INTRODUCTION
Executive functions (EF) play a crucial role in early cognitive, behavioral and socio-emotional development (Carlson, Davis, & Leach, Reference Carlson, Davis and Leach2005; Isquith, Crawford, Espy, & Gioia, Reference Isquith, Crawford, Espy and Gioia2005). EF is an umbrella term used to describe the processes needed to achieve novel, purposeful and goal-directed action; these include, among others, inhibition, cognitive flexibility, planning and abstraction (Garon, Bryson, & Smith, Reference Garon, Bryson and Smith2008; Lezak, Reference Lezak1982). The development of EF has been linked in part to the maturation of prefrontal brain regions (Goldberg & Bougakov, Reference Goldberg and Bougakov2005; Jacobs, Harvey, & Anderson, Reference Jacobs, Harvey and Anderson2007; Wood & Smith, Reference Wood and Smith2008). During the first 5 years of life, the rapid growth of prefrontal brain regions translates into significant progress in EF (Anderson, Reference Anderson2002; Durston & Casey, Reference Durston and Casey2006; Garon et al., Reference Garon, Bryson and Smith2008; Tsujimoto, Reference Tsujimoto2008). The preschool period is marked by the acquisition of core EF (e.g., inhibition, cognitive flexibility), while more complex skills (e.g., problem solving, goal setting, reasoning) develop in middle childhood and adolescence (Anderson, Reference Anderson1998, Reference Anderson2002). Studies that have focused on mapping the developmental trajectories of EF indicate that earlier developing core EF underlie and predict more complex executive abilities (Collins & Koechlin, Reference Collins and Koechlin2012; Diamond, Reference Diamond2013; Garon et al., Reference Garon, Bryson and Smith2008). As such, if the integrity of the brain is altered during early childhood, a critical time for the establishment and emergence of executive skills, there is likely to be increased risk for EF impairments and cumulative deficits.
Given that EF are mainly governed by prefrontal regions, they are particularly vulnerable to frontal insults, such as those typically observed after traumatic brain injury (TBI), which causes the brain to move back and forth within the skull due to accelerating and decelerating forces (Mustafa & Alshboul, Reference Mustafa and Alshboul2013). Accordingly, EF deficits after pediatric TBI are well documented in school-aged children and adolescents (Levin & Hanten, Reference Levin and Hanten2005). Such executive deficits are generally more pronounced following moderate-severe TBI (Nadebaum, Anderson, & Catroppa, Reference Nadebaum, Anderson and Catroppa2007), but in some cases, they may manifest after mild TBI (mTBI; Loher, Fatzer, & Roebers, Reference Loher, Fatzer and Roebers2014). However, only a handful of studies have investigated EF following TBI in early childhood. Those that have addressed the question report inhibition, flexibility and working memory deficits that are generally more pronounced after more severe injuries (Catroppa, Anderson, Morse, Haritou, & Rosenfeld, Reference Catroppa, Anderson, Morse, Haritou and Rosenfeld2007; Crowe, Catroppa, Babl, & Anderson, Reference Crowe, Catroppa, Babl and Anderson2013; Ewing-Cobbs, Prasad, Landry, Kramer, & DeLeon, Reference Ewing-Cobbs, Prasad, Landry, Kramer and DeLeon2004; Ganesalingam et al., Reference Ganesalingam, Yeates, Taylor, Walz, Stancin and Wade2011; Nadebaum et al., Reference Nadebaum, Anderson and Catroppa2007).
Despite these results, several methodological issues preclude clear conclusions about the impact of preschool TBI on EF. First, some studies group preschoolers with school-age children, making it impossible to draw conclusion specific to the younger age group (Catroppa et al., Reference Catroppa, Anderson, Morse, Haritou and Rosenfeld2007; Nadebaum et al., Reference Nadebaum, Anderson and Catroppa2007). Others group accidental and inflicted TBI together (Ewing-Cobbs et al., Reference Ewing-Cobbs, Prasad, Landry, Kramer and DeLeon2004), though the two forms are associated with distinct brain pathology and psychosocial factors (Ennis & Henry, Reference Ennis and Henry2004; Keenan, Runyan, Marshall, Nocera, & Merten, Reference Keenan, Runyan, Marshall, Nocera and Merten2004). In addition, some focus only on longer-term outcomes (e.g., middle childhood) of preschool injuries or do not standardize the time elapsed since the injury (Catroppa et al., Reference Catroppa, Anderson, Morse, Haritou and Rosenfeld2007; Crowe et al., Reference Crowe, Catroppa, Babl and Anderson2013; Nadebaum et al., Reference Nadebaum, Anderson and Catroppa2007). Finally, some focus specifically on the effects of moderate–severe injuries, and do not include mTBI (Ewing-Cobbs et al., Reference Ewing-Cobbs, Prasad, Landry, Kramer and DeLeon2004; Ganesalingam et al., Reference Ganesalingam, Yeates, Taylor, Walz, Stancin and Wade2011), although mild injuries constitute the majority (approximately 90%) of TBI (Cassidy et al., Reference Cassidy, Carroll, Peloso, Borg, von Holst, Holm and Coronado2004).
In addition to showing susceptibility to brain insults such as TBI, altered EF has been linked to broader disorders of biological functions, such as sleep disturbances. Beyond the negative effects of sleep problems on general cognitive functioning, substantive literature has identified EF as especially vulnerable to poor sleep. Although the underlying neuronal and neurochemical mechanisms of sleep-related EF dysfunction remain unclear, it has been suggested that the effect of poor sleep is more potent for EF because the prefrontal cortex is especially sensitive to sleep curtailment (Dahl, Reference Dahl1996; Horne, Reference Horne1993; Jones & Harrison, Reference Jones and Harrison2001; Muzur, Pace-Schott, & Hobson, Reference Muzur, Pace-Schott and Hobson2002). It is hypothesized that the prefrontal regions largely benefit from the regenerating role of sleep due to their intense activity in waking humans, and that their functioning essentially rests with proper sleep (Horne, Reference Horne1993; Ingvar, Reference Ingvar1979).
