation at maximum intensity and were less easily conditioned to tolerate the stimulation.Reference Alon and Smith 31 Gorham-Rowan et al. found that women were more likely to report symptoms of fatigue and muscle soreness or delayed-onset muscle soreness following 1 hour of neuromuscular electrical stimulation, which was attributed to personality differences and higher levels of subcutaneous fat in the female participants.Reference Gorham-Rowan, Fowler and Hapner 11 Subcutaneous fat impedes the flow of the electrical current, and redirects the current back towards the skin and peripheral pain receptors, thus resulting in an increase in pain perception.Reference Petrofsky 32 Given that all participants in the current study were female, one may speculate a higher chance of discomfort or fatigue being reported.
Furthermore, some of the participants who reported muscular soreness after 15 minutes of neuromuscular electrical stimulation later stated that they may have been reacting to a sensation of increased tightness of the skin covering the laryngeal area, rather than actual fatigue or pain. The placement of the VitalStim unit surface electrodes involves a small amount of adhesive material, used to insure adherence of the electrode to the skin; the adhesive material likely contributed to the sensation of increased skin tightness.
Experiment two
The second study was undertaken to determine the extent to which a 15-minute session of laryngeal neuromuscular electrical stimulation would mitigate experimentally induced muscle tension in speakers who exhibit normal phonation. Neuromuscular electrical stimulation may be used to either improve muscle strength and contractile force or to reduce excessive muscular tension and promote recovery. Neric and colleagues demonstrated that electrical stimulation was effective in clearing accumulated blood lactate among elite swimmers and thus served as an alternative method for muscle recovery following prolonged exercise.Reference Neric, Beam, Brown and Wiersma 33 However, other studies have found no significant difference when recovery using neuromuscular electrical stimulation was compared to active or passive muscle recovery in different athlete populations (e.g. triathletes, cyclists and runners),Reference Lattier, Millet, Martin and Martin 34 – Reference Malone, Coughlan, Crowe, Gissane and Caulfield 36 although Lattier et al.Reference Lattier, Millet, Martin and Martin 34 and Leeder et al.Reference Leeder, Spence, Taylor, Harrison and Howatson 35 reported a trend towards improved muscle function following a period of neuromuscular electrical stimulation.
Electrical stimulation has been used to help heal muscles, and a number of studies have demonstrated that the use of electrical stimulation reduces excessive muscle tension (e.g. spasticity), and promotes an appropriate muscle balance ratio following surgery, trauma or stroke, or in individuals with cerebral palsy.Reference Al-Abdulwahab and Al-Khatrawi 37 – Reference Wright, Durham, Ewins and Swain 39 Cho and colleagues utilised electrical stimulation to reduce spasticity in the lower extremities of post-stroke patients, noting a 29 per cent reduction in spasticity compared to a 13–19 per cent reduction in the control group who received physical therapy only.Reference Cho, In, Cho and Song 40 Cho and colleagues suggested that the application of electrical stimulation to the muscle bellies of the gastrocnemius muscles in the lower leg enhanced pre-synaptic inhibition of the nerve for this muscle.
In individuals with muscle tension dysphonia, sustained contraction of the extrinsic laryngeal muscles results in improper muscle use and imbalance, contributing either to prolonged elevation or depression of the larynx. Patients with muscle tension dysphonia exhibit disordered voice quality, which may be characterised as breathy, rough and/or strained. Additionally, these individuals often report symptoms of vocal fatigue, in which the voice wears out with continued talking and/or at the end of a day, as well as physical symptoms of neck pain and throat soreness. These symptoms are thought to be associated with inappropriate use of the extrinsic musculature in an effort to compensate for vocal fold dysfunction. Specifically, excessive contraction of the thyrohyoid muscle has been reported prior to and during phonation among individuals with muscle tension dysphonia, as well as co-contraction of the submental and infrahyoid muscles in some patients with muscle tension dysphonia.Reference Angsuwarangsee and Morrison 41 , Reference Hočevar-Boltežar, Janko and Žargi 42 This pattern may occur in conjunction with chronic, acute or temporary changes to the vocal folds, such as nodules or viral-induced oedema or erythema, or in the absence of vocal fold anomalies.
Given the reported benefits of the use of electrical stimulation in reducing excessive muscular tension and restoring muscle balance among individuals with neurogenic dysfunction, it was hypothesised that neuromuscular electrical stimulation would also be beneficial in restoring appropriate muscle function in healthy individuals with functional disorders, specifically muscle tension dysphonia.
