INTRODUCTION
Island ecosystems are particularly vulnerable to invasive mammals because insular flora and fauna have generally evolved in the absence of terrestrial mammals, and thus many island species lack morphological, chemical, behavioural and life history defences against mammalian predation and herbivory (Brown Reference Brown1997; Kress Reference Kress, Marduff and Sallabanks1998; Billing Reference Billing2000; Atkinson Reference Atkinson2001). Two-thirds of all currently threatened birds nest on islands, and at least 92% of bird extinctions over the past 400 years were island nesters (Kress Reference Kress, Marduff and Sallabanks1998).
One of the most damaging and widespread groups of introduced mammals are the three species of commensal rats (Rattus exulans, R. rattus and R. norvegicus). One or more of these species have been introduced to about 82% of the world's island groups (Atkinson Reference Atkinson and Moors1985) and are cited as the cause of an estimated 40–60% of bird and reptile extinctions worldwide (Groombridge Reference Groombridge1992; see Donlan et al. Reference Donlan, Howald, Tershy and Croll2003). Compared to endemic landbirds, seabirds tend to breed as metapopulations across several islands, making them less vulnerable to extinction from introduced predators. However, seabird populations are highly vulnerable to local extinctions caused by rats (Atkinson Reference Atkinson and Moors1985), and this is exacerbated by general life history traits of low adult mortality, delayed reproduction, low fecundity and long incubation periods (Russell Reference Russell1999). Burrow or crevice nesters with relatively small body sizes and high nest site fidelity may be particularly vulnerable to introduced rats because they prefer similar habitats (Bertram & Nagorsen Reference Bertram and Nagorsen1995; Seto & Conant Reference Seto and Conant1996; Zino et al. Reference Zino, Oliveira, King, Buckle, Biscoito, Neves and Vasconcelos2001; Jouventin et al. Reference Jouventin, Bried and Micol2003).
The impacts of introduced rats on island nesting seabird populations can be separated into two categories: (1) direct impacts because of egg, chick and adult predation (Moors & Atkinson Reference Moors, Atkinson, Croxall, Evans and Schreiber1984; Atkinson Reference Atkinson and Moors1985) and (2) indirect impacts through disturbance, nest abandonment, higher mortality in colonies leading to increased divorce rates and burrow-switching (Jouventin et al. Reference Donlan, Howald, Tershy and Croll2003). These impacts can best be demonstrated with manipulative studies, as have been conducted for landbirds (for example Penloup et al. Reference Penloup, Martin, Gory, Brunstein and Bretagnolle1997; Brown et al. Reference Brown, Moller, Innes and Jansen1998; Robinet et al. Reference Robinet, Craig and Chardonnet1998; VanderWerf Reference VanderWerf2001). However, few such studies have been conducted on island nesting seabirds. Thus, while it is widely accepted that invasive rats are detrimental, the extent of their effects on seabirds has rarely been quantified experimentally (Towns et al. Reference Towns, Simberloff and Atkinson1997; Parker et al. Reference Parker, Simberloff, Lonsdale, Goodell, Wonham, Kareiva, Williamson, Von Holle, Moyle, Byers and Goldwasser1999; but see Pierce Reference Pierce2002; Stapp Reference Stapp2002; Jouventin et al. Reference Jouventin, Bried and Micol2003).
Murrelets (family Alcidae) of the genus Synthliboramphus are a group of long-lived burrow or crevice nesting seabirds with low fecundity, high nest-site fidelity and an incubation strategy where adults leave eggs unattended for extended periods (Gaston & Jones Reference Gaston and Jones1998). For these reasons they are particularly vulnerable to introduced rats. In this study we use a two-phase rat eradication effort to: (1) quantify the effects of black rat predation on Xantus's murrelet (Synthliboramphus hypoleucus scrippsi) eggs and (2) examine the relative depredation of introduced black rats versus endemic deer mice (Peromyscus maniculatus anacapae) on murrelet eggs.
Study area
Anacapa Island, California, USA (Fig. 1) is located c. 15 km off the coast of Southern California and is included in Channel Islands National Park. It comprises three distinct islets (East Anacapa Islet [45 ha], Middle Anacapa Islet [60 ha] and West Anacapa Islet [179 ha]) separated by narrow channels. Steep and rugged 50–200 m basalt cliffs surround the island and provide abundant breeding habitat for hole-nesting seabirds (McChesney et al. Reference McChesney, Gress, Carter and Whitworth2000).
Figure 1 Artificial nest study sites on Anacapa's three islets (East, Middle and West), located off the coast of southern California, USA. Black dots indicate artificial nest deployment areas. Note: scale applies only to the visually enhanced portion of the Anacapa islets.