In typically developing school-aged children and adolescents, associations between poor sleep and EF impairments have been identified through both correlational studies (Friedman, Corley, Hewitt, & Wright, Reference Friedman, Corley, Hewitt and Wright2009; Gregory, Caspi, Moffitt, & Poulton, Reference Gregory, Caspi, Moffitt and Poulton2009; Sadeh, Reference Sadeh2007; Sadeh, Gruber, & Raviv, Reference Sadeh, Gruber and Raviv2002), and experimental sleep restriction paradigms (Gruber et al., Reference Gruber, Wiebe, Montecalvo, Brunetti, Amsel and Carrier2011; Randazzo, Muehlbach, Schweitzer, & Walsh, Reference Randazzo, Muehlbach, Schweitzer and Walsh1998; Sadeh, Gruber, & Raviv, Reference Sadeh, Gruber and Raviv2003). A small number of studies have investigated the association between sleep and EF in the preschool period. In a longitudinal study, sleep at 1 year of age was shown to predict EF performance at 18, 24 and 48 months of age, independently of general cognitive abilities and language skills (Bernier, Beauchamp, Bouvette-Turcot, Carlson, & Carrier, Reference Bernier, Beauchamp, Bouvette-Turcot, Carlson and Carrier2013; Bernier, Carlson, Bordeleau, & Carrier, Reference Bernier, Carlson, Bordeleau and Carrier2010). Similarly, Nelson, Nelson, Kidwell, James, and Espy (Reference Nelson, Nelson, Kidwell, James and Espy2015) showed that sleep problems were negatively associated with subsequent performance on suppression inhibition and working memory tasks in a sample of typically developing preschoolers.
Despite growing literature on the effects of poor sleep and EF, few studies have been conducted in children with TBI. This is concerning given that children with TBI are at increased risk for sleep disturbances (Gagner, Landry-Roy, Laine, & Beauchamp, Reference Gagner, Landry-Roy, Laine and Beauchamp2015), which could exacerbate putative post-injury EF impairments. The preschool period, marked by the development of core EF, is of particular interest since it witnesses the highest prevalence of TBI (Crowe, Babl, Anderson, & Catroppa, Reference Crowe, Babl, Anderson and Catroppa2009; Hawley, Ward, Long, Owen, & Magnay, Reference Hawley, Ward, Long, Owen and Magnay2003). To our knowledge, only one study has examined the relation between sleep disturbances and EF in preschoolers with TBI of various severity at 6, 12, and 18 months post-injury (Shay et al., Reference Shay, Yeates, Walz, Stancin, Taylor, Beebe and Wade2014). Most analyses failed to reveal significant associations between sleep disturbances and executive functions. However, the authors observed that children with severe TBI who experienced a high level of sleep disturbance tended to display poorer performance on inhibition and flexibility measures at 6 and 12 months post-injury, but this finding must be interpreted with caution as it only approached significance. In contrast, sleep disturbance was not associated with any of the other tests of cognitive ability included. Shay and colleagues (2014) also reported that children with more sleep problems demonstrated poorer executive functions in everyday situations, but this relation held across both TBI and control groups.
The first objective of the current study was to assess EF in preschoolers with mTBI. It was expected that children with mTBI would display poorer EF than typically developing controls. The second objective was to determine the role of sleep in the links between preschool mTBI and executive functioning. Given that sleep impacts EF in normative pediatric populations, it was expected that sleep would interact with mTBI in the prediction of EF, such that children with mTBI and poorer sleep would exhibit poorer EF.
METHODS
Participants
This study was approved by the institutional review board of Sainte-Justine Hospital and conducted in accordance with the Helsinki declaration. The sample included 167 children divided in two groups: children who sustained accidental mTBI (n=84) and typically developing children (TDC, n=83). The present sample was drawn from a broader longitudinal study investigating cognitive and social outcomes of preschool TBI (LION study). As part of this larger study, children who sustained orthopedic injuries (OI) were also recruited. However, they were not included in the current paper given that only a small proportion of OI had available sleep data. We confidently used TDC for the current analyses, having previously shown that the TDC and OI groups in the study had similar demographic profiles, development and medical history, behavioral and adaptive profiles, family functioning, and cognition (Beauchamp, Landry-Roy, Gravel, Beaudoin, & Bernier, Reference Beauchamp, Landry-Roy, Gravel, Beaudoin and Bernier2017).
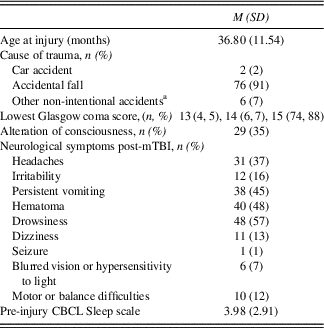
Children with mTBI were recruited based on the definition reported by Osmond et al. (Reference Osmond, Klassen, Wells, Correll, Jarvis, Joubert and Stiell2010). Inclusion criteria were: (a) age at injury between 18 and 60 months; (b) closed accidental TBI leading to an emergency consultation with a Glasgow Coma Scale (GCS) score between 13 and 15, and at least one of the following symptoms: persistent vomiting (≥3), excessive irritability, loss or alteration of consciousness, amnesia, headaches that worsen over time, drowsiness, dizziness, motor or balance difficulties, blurred vision, hypersensitivity to light and/or seizures; (c) child and at least one parent fluent in English or French. Injury characteristics are detailed in Table 1. Most mTBIs were caused by an accidental fall (93%), resulting in a GCS score of 15 in 88% of cases. The inclusion criteria for the TDC were: (a) age between 24 and 66 months at time of recruitment (to ensure that the two groups were comparable at time of assessment); (b) child and at least one parent fluent in English or French.
Table 1 Pre-injury and injury characteristics for the mTBI group

a Other non-intentional accidents were mainly collision-related (being struck by or against).
The following exclusion criteria were applied to all participants: (a) diagnosed congenital, neurological, developmental, psychiatric, metabolic, or sleep condition; (b) less than 36 weeks of gestation; (c) prior TBI.
Procedure
Children with mTBI were recruited in an urban tertiary care pediatric emergency department between 2011 and 2015. A research nurse approached eligible children and obtained their verbal consent to be contacted by the research coordinator. Families who agreed to participate were mailed a sociodemographic questionnaire and pre-injury questionnaires (including the Child Behavior Checklist, see description below) immediately after consenting. Parents were asked to answer the questionnaires based on their child’s functioning before the TBI to provide baseline information. Meanwhile, a standardized case report form was completed by the research nurse to ensure that children satisfied inclusion and exclusion criteria and to document clinical and injury-related details. Six months later (M=6.65, SD=0.86), parents were asked to complete the same questionnaire booklet and the Postconcussive Symptom Interview (see description below), and children completed a 3-hour neuropsychological assessment.