Prior to testing the use of neuromuscular electrical stimulation on individuals with muscle tension dysphonia, it was deemed appropriate to examine its effects on individuals who experienced experimentally induced vocal fatigue. Vocal loading, defined as prolonged voice use,Reference Vintturi, Alku, Lauri, Sala, Sihvo and Vilkman 43 may be used to simulate vocal fatigue, as it requires prolonged, forceful use of the intrinsic laryngeal muscles. A prolonged reading task at an increased loudness level was used as a vocal loading task. This type of task results in temporary vocal fatigue associated with overuse of the intrinsic laryngeal musculature and subsequent declines in muscle strength and contraction speed.Reference Titze 44 Neuromuscular electrical stimulation was applied following the reading task to assess its effects in reducing subjective and objective measures of fatigue and soreness.
Materials and methods
Participants
Twenty-two women, aged 20–30 years, were recruited as participants. Approval from the Valdosta State University Institutional Review Board was obtained prior to enrolment of these participants. The inclusion of all female participants was suitable, as vocal dysfunction, including muscle tension dysphonia, is more common in women than in men.Reference Dietrich, Verdolini Abbot, Gartner-Schmidt and Rosen 45 All the women were non-smokers, in good general health and reported hearing acuity to be within normal limits. All participants exhibited a normal vocal quality, as determined by a certified speech and language pathologist. Information concerning age, BMI and intensity of current used during neuromuscular electrical stimulation is provided in Table III.
Table III Participant information by group

NMES = neuromuscular electrical stimulation; BMI = body mass index
Procedures
Each participant read aloud for 45 minutes at 65–75 dB, with a mouth-to-microphone distance of 30 cm. This vocal loading task is similar to that described by Laukkanen et al.Reference Laukkanen, Ilomäki, Leppänen and Vilkman 16 Loudness level was monitored by one of the investigators using a hand-held digital sound level meter (CEM™ DT-805). The participants were cued to increase or decrease loudness as needed during the reading task.
Following the prolonged reading task, 11 of the participants received 15 minutes of neuromuscular electrical stimulation at the minimum motor level of stimulation, defined as the minimum intensity at which the participant perceives a muscle contraction. The remaining 11 participants sat quietly for 15 minutes, serving as a control group. Neuromuscular electrical stimulation was administered via the VitalStim electrotherapy unit using two pairs of bipolar surface electrodes. Each pair of electrodes was placed on the anterior neck, lateral to the midline. One electrode on each side was placed in the thyrohyoid space, as determined via palpation of the neck. The second pair of electrodes was placed inferior to this position. These placements were used in an effort to recruit infrahyoid muscle fibres.
Acoustic data
Voice recordings were obtained before reading, after reading, and after neuromuscular electrical stimulation or rest. Similar to the procedure followed for experiment one (described above), each participant was asked to produce three repetitions of the first two sentences of the Rainbow Passage.Reference Fairbanks 24 The voice samples were obtained using the Audio-Technica AT3032 omnidirectional microphone connected to a desktop computer, and the samples were digitised directly into the computer via the Computerized Speech Lab system.
As for experiment one, measurements of speaking fundamental frequency and relative loudness level were obtained using the Computerized Speech Lab software, and measures of cepstral peak prominence and low/high spectral ratio were obtained via Analysis of Dysphonia in Speech and Voice software. All recordings were completed at a pitch and loudness level perceived as comfortable by the participant. Given that pitch and loudness levels may change following vocal loading tasks,Reference Laukkanen, Ilomäki, Leppänen and Vilkman 16 it was important that the participants determined their individual recording levels.
Statistical analyses were performed using a repeated measures ANOVA, with the three time periods of: before reading, after reading, and following 15 minutes of neuromuscular electrical stimulation or rest. The application of neuromuscular electrical stimulation was included as a between-group factor.
Perceptual ratings
Participants were asked again to provide ratings of muscular soreness and vocal fatigue using a 100 mm visual analogue scale, at the following time points: before and after reading, after 15 minutes of neuromuscular electrical stimulation or rest, and 24 hours following neuromuscular electrical stimulation or rest.