Study species
Xantus's murrelets are small (148–167 g), relatively long-lived seabirds (Drost & Lewis Reference Drost and Lewis1995). Due to their small and restricted population (around 10 000 breeding individuals on the US Channel Islands and three island groups in Mexico) and potential threats (such as oil spills and introduced predators; Drost & Lewis Reference Drost and Lewis1995; Carter et al. Reference Carter, Whitworth, Takekawa, Keeney, Kelly, Browne, Mitchell and Chaney1999; Wolf Reference Wolf2002), Xantus's murrelets are listed as threatened under the California State Endangered Species Act (E. Burkett & J. Ugoretz, unpublished data 2004). Xantus's murrelets spend most of their life on the open ocean, only coming ashore to nest. Eggs are laid on bare ground in holes, crevices or beneath shrubs (Drost & Lewis Reference Drost and Lewis1995).
Adults only enter and leave the nesting colonies at night when there is little or no moon. Xantus's murrelets typically arrive on the Channel Islands in mid-February. Nesting persists through mid-June with peak nest initiation from late March to late April (Drost & Lewis Reference Drost and Lewis1995). A clutch consists of two eggs typically laid eight days (and up to 19 days) apart. Birds nest annually and replacement of lost clutches is unusual. Incubation begins after the second egg is laid and continues for approximately 34 days (Drost & Lewis Reference Drost and Lewis1995). Chicks are precocial and leave the nest with their parents one to two days after hatching (Drost & Lewis Reference Drost and Lewis1995). Black rats, the only non-native mammals present on Anacapa, occur on all three Anacapa islets and were probably introduced between the mid-1800s and early 1900s (Collins Reference Collins and Power1979; McChesney & Tershy Reference McChesney and Tershy1998). At that time Xantus's murrelets were thought to have been abundant breeders on the island (Howell Reference Howell1917), but surveys in the late 1990s and early 2000s revealed the breeding population was small, with all signs of breeding limited to steep cliffs and sea caves where rats presumably have limited access (McChesney & Tershy Reference McChesney and Tershy1998). Nevertheless, even these sites have shown high rates of rat predation on murrelet eggs (Whitworth et al. Reference Whitworth, Keitt, Carter, McChesney, Mason, McIver and Hebshi2003). There are no recent records of Xantus's murrelets breeding under shrubs on top of Anacapa Island, a habitat used frequently by this species on nearby, rat-free Santa Barbara Island (Drost & Lewis Reference Drost and Lewis1995).
Endemic Anacapa deer mice, western gulls (Larus occidentalis) and common ravens (Corvus corax) occur on all three Anacapa islets, and are the only natural Xantus's murrelet egg predators on Anacapa. Deer mice populations were established long before rats were introduced on Anacapa, precluding them from consideration as the cause of Xantus's murrelet declines. Although there are no data for Anacapa Island prior to rat introduction, deer mice coexist with Xantus's murrelets on nearby rat-free Santa Barbara Island, where deer mouse predation rates are estimated at around 20–30% of Xantus's murrelet eggs in years of average mice densities (Schwemm & Coonan Reference Schwemm and Coonan2001).
METHODS
Predation on artificial nests was measured during spring 2002 and 2003, coinciding with a successful rat eradication effort on the three islets of Anacapa Island (Howald et al. Reference Howald, Faulkner, Tershy, Keitt, Gellerman, Creel, Grinnell, Ortega, Croll, Garcelon and Schwemm2005). We took advantage of the staged eradication: a 25-ppm brodifacoum rodenticide bait was dropped on East Anacapa Islet in December 2001 (prior to the 2002 murrelet breeding season), followed by similar treatments of Middle and West Islets in November 2002 (prior to the 2003 murrelet breeding season) (Table 1). Artificial nests were deployed on all islets during the peak Xantus's murrelet breeding seasons of 2002 and 2003 (approximately 4–5 months after the rodenticide applications). By establishing artificial nests across East, Middle and West islets in 2002 and 2003, we were able to: (1) compare rat predation in 2002 on artificial nests on treated East Islet with untreated Middle and West Islets, and (2) compare rat predation of artificial nests on untreated Middle and West Islets in 2002 with predation on treated Middle and West Islets in 2003.
Table 1 Timetable of artificial nest deployment and eradication activities on Anacapa Island from 2001 to 2003.

Prior to application of the rodenticide, endemic deer mice were captured and held in captivity (Pergams et al. Reference Pergams, Lacy and Ashley2000). Populations of wild deer mice were dramatically reduced or extirpated following treatment with rodenticide. The captive animals were then released back into the wild and their populations recovered to at or above pre-rat eradication levels (H. Gellerman, personal communication 2004). This enabled us to determine the relative rates of nest predation by introduced rats and endemic mice.