Children in the TDC group were recruited in local daycare centers. Given the absence of injury, children were invited for assessment as soon as possible after recruitment, and parents completed the sociodemographic questionnaire, the questionnaire booklet, and the Postconcussive Symptom Interview.
At the end of the neuropsychological assessment, a subset of children (n=68; 33 mTBI and 35 TDC) were asked to wear an actigraph to assess their sleep objectively. Actigraphs could not be proposed to all participants as the sleep sub-study was introduced after the initial start of recruitment. After the sub-study was introduced, all participants were offered an actigraph, unless they lived outside the urban area, which was not easily serviced by courier. There were no differences between those who were offered participation in the sleep sub-study and those who were not in terms of age (t(165)=0.20; p=.84), sex (χ 2 (1, N=167)=0.67; p=.42), or socioeconomic status (SES) (t(155)=−0.144; p=.89). Of the 68 families to whom actigraphs were offered, 2 families refused participation (either the parent or the child refused).
Measures
Participant and injury characteristics
Sociodemographic questionnaire
This questionnaire provides demographic information such as age, ethnicity, family constellation, parent’s education and occupation. Parental education was obtained by averaging both parents’ highest educational attainment, ranging from 1 (doctoral level) to 8 (less than 7 years of school). When parental occupation and/or highest educational attainment was available for only one parent, or in the case of single parent families, no average was computed. If a child lived with one biological parent and a step-parent, we used the step-parent’s information in the average. Parent occupation allows calculation of the Blishen Socioeconomic Index (Blishen, Carroll, & Moore, Reference Blishen, Carroll and Moore1987). In light of their intercorrelation (r=−.51; p<.001), parental education and Blishen scores were standardized and averaged into a global family SES index.
Case Report Form
Information obtained from medical files for the mTBI group included: cause of the accident, lowest GCS, presence of neurological signs (headaches, irritability, persistent vomiting, hematoma, drowsiness, dizziness, seizure, blurred vision or hypersensitivity to light, and motor or balance difficulties) and alteration of consciousness (defined as the presence of either confusion or loss of consciousness).
Postconcussive Symptom Interview (PCS-I) (Mittenberg, Wittner, & Miller, Reference Mittenberg, Wittner and Miller1997)
The PCS-I assesses 15 cognitive, somatic, sleep, and affective post-concussive symptoms. Parents must say if the symptoms were present or absent during the preceding week.
Executive functions
Delay of Gratification (Beck, Schaefer, Pang, & Carlson, Reference Beck, Schaefer, Pang and Carlson2011)
In this inhibition task, children must make a series of choices as to whether they want a smaller, immediate reward or a larger, delayed reward. Rewards consist of stickers, crackers and pennies presented in small plastic containers. There are three trials for each type of reward, for a total of nine trials. For example, the child must choose between a cracker to eat now, or six crackers to eat later “when all games are completed and you return home”. One point is awarded every time the child selects the delayed reward for a maximum of nine points, and a higher score indicates better performance. This task has satisfactory test–retest reliability (r=.76; Beck et al., Reference Beck, Schaefer, Pang and Carlson2011).
Conflict Scale (Zelazo, Reference Zelazo2006)
This cognitive flexibility task consists of four levels of increasing difficulty (Categorization/Reverse Categorization, Dimensional Change Card Sort (DCCS)–Separated, DCCS–Integrated, and DCCS–Advanced), and children begin the task at the appropriate level for their age. Children are asked to categorize items, either plastic animals or cards, according to a rule, and if they succeed on five trials out of six, the rule is changed in a post-switch phase. For example, children are first instructed to sort cards depicting trucks and stars according to color (blue or red). Then, the experimenter announces that they will stop playing the “color game” and now play the “shape game”. Children must then sort cards according to shape (truck or star), regardless of color. There are 12 trials per level, for a maximum of 48 points, and a higher score indicates higher cognitive flexibility. This measure has excellent test–retest reliability (r=.94; Beck et al., Reference Beck, Schaefer, Pang and Carlson2011).
Shape Stroop (Carlson, Reference Carlson2005; Kochanska, Murray, & Harlan, Reference Kochanska, Murray and Harlan2000)
In this task of inhibition and cognitive flexibility, children are first shown six cards depicting three fruits (three large and three small fruits) and asked to identify each fruit. Then, children are shown cards depicting a small fruit embedded in a large fruit (e.g., small banana embedded in a large orange), and asked to point to each small fruit (e.g., “show me the small banana”). A total of three cards are presented, for a maximum of three points, and a higher score indicates better performance.
Sleep measures
Child Behavior Checklist (CBCL), 1.5–5 year version (Achenbach & Rescorla, Reference Achenbach and Rescorla2001)
The CBCL is a parent-report questionnaire pertaining to children’s behavioral difficulties and includes a Sleep scale that can be generated from seven items designed to document sleep problems; (1) “Does not want to sleep alone,” (2) “Has trouble getting to sleep,” (3) “Nightmares,” (4) “Resists going to bed at night,” (5) “Sleeps less than most children during day and/or night,” (6) “Talks or cries out in sleep,” and (7) “Wakes up often at night”. Each item is rated on a 3-point scale (0=Not true, 1=Somewhat or sometimes true, 2=Very true or often true), and a higher score indicates a higher level of sleep disturbances. Following the recommendation of Thurber and Sheehan (Reference Thurber and Sheehan2012), raw scores were used in statistical analyses.
Although the specific CBCL Sleep scale has not been validated independently of the overall CBCL questionnaire, it shows good convergent validity (r=.39–.55) with other validated sleep scales, such as the Children Sleep Habit Questionnaire, the Sleep Disorders Inventory for Students, and the Adolescent the Sleep-Wake Cycle questionnaire (Becker, Ramsey, & Byars, Reference Becker, Ramsey and Byars2015). The internal consistency of the 7-item Sleep scale in the current study was 0.73.
Actigraphy and sleep diary
Children were asked to wear an Actiwatch 2 actigraph (Phillips Respironics) for 5 consecutive days and nights. Actigraphy data were collected in 1-minute epochs, and data were analysed using the Phillips Respironics Actiware software version 5.70. The automated manufacturer’s low sensitivity threshold algorithm (80 activity counts per epoch), which has been shown to be appropriate for preschoolers (Bélanger, Bernier, Paquet, Simard, & Carrier, Reference Bélanger, Bernier, Paquet, Simard and Carrier2013; Meltzer, Walsh, Traylor, & Westin, Reference Meltzer, Walsh, Traylor and Westin2012), was used to determine minute-by-minute sleep-wake status.