Results
Acoustic data
The repeated measures ANOVA revealed a significant effect of time for speaking fundamental frequency (F (1, 40) = 38.136, p < 0.0001), relative loudness level (F (1, 40) = 51.830, p < 0.0001) and cepstral peak prominence (F (1, 40) = 18.780, p < 0.0001), but there was no significant effect of time for low/high spectral ratio (F (1, 40) = 3.064, p = 0.058). Both the neuromuscular electrical stimulation and non-neuromuscular electrical stimulation participants exhibited an increase in speaking fundamental frequency, relative loudness level and cepstral peak prominence post-reading, as well as a subsequent downward shift for these parameters following 15 minutes of neuromuscular electrical stimulation or rest (Figures 1–3). There was a significant effect of neuromuscular electrical stimulation for cepstral peak prominence (F (1, 20) = 6.840, p = 0.017). The neuromuscular electrical stimulation group exhibited a higher average cepstral peak prominence for all time periods combined, compared to the non-neuromuscular electrical stimulation group (6.48 dB vs 5.90 dB).

Fig. 1 Speaking fundamental frequencies pre- and post-reading, and following 15 minutes of (a) neuromuscular electrical stimulation or (b) rest (no neuromuscular electrical stimulation). SFF = speaking fundamental frequency; no. = number; min = minutes

Fig. 2 Relative loudness level pre- and post-reading, and following 15 minutes of (a) neuromuscular electrical stimulation or (b) rest (no neuromuscular electrical stimulation). RLL = relative loudness level; no. = number; min = minutes

Fig. 3 Cepstral peak prominence pre- and post-reading, and following 15 minutes of (a) neuromuscular electrical stimulation or (b) rest (no neuromuscular electrical stimulation). CPP = cepstral peak prominence; no. = number; min = minutes
Perceptual ratings
As depicted in Figure 4a and b, both groups reported moderate fatigue and muscle soreness immediately after reading. There were decreases in both parameters after 15 minutes of neuromuscular electrical stimulation or rest, and 24 hours following neuromuscular electrical stimulation or rest. Examination of the data revealed a trend towards a greater reduction in fatigue and muscle soreness or delayed-onset muscle soreness ratings in participants who had 15 minutes of rest compared to those who had 15 minutes of neuromuscular electrical stimulation. However, the results of a repeated measures ANOVA revealed no significant differences in vocal fatigue ratings according to time (F (1, 19) = 2.958, p = 0.102) or neuromuscular electrical stimulation (F (1, 19) = 0.183, p = 0.674). Similarly, no significant differences in ratings of delayed-onset muscle soreness were found for time (F (1, 19) = 3.296, p = 0.085) or neuromuscular electrical stimulation (F (1, 19) = 0.292, p = 0.595).

Fig. 4 Average perceptual ratings of (a) fatigue and (b) muscle soreness or delayed-onset muscle soreness before and after reading, after 15 minutes of neuromuscular electrical stimulation or rest, and 24 hours following neuromuscular electrical stimulation or rest. NMES = neuromuscular electrical stimulation; min = minutes; h = hours
Discussion
This second study was designed to determine whether the application of neuromuscular electrical stimulation to the laryngeal area contributed to muscle recovery following a vocal loading task. In order to answer this question, we needed a task that provided sufficient muscular stress to the laryngeal system. The finding that participants in both groups exhibited an increase in speaking fundamental frequency and relative loudness level following the prolonged reading task at an elevated loudness level indicates that the task was sufficient to induce muscle fatigue and soreness. Prior reports have demonstrated similar acoustic changes following a prolonged reading taskReference Laukkanen, Järvinen, Artkosi, Waaramaa-Mäki-Kulmala, Kankare and Sipolla 15 , Reference Vintturi, Alku, Lauri, Sala, Sihvo and Vilkman 43 and in teachers complaining of vocal fatigue.Reference Laukkanen, Ilomäki, Leppänen and Vilkman 16 The increase in cepstral peak prominence following the vocal loading task is associated with an increase in relative loudness level and reflects greater vocal strength.Reference Awan, Giovinco and Owens 46
Both the neuromuscular electrical stimulation and non-neuromuscular electrical stimulation participants exhibited lower values for all parameters following the 15 minutes of neuromuscular electrical stimulation or rest; however, the post-recovery data remained slightly above pre-reading levels. This pattern is in agreement with findings reported by Vintturi et al., whose participants demonstrated lower fundamental frequencies and SPLs following a ‘short vocal rest’ period of 15 minutes subsequent to a 45-minute vocal loading task.Reference Vintturi, Alku, Lauri, Sala, Sihvo and Vilkman 43
The vocal loading task induced a temporary state of muscle fatigue as a result of overuse of the intrinsic laryngeal muscles. The interarytenoid and cricothyroid muscles are likely contracted maximally during vocal loading in an effort to increase vocal loudness, as both are used to increase subglottic pressure. Muscular fatigue results from continued and forceful contraction of these muscles. Although laryngeal muscles have a higher proportion of fatigue-resistant fibres,Reference Schiaffino and Reggiani 47 they may still weaken during prolonged voice use at loud levels.