Artificial nests were deployed in crevices, under boulders, and under plants in typical nest locations in known Xantus's murrelet nesting habitat (D. Whitworth, personal communication 2002). Xantus's murrelets do not construct nests, thus deployment was simply placing the two eggs in the chosen habitats. To encompass all possible nesting habitats, artificial nests were placed both at shore sites and on top of the island. In 2002, 132 artificial nests (n = 62, 39 and 31 for East, Middle and West Anacapa Islets, respectively) were deployed for 23 days. In 2003, 102 artificial nests (n = 43, 40 and 19 for East, Middle and West Anacapa Islets, respectively) were deployed for 35 days. We considered differences in deployment duration conservative, as the lengthened deployment time in 2003 would overestimate predation rates in treated areas compared to rates in untreated areas. Nests were placed in all available and accessible known Xantus's murrelet nesting habitat, resulting in uneven numbers of nests on each islet, but an accurate portrayal of true Xantus's murrelet nest vulnerability.
Each nest consisted of two eggs: one brown chicken egg to attract predators via smell and one brown plasticine clay egg to preserve evidence of depredation and allow identification of predators. Both types of eggs were of very similar size and shape to those of Xantus's murrelets. Latex gloves were worn while shaping the plasticine eggs and handling time in the field was limited to prevent predation overestimates due to human scent on eggs. Nests were considered depredated if there were signs of rodent chews or bird (gull or raven) beak indents on either egg. Eggs with incisor marks that could not be distinguished as either introduced rat or native mouse were designated unknown predator.
Pearson χ2 statistical analyses were performed using Systat 10 to compare frequency distributions of depredated and non-depredated nests between years and treatments. Because Middle and West Anacapa Islets are located adjacent to one another, have identical geological histories, have similar geomorphology and received identical treatments with rodenticide, data from artificial nests on these two islets were pooled for analyses.
RESULTS
In 2002, nest predation was considerably higher at the untreated islets (Middle/West Anacapa Islets; 96%) than at our treated site (East Anacapa Islet; 8%) (Fig. 2). Native deer mice (n = 4) and birds (n = 1) accounted for all the artificial nest predation at East Anacapa Islet in 2002, while rats depredated the majority of nests at Middle and West Anacapa Islets (n = 46; 69%). In reality, the number of rat-depredated nests was likely higher as 24% of artificial nest predators on Middle and West Anacapa Islets could not be determined as either rat or mouse because of indistinguishable incisor marks (n = 17).
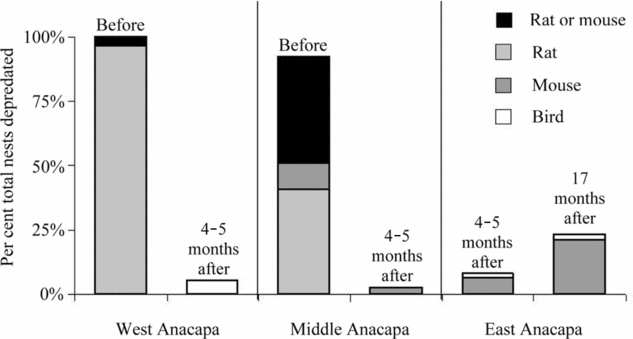
Figure 2 Depredation of Xantus's murrelet nests on the islets of Anacapa Island, Channel Islands, California, both before and after rat eradication. ‘Rat or mouse’ nests are those that contained chewed eggs where we could not distinguish between damage caused by rat or mouse incisors.
Rat predation was significantly lower on East Islet compared to Middle and West Islets in 2002 (Fig. 2; χ2 = 101.878, df = 1, p < 0.001). Rat predation was also significantly lower on Middle and West Islets in 2003 compared to 2002 (χ2 = 109.691, df = 1, p < 0.001). Nest depredation on ratfree East Anacapa Islet was lower in the first year of rat eradication, when neither rats nor mice were present, than in the second year, when endemic deer mouse populations had recovered to record levels (Fig. 2; χ2 = 4.785, df = 1, p < 0.05). Even with mice at record numbers on East Islet, rat predation on Middle and West Islets in 2002 was significantly higher than mouse predation on East Islet (χ2 = 21.385, df = 1, p < 0.001).