To corroborate actigraphy data and identify potential artefacts, parents completed a sleep diary for the 5 days during which the child wore the actigraph. They were asked to indicate for each half-hour of the day whether the child was awake or asleep, and where he or she slept (e.g., child’s bedroom, car). They were also instructed to document periods when the child did not wear the actigraph (e.g., bath) and to report any unusual events (e.g., illness). When the child had an unusual night, was not wearing the actigraph or was co-sleeping, nights were excluded on a case-by-case basis. Furthermore, actigraphic data were discarded when they showed poor correspondence with the sleep diary or when no diary was available. Valid actigraphic data were available for 5 nights for 25 participants (11 mTBI and 14 TDC), for 4 nights for 8 participants (5 mTBI and 3 TDC), for 3 nights for 9 participants (4 mTBI and 5 TDC), for 2 nights for 4 participants (2 mTBI and 2 TDC), and for 1 night for 3 participants (3 mTBI).
Sleep onset and sleep offset were determined based on visual examination of the actogram and guided by the diary. Sleep onset was determined by starting to examine the actogram 30 minutes before the sleep onset reported in the diary. Similarly, the starting point was set 30 minutes after sleep offset, as indicated on the diary, to identify sleep offset. Two actigraphic variables were derived across the available assessment period: nighttime sleep duration (total number of minutes from sleep onset to sleep offset that were scored as sleep) and sleep efficiency (sleep minutes at night / (sleep minutes at night + wake minutes at night) * 100). The stability of sleep parameters across the 5 days was moderate to high (α=.79 for nighttime sleep duration, and α=.86 for sleep efficiency). To increase the reliability of estimates, data were averaged across days for each sleep parameter.
The actigraph was usually worn on the non-dominant wrist, but when the child felt uncomfortable, parents were told that the actigraph could be worn on the ankle. Consistent with a previous study in preschoolers (Bélanger et al., Reference Bélanger, Bernier, Paquet, Simard and Carrier2013), there was no significant location difference for nighttime sleep duration (t(47)=−1.35; p=.19) or sleep efficiency (t(47)=1.37; p=.18). There were no difference in nighttime sleep duration (t(39)=0.22; p=.83) or sleep efficiency (t(39)=0.31; p=.76) between weekdays and weekends.
Actigraphy data collected could not be analyzed for 17 participants. Data were missing at random for the following reasons: the actigraph was worn but the parent did not complete or return the sleep diary (n=2; 2 TDC), the actigraph was worn but potential artefacts were identified (n=1; 1 TDC), the child refused to wear the actigraph (n=11; 5 mTBI and 6 TDC), co-slept with someone (n=2; 2 mTBI) or was ill (n=1; 1 TDC) throughout the entire data collection.
Statistical Analyses