Several studies have suggested that passive recovery or rest contributes to greater muscle recovery and improved performance during repeated bouts of high-intensity exercise.Reference Ben Abderrahman, Zouhal, Chamari, Thevenet, de Mullenheim and Gastinger 48 , Reference McAinch, Febbraio, Parkin, Zhao, Tangalakis and Stojanovaska 49 Evidence regarding the use of neuromuscular electrical stimulation as a means of muscle recovery has been conflicting, with some studies reporting that neuromuscular electrical stimulation aids muscle recoveryReference Neric, Beam, Brown and Wiersma 33 and others reporting no effect.Reference Lattier, Millet, Martin and Martin 34
In the present study, there were no significant differences in the objective parameters following neuromuscular electrical stimulation assisted recovery compared to passive recovery. Both groups exhibited a trend towards baseline for all parameters, although none of the participants returned to baseline. Based on these data, one may conclude that the use of neuromuscular electrical stimulation to restore muscle function and balance to the laryngeal system is not an effective method for improving muscle performance.
However, as in prior studies that investigated the effect of neuromuscular electrical stimulation upon healthy participants, individual reactions were mixed. While participants in both groups reported sensations of fatigue and soreness post-reading, six of the participants who received neuromuscular electrical stimulation stated that their voice felt better or ‘stronger’ after 15 minutes of electrical stimulation. Two participants reported that they were still sore or fatigued after neuromuscular electrical stimulation, but they did not elaborate further. One participant stated that the neuromuscular electrical stimulation was painful and her neck remained sore following the brief session of electrical stimulation, while another participant felt an ‘odd’ sensation on her neck after neuromuscular electrical stimulation. The last participant reported that she was initially nervous about using neuromuscular electrical stimulation, but subsequently felt fine. These findings are similar to those reported by Fowler et al.Reference Fowler, Gorham-Rowan and Hapner 10 , Reference Fowler, Awan, Gorham-Rowan and Morris 12 and Gorham-Rowan et al.,Reference Gorham-Rowan, Fowler and Hapner 11 as well as the experiment one findings.
In contrast, six of the participants in the non-neuromuscular electrical stimulation group continued to experience slight to moderate levels of fatigue and soreness following 15 minutes of rest. While none of the participants reported fatigue or soreness 24 hours post-reading, these findings suggest that the use of neuromuscular electrical stimulation may be beneficial in the short-term to reduce muscle tension or at least the perception of excessive tension. This perceptual improvement is key in the recovery process.
The perception of muscle fatigue and soreness impairs muscle performance and limits an individual's ability to perform at peak capacity. It is also likely to encourage maladaptive behaviours, used to compensate for these sensations. In muscle tension dysphonia patients, the sensation of fatigue and soreness leads to inappropriate use of the extrinsic musculature in an effort to minimise these sensations. However, these compensatory strategies generally exacerbate muscle misuse and contribute to further vocal problems. In the absence of these negative sensations, one can learn the appropriate techniques more easily; for muscle tension dysphonia patients, this would allow for an easier transition to resonant voice production.
-
• Neuromuscular electrical stimulation applied to the laryngeal area for 30–60 minutes can result in measurable voice changes in normophonic speakers
-
• Some report vocal warm-up (or no effect) following this electrical stimulation; others report vocal fatigue and/or muscle soreness
-
• A shorter laryngeal neuromuscular electrical stimulation time (15 minutes) still results in measurable voice changes
-
• Factors other than neuromuscular electrical stimulation may account for perceived fatigue and muscle soreness symptoms
-
• Laryngeal neuromuscular electrical stimulation may be used to facilitate muscle recovery after a vocally stressful task
Based on this premise, one may speculate that neuromuscular electrical stimulation may be beneficial for some patients to enhance rehabilitative efforts. Further investigation is warranted to determine the applicability of laryngeal neuromuscular electrical stimulation in voice therapy.
Acknowledgements
The authors express their gratitude to the volunteers who participated in the study, and thank Linda Fowler, Dee Kachra, Joanne Brogdon, Hayley Holbrook and Miranda Larimore for their assistance in study design, data collection and analysis.