DISCUSSION
In 2003, following eradication efforts on Middle and West Anacapa Islets, predation on artificial nests was drastically reduced, with numbers rebounding from nearly complete artificial nest failure (96% depredated) to nearly complete survival (97% survived). However, the overall predation rates measured on Middle/West Anacapa Islets in 2003 and on East Anacapa Islet in 2002 are likely underestimates because artificial nests were deployed just 4–5 months after rodenticide bait was broadcast on the islets, before the native deer mice had time to recover. The predation rates on East Anacapa Islet in 2003 include this source of predation and may be more realistic as the native deer mice had 17 months of recovery prior to nest deployment. However, rodent studies conducted on East Anacapa Islet in 2003 (H. Gellerman, personal communication 2004) found three-fold higher population densities of native deer mice than had ever been recorded on the islet, as a result of exceptionally good conditions and possibly the absence of rat competition. Deer mouse populations have since dropped to about 25% higher than densities recorded when introduced rats were on the island. As a result of the post-eradication recovery of deer mice on East Anacapa Islet, artificial nest depredation increased from 8% in 2002 to 23% in 2003, but remained well below the levels recorded in the presence of introduced rats (Fig. 2) and similar to deer mouse predation rates on rat-free Santa Barbara Island (Schwemm & Coonan Reference Schwemm and Coonan2001). Even with deer mice population density at its peak, rat predation rates prior to eradication (69% of artificial nests) far exceeded those of mice following their recovery (23% of artificial nests).
Recent papers have criticized mainland-based artificial nest studies as: (1) having unnaturally high nest densities, (2) poorly mimicking the construction of natural nests with live adults present and (3) likely to attract a different array of predators than natural nests (Faaborg Reference Faaborg2004; Thompson & Burhans Reference Thompson III and Burhans2004). None of these three problems apply to this study because Xantus's murrelets, like many other island-nesting seabirds, nest at relatively high densities compared to many landbirds, construct no nests, have long periods with no adult nest attendance, and are threatened by only one introduced and two or three native nest predators. Thus, for the Xantus's murrelet, like many island-breeding seabirds, artificial nest studies can provide a means to quickly document the impact of introduced rats on both extant and extirpated island-nesting seabirds. Furthermore, because Xantus's murrelet chicks, like chicks of all Synthliboramphus species, leave the colony within a few days after hatching, artificial nest experiments may provide a relatively accurate index of overall egg and chick mortality caused by introduced rats. However, it is also possible that chicks are particularly vulnerable to rat predation during their short time on the nest and when they walk toward the sea for fledging.
Whatever potential bias existed in our estimates of nest predation rates using artificial nests, they were consistent between years and across islands, suggesting that our study provides an accurate assessment of the conservation benefit of rat removal to Xantus's murrelets. The observed increase in nest survival from 4% in the presence of introduced rats and native mice to 77% in the presence of native mice alone (based on data from Middle and West Anacapa islets) provides experimental evidence for a clear benefit of introduced rat removal to seabird conservation. Xantus's murrelet nest surveys following rat removal revealed a marked increase in nest success, with successful nests increasing from a previous high in 2003 (17 nests) to even more in 2005 (25 nests) (D. Whitworth, personal communication 2005). Many of the successful nests are in the exact locales of our artificial nest sites, indicating our study design closely mimicked actual Xantus's murrelet nesting habitat.
On a global scale, introduced species are thought to have the largest impact on seabirds, with invasive predatory mammals having the greatest effects (Taylor Reference Taylor2000). While eradication of introduced species from islands is a powerful tool to protect threatened seabirds, it is underused (Donlan et al. Reference Donlan, Howald, Tershy and Croll2003). One of the reasons eradication is an underused seabird conservation tool may be the many years it often takes to detect a positive response in seabird populations following eradication efforts. This is supported by the relatively small number of studies showing seabird recovery (for example Lorvelec & Pascal Reference Lorvelec and Pascal2005) compared to the hundreds of invasive mammal eradications that have been successfully undertaken worldwide (Tershy et al. Reference Tershy, Donlan, Sanchez-Pacheco, Wood, Howald, Hermosillo, Biavaschi, Veitch and Clout2002; Towns & Broome Reference Towns and Broome2003). Seabirds are inherently slow to respond to predator release because they have low reproductive rates, delayed onset of reproductive maturity and relatively strong Allee effects (Jouventin et al. Reference Jouventin, Bried and Micol2003). Artificial nest studies can be a quick and effective means of measuring the likely response of targeted seabird species, and they can also be useful in evaluating (1) eradication efficacy, (2) compensatory predatory response of native egg predators and recovery of native predators following invasive rodent eradications and (3) historical impacts of introduced egg predators on breeding seabird populations that have long been extirpated.
ACKNOWLEDGEMENTS
We thank Channel Islands National Park (Kate Faulkner, Steve Ortega, Randy Bidwell, Bill Struble, Doretta Burgess and especially Fred Rodriguez), the American Trader Trustee Council (Jennifer Boyce [NOAA], Carol Gorbics [FWS], and Paul Kelly [CDFG]), the staff of Conservación de Islas and Island Conservation (Brad Keitt, Kris Hulvey, Noah Biavaschi, Matt Grinnell, Tosha Comendant, Holly Gellerman, Eileen Creel, Jacob Sheppard and especially Virginia McDermott), Darrell Whitworth and Abe Jayson for their support and contributions to this project. We thank Michel Pascal and two anonymous reviewers for their valuable comments on this manuscript.