All data were analyzed using SPSS statistical software (version 24.0; SPSS, Inc., Chicago, IL) and screened for violations of normality. For all analyses, an alpha of .05 was used and effect sizes are reported using Cohen’s d for continuous data, and Cramer’s V for categorical data. Available-case analyses were performed to preserve statistical power, given that not all participants completed the actigraphy assessment or that data was missing on various outcome measures (some parents failed to complete the questionnaire booklet or the cognitive assessment could not be completed due to lack of child cooperation).
Group comparisons (mTBI vs. TDC) were conducted on demographic variables (age, sex, SES, ethnicity). Independent sample t tests were used for continuous variables, and chi-square tests were conducted for categorical variables. To investigate group differences on executive functions, independent sample t tests were performed. Then, a series of multiple hierarchical regressions were performed to assess the interactive effects of children’s group (independent variable: mTBI or TDC) and sleep (moderator) on executive functions (outcomes: Delay of Gratification, Conflict Scale). In each equation, group and a sleep measure (CBCL Sleep scale, actigraphy nighttime sleep duration, or actigraphy sleep efficiency) were entered in the first block, followed by their interactive product in the second block. Sleep measures were centered before the formation of the interactive term. Then, significant interactions were decomposed following the Preacher, Curran, and Bauer (Reference Preacher, Curran and Bauer2006) guidelines.
RESULTS
Final Sample
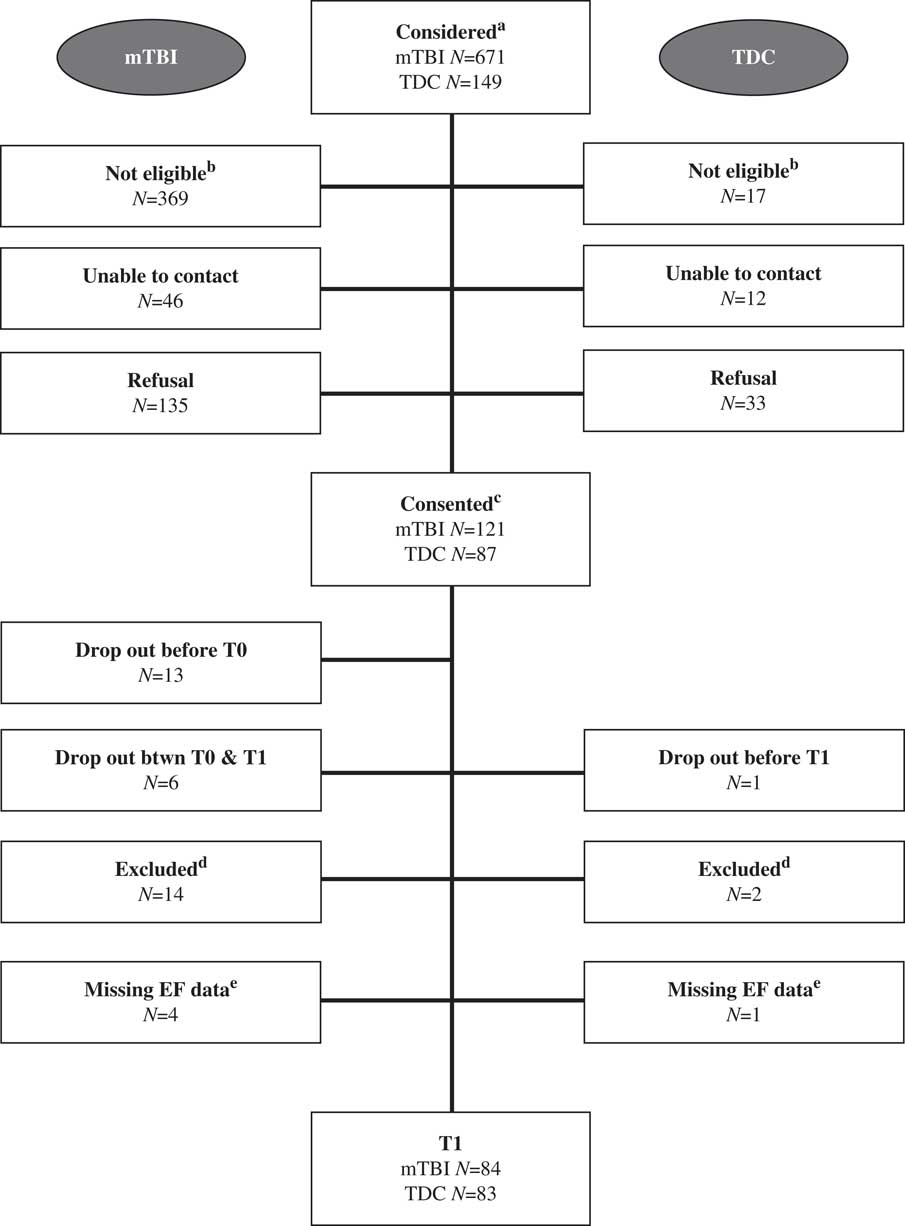
Information on recruitment and follow-up is presented in Figure 1. For both groups, there was no difference in terms of age at recruitment (mTBI: t(233)=0.66; p=.51; TDC: t(110)=0.44; p=.66) and sex (mTBI: χ 2 (1, N=238)=0.11; p=.74; TDC: χ 2(1, N=117)=2.30; p=.13) between those who participated in the study and those who refused. In terms of attrition, there was no difference in terms of age at injury (t(101)=0.41; p=.68) and sex (χ 2 (1, N=238)=0.11; p=.74) between those who dropped out after recruitment and those who maintained their participation in the mTBI group. In the TDC group, only one participant dropped out, therefore, no attrition statistics were computed.
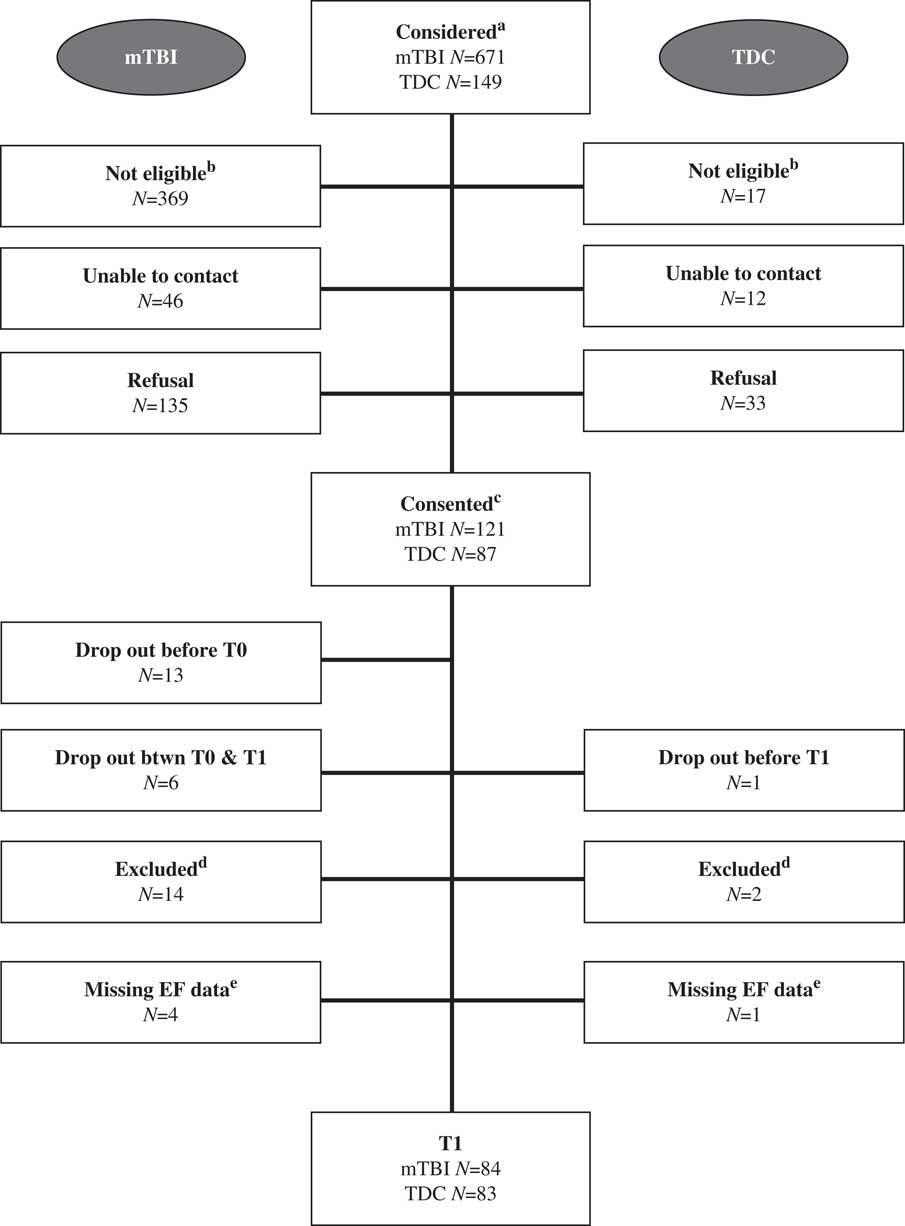
Fig. 1 Recruitment and follow-up flowchart. aThe following emergency department diagnoses were considered for participation in the study: mTBI group: traumatic brain injury, head fracture, concussion, intracranial bleeding/hemorrhage, polytrauma. TDC participants were considered if they received a pamphlet of our study at the local daycare and gave their verbal consent to be contacted by the research coordinator. bPotential participants were not eligible because they did not satisfy an inclusion and/or exclusion criterion. cConsented refers to those participants whose parents signed a consent form. dThese participants were excluded at T1 because they did not satisfy an inclusion and/or exclusion criteria that had not been detected before testing. eMissing EF data due to failure to complete the evaluation or lack of collaboration.
Preliminary Analyses
Preliminary analyses of the Shape Stroop data revealed a ceiling effect: 62% of the participants obtained the highest possible score. Therefore, these data were not further considered in the statistical models to prevent bias in the results (Uttl, Reference Uttl2005; Wang, Zhang, McArdle, & Salthouse, Reference Wang, Zhang, McArdle and Salthouse2009).
Main Analyses
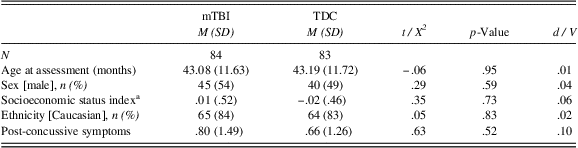
As shown in Table 2, the two groups did not differ in terms of their age at assessment, sex, SES, ethnicity, or post-concussive symptoms. In addition, there was no significant difference between the two groups on executive function measures (Delay of Gratification, Conflict Scale) and sleep measures (CBCL Sleep scale, actigraphy nighttime sleep duration and sleep efficiency) 6 months post-injury (see Table 3).
Table 2 Participant characteristics
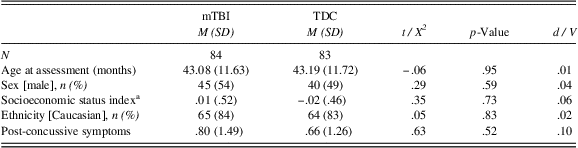
a Socioeconomic status index is a standardized composite of parental education and Blishen scores.
Table 3 Group comparisons on executive function and sleep measures 6 months post-injury
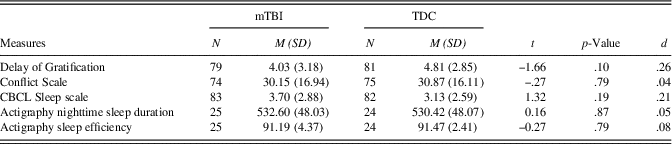
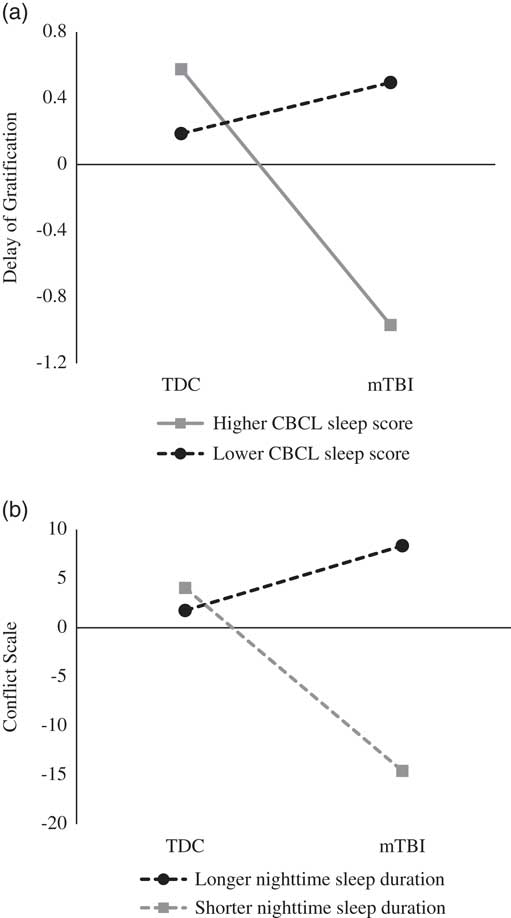
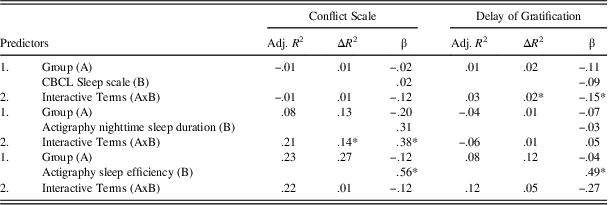
Table 4 presents the bivariate correlations among all continuous variables under study. As expected, CBCL Sleep scale scores were not correlated with actigraphy-derived variables, suggesting that these instruments measure distinct aspects of sleep (Bélanger, Simard, Bernier, & Carrier, Reference Bélanger, Simard, Bernier and Carrier2014). The results of the regression models are displayed in Table 5. The analyses first revealed a significant interaction effect of children’s group and CBCL Sleep scale scores on Delay of Gratification (β=−0.15; p=.05). Furthermore, children’s group interacted with nighttime sleep duration in the prediction of Conflict Scale (β=0.38; p=.008). For these two significant interactions, fitted regression lines were plotted at high (+1 SD) and low (−1 SD) values of the moderator (CBCL Sleep scale or actigraphy nighttime sleep duration) as depicted in Figure 2.
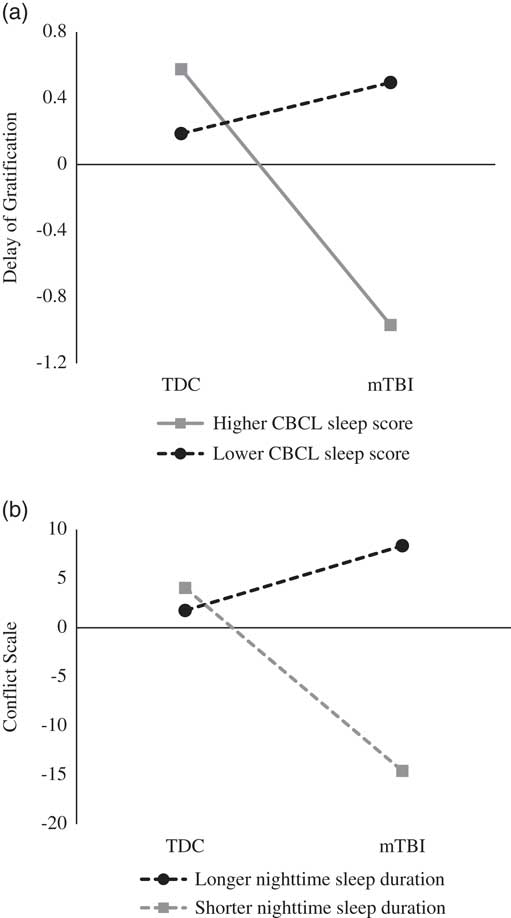
Fig. 2 (a) Interactive effects of children’s group and CBCL sleep score onto Delay of Gratification score. (b) Interactive effects of children’s group and actigraphy nighttime sleep duration onto Conflict Scale score.
Table 4 Zero-order correlations among executive function and sleep measures 6 months post-injury

*p<.05.
**p<.01.
Table 5 Summary of regression analyses
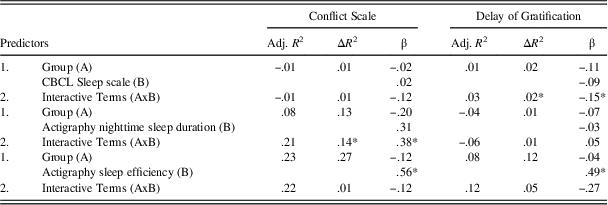
*p<.05.
The results revealed a significant group difference for Delay of Gratification performance for children with higher levels of sleep problems on the CBCL (β=−0.77; t=−2.24; p=.03), whereas the group difference for children with lower levels of sleep problems was not significant (β=0.15; t=.45; p=.66). Thus, children with mTBI had poorer performance than the TDC group on Delay of Gratification only if they had higher levels of sleep problems. Likewise, post hoc analyses yielded a significant group difference on Conflict Scale performance for children with shorter nighttime sleep duration (β=−9.31; t=3.02; p=.004), whereas the difference was not significant for children with higher nighttime sleep duration (β=3.30; t=1.06; p=.30), indicating that children with mTBI exhibited poorer Conflict Scale performance than those in the TDC group only if they had shorter nighttime sleep duration.
To further investigate the relation between sleep and executive functioning, additional analyses were performed in the mTBI group using the pre-injury and 6-month post-injury CBCL Sleep scale, which are intercorrelated (r=.68; p=.001). There was no significant difference between pre- and post-injury CBCL Sleep scale scores (t(79)=0.44; p=.66). Also, both pre-injury and 6-month post-injury CBCL Sleep scale scores significantly predicted Delay of Gratification performance (respectively, β=-0.28; t=−2.47; p=.02; β=−0.24; t=−2.18; p=.03). This suggests that sleep quality, regardless of when it is documented, is important in predicting executive functioning in children with mTBI.
DISCUSSION
The primary goal of the current study was to investigate executive functioning after early mTBI. The performance of preschoolers with mTBI was compared to that of typically developing same-age children. Contrary to our hypothesis, there were no significant group differences on measures of inhibition and cognitive flexibility 6 months post-injury. These findings differ from previous reports of EF impairments after early TBI, and might be explained by several reasons. First, children in the current study had mild injuries whereas most previous studies have focused on moderate–severe TBI (Ewing-Cobbs et al., Reference Ewing-Cobbs, Prasad, Landry, Kramer and DeLeon2004; Ganesalingam et al., Reference Ganesalingam, Yeates, Taylor, Walz, Stancin and Wade2011). Nonetheless, our findings may not merely reflect a general absence of alterations following mild injury, as difficulties in other complex constructs (e.g., social cognition, behavior, parent–child relations) have previously been identified in the same cohort of children (Bellerose, Bernier, Beaudoin, Gravel, & Beauchamp, Reference Bellerose, Bernier, Beaudoin, Gravel and Beauchamp2015, Reference Bellerose, Bernier, Beaudoin, Gravel and Beauchamp2017; Gagner, Landry-Roy, Bernier, Gravel, & Beauchamp, Reference Gagner, Landry-Roy, Bernier, Gravel and Beauchamp2017; Lalonde, Bernier, Beaudoin, Gravel, & Beauchamp, Reference Lalonde, Bernier, Beaudoin, Gravel and Beauchamp2016). Furthermore, although no EF deficits were identified 6 months post-injury, we cannot rule out the possibility that children exhibited EF difficulties shortly after the injury, which may have resorbed before the 6-month timepoint. It is also possible that EF difficulties may subsequently emerge in middle and late childhood. Although EF are rapidly developing during early childhood, such abilities are still immature, even in healthy children. The impact of brain insult on EF may become apparent only later in development when EF become fully functional and mature (e.g., school setting; Anderson, Reference Anderson2002; Anderson & Catroppa, Reference Anderson and Catroppa2005). One study previously reported EF impairments in attentional control after early mTBI, but children were assessed 2 years post-injury, which might explain discrepancies with the current results (Crowe et al., Reference Crowe, Catroppa, Babl and Anderson2013). Further follow-up is required as children mature into middle childhood, especially given that executive abilities have a strong bearing on school success. In addition, this study focused specifically on inhibition and flexibility, but other subdomains of EF may also be relevant in future work.
Early mTBI was not associated with executive deficits or with sleep disturbances 6 months post-injury. No group differences were found in parental ratings of sleep problems or for actigraphy-derived nighttime sleep duration and sleep efficiency, as previously demonstrated (Landry-Roy et al., Reference Landry-Roy, Bernier, Gravel and Beauchamp2017). However, the data indicate that a combination of mTBI and poor sleep results in poorer EF outcomes, which supports a “double hazard” effect (Escalona, Reference Escalona1982). Children with mTBI were found to have poorer inhibition and flexibility only if they experienced higher levels of sleep problems (measured by CBCL parental report) or shorter actigraphy nighttime sleep duration. This contributes to explaining our failure to find EF differences at the group level, as EF difficulties post-TBI appear to manifest only among children with sleep disturbances or insufficient sleep. The results align with previous studies in children and adults with TBI, in which high levels of sleep problems reported on sleep questionnaires was associated with poorer EF performance (Mahmood, Rapport, Hanks, & Fichtenberg, Reference Mahmood, Rapport, Hanks and Fichtenberg2004; Shay et al., Reference Shay, Yeates, Walz, Stancin, Taylor, Beebe and Wade2014). To our knowledge, this is the first study to depict that sleep disturbances exacerbate EF difficulties in children with mTBI, and through both subjective and objective sleep instruments. This is a promising first step, but independent replication is necessary. Although sleep questionnaires are often criticized for being susceptible to respondent bias (Sadeh, Reference Sadeh2015), our results suggest that both subjective and objective measures are likely to be useful in predicting EF post-TBI, and could be used complementarily. The CBCL sleep scale and actigraphy-derived nighttime sleep duration tap into different aspects of sleep, respectively, sleep problems and sleep quantity. Therefore, it is perhaps not surprising that they were related to different EF measures.
Of note, regressions performed with sleep efficiency revealed no significant interaction for any of the EF tasks. Therefore, it appears that sleep quantity, regardless of the number of nocturnal awakenings, is more predictive of daytime executive functioning than efficiency per se. Our results also indicate that sleep, both pre- and post-injury, is associated with EF after mTBI. This suggests that children with pre-injury sleep problems may be at risk for poorer executive functioning if they sustain a mTBI, and that quality of sleep is important for executive functioning following mTBI in young children.
Two underlying mechanisms are generally proposed to explain the cognitive deficits associated with sleep disturbances. Some theorists argue that sleep disturbances lead to a global decrease in arousal level and alertness, which results in global cognitive impairments, especially on long and simple tasks requiring vigilance (Kjellberg, Reference Kjellberg1977; Koslowsky & Babkoff, Reference Koslowsky and Babkoff1992). On the other hand, some argue that sleep disturbances affect specific brain structures and functions (Alhola & Polo-Kantola, Reference Alhola and Polo-Kantola2007). Notably, it has been proposed that sleep disturbances impair cognitive abilities that depend on the prefrontal brain regions, such as EF (Harrison & Horne, Reference Harrison and Horne2000; Jones & Harrison, Reference Jones and Harrison2001). This theory assumes that performance is impaired on tasks that are subserved by the prefrontal cortex, even if they are interesting and of short duration. Our results are consistent with this latter hypothesis.
The role of sleep in neuroplasticity and neurogenesis has been well established (Benington & Frank, Reference Benington and Frank2003; Kreutzmann, Havekes, Abel, & Meerlo, Reference Kreutzmann, Havekes, Abel and Meerlo2015; Meerlo, Mistlberger, Jacobs, Heller, & McGinty, Reference Meerlo, Mistlberger, Jacobs, Heller and McGinty2009). It is, therefore, possible that some aspects of sleep support brain recovery post-TBI. As such, we speculate that poor sleep, whether pre- or post-injury, could impede cognitive recovery post-TBI. In addition, sleep plays an active role in brain maturation throughout development via brain plasticity processes (Sadeh, Reference Sadeh2007). The putative impact of sleep is particularly important in the preschool period, which is marked by rapid neural and cognitive development (Anderson, Reference Anderson2002; Durston & Casey, Reference Durston and Casey2006). During this developmental period, sleep impairments or brain insult, such as TBI, could possibly increase the risk for cognitive problems. As such, preschoolers who sustain mTBI and present with poor sleep, pre- or post-injury, may be especially at risk for EF difficulties. Finally, it should be kept in mind that other factors (e.g., parental practices, socio-emotional difficulties, fatigue) may account for both sleep disturbances and executive difficulties, and these should be explored in future work (Astill, Van der Heijden, Van Ijzendoorn, & Van Someren, Reference Astill, Van der Heijden, Van Ijzendoorn and Van Someren2012; Byars, Yeomans-Maldonado, & Noll, Reference Byars, Yeomans-Maldonado and Noll2011; Owens, Spirito, & McGuinn, Reference Owens, Spirito and McGuinn2000).
Several methodological limitations must be considered when interpreting the current results. First, it is important to acknowledge that regression analyses preclude the establishment of causality between poor sleep and executive functioning. A further limitation of this study is that regression analyses were limited by a modest sample size, which could explain the failure to find certain significant interactions. Also, some participants did not complete all 5 days of actigraphy data collection. Due to challenges associated with actigraphy compliance, it is not unusual to have fewer than 5 nights of assessment when working with young children (Bélanger, Bernier, Simard, Desrosiers, & Carrier, Reference Bélanger, Bernier, Simard, Desrosiers and Carrier2018; Sazonov, Sazonova, Schuckers, & Neuman, Reference Sazonov, Sazonova, Schuckers and Neuman2004; Scher, Hall, Zaidman-Zait, & Weinberg, Reference Scher, Hall, Zaidman-Zait and Weinberg2010; Ward, Gay, Alkon, Anders, & Lee, Reference Ward, Gay, Alkon, Anders and Lee2008). However, it would be ideal to exclude children with less than 3 nights of actigraphy in future replications (Acebo et al., Reference Acebo, Sadeh, Seifer, Tzischinsky, Wolfson, Hafer and Carskadon1999). Besides, we used raw scores for the sleep data, but the use of a normed sleep scale would be helpful in future work to determine the contributions of sleep problems to EF. Finally, we used a typically developing control group to compare children with mTBI to the peers they are likely to interact with and be compared to in everyday life. An injury comparison group could be beneficial to further ensure that the current results represents brain-injury specific effects rather than more general injury effects. We previously showed that the TDC group included in this sample are highly comparable on a very large range of measures to an orthopedic controls group recruited as part of the larger study (Beauchamp et al., Reference Beauchamp, Landry-Roy, Gravel, Beaudoin and Bernier2017), but sleep variables were not included in those comparisons. Nevertheless, this study improves on previous work through methodological strengths, such as conducting assessments at 6 months post-injury, the use of direct cognitive measures of EF and reliance on both subjective and objective measures of sleep. Although previous studies have investigated EF after pediatric TBI, few have done so in children injured before the age of 6 years, despite the fact that the young developing brain is particularly vulnerable to such insults and is undergoing major cognitive maturation (Crowe et al., Reference Crowe, Babl, Anderson and Catroppa2009; Tsujimoto, Reference Tsujimoto2008).
CONCLUSION
This study investigated EF and sleep in preschoolers with mTBI using objective measures of executive functions, a sleep questionnaire and actigraphy data. If replicated, these results would suggest that the presence of a mTBI interacts with sleep in the prediction of executive functioning. This study highlights the importance of documenting sleep in children with TBI, as sleep difficulties place children at risk for later executive dysfunction, which may subsequently impact other spheres of functioning, such as school performance and peer relationships (Asikainen, Kaste, & Sarna, Reference Asikainen, Kaste and Sarna1996; Beauchamp et al., Reference Beauchamp, Catroppa, Godfrey, Morse, Rosenfeld and Anderson2011; Nadebaum et al., Reference Nadebaum, Anderson and Catroppa2007). Early recognition of sleep disturbances may allow clinicians to intervene in the early course of recovery and potentially reduce the negative impact on EF.
ACKNOWLEDGEMENTS
The authors declare no conflicts of interests. This study was funded by the Canadian Institutes of Health Research (MOP11036 to M.H.B.). Thank you to the lab members for their collaborative efforts.