Introduction
Stem borers are regarded as a major limiting factor to the production of maize, Zea mays L. in India (Panwar, Reference Panwar, Zaidi and Singh2005). Among them, the spotted stem borer, Chilo partellus (Swinhoe) (Lepidoptera: Crambidae), is the most serious and prevalent pest of maize, causing 27–80% yield losses in different agro climatic regions of the country (Kanta et al., Reference Kanta, Dhillon, Sekhon and Mihm1997). The recommended chemical measures are often not adopted by the farmers to the desired extent for various reasons. Recent studies have shown that the high grass diversity surrounding maize fields is important in stem borer management (Mohamed et al., Reference Mohamed, Khan, Overholt and Elizabeth2004). These grasses either acted as trap plants or they stabilized the system for both the pest and natural enemies (Schulthess et al., Reference Schulthess, Chabi-Olaye and Georgen2001). Plants that are highly attractive for oviposition had been used in habitat management systems (also referred to as push-pull systems) for C. partellus in Africa (Khan et al., Reference Khan, Pickett, van den Berg, Wadhams and Woodcock2000). C. partellus females can oviposit even on non-host plants (Kumar & Saxena, Reference Kumar and Saxena1985; Mohamed et al., Reference Mohamed, Khan, Overholt and Elizabeth2004), but preference for certain plants such as Napier grass, Pennisetum purpureum Schmach. and sorghum, Sorghum bicolor (L.) Moench, has been established under choice tests (Mohamed et al., Reference Mohamed, Khan, Overholt and Elizabeth2004; Khan et al., Reference Khan, Midega, Hutter, Wilkins and Wadhams2006; van den Berg, Reference van den Berg2006). These grass species were utilized as trap crops (pull effect) against C. partellus. Besides being highly preferred for egg laying, Napier grass has also been found detrimental for the survival of C. partellus larvae (Khan et al., Reference Khan, Midega, Hutter, Wilkins and Wadhams2006; van den Berg, Reference van den Berg2006), hence, could be termed a dead-end trap crop (Shelton & Nault, Reference Shelton and Nault2004).
Burton (Reference Burton1944) showed that Napier grass and pearl millet, Pennisetum glaucum (L.) R.Br, could be readily crossed and the resultant interspecific hybrids were more vigorous than the parent species. In Punjab state, two such Napier millet hybrids have been recommended for fodder purposes and are popular among farmers. We hypothesized that being derivatives of Napier grass, these hybrids might have the potential to be used as trap crops against C. partellus. Earlier, van den Berg (Reference van den Berg2006) determined that Bana grass, an interspecific hybrid between P. purpureum and P. glaucum, was preferred for oviposition by C. partellus, while larval survival was poor on this hybrid. The objective of the current study was to determine oviposition preference of C. partellus for Napier millet and sorghum trap crops and to assess larval survival on these host plants. Further, larval dispersal from Napier millet hybrids to adjacent grown maize was assessed under field conditions.
Materials and methods
Plants
The test plants were two graminaceous fodder crops commonly grown in India. Two recommended Napier millet (P. purpureum×P. glaucum) hybrids, namely PBN 83 and PBN 233, and one sorghum (S. bicolor) variety, SL 44, were evaluated as trap crops against C. partellus. These hybrids have long and broad leaves, while PBN 233 gives higher fodder yield than PBN 83. The maize cultivar, PMH 1, was used as control, and it was reported to be susceptible against stem borer (Anonymous, 2004). Napier millet plants were propagated from stem cuttings, whereas both sorghum and maize were planted as seeds. The plants were maintained in an insect-proof screen house at Punjab Agricultural University, Ludhiana in India. The experimental station is situated at 30°45′ N by 75°40′ E at an altitude of approximately 247 m above sea level. Normal agronomic practices were followed for raising the plants, and no insecticide was applied on the test plants. All the plants used in experiments were approximately of the same biomass.
Insects
Stem borer larvae (3rd–4th instar) were regularly collected from the maize fields and introduced into the laboratory culture to maintain the genetic diversity of the colony. The C. partellus culture was maintained on a moong-based artificial diet (Kanta & Sajjan, Reference Kanta and Sajjan1992) following Siddiqui et al. (Reference Siddiqui, Sarup, Panwar and Marwaha1977). More than 150 black-head stage eggs (5–6 egg masses on wax paper strips) were placed in each diet jar (15×10-cm dia., 2.5 cm diet layer), which were wrapped all around, except the bottom, with black paper sheets. These were kept in the insect rearing room at 25±2°C and 70% RH. The larvae were allowed to feed undisturbed within the jars until pupation. The emerged moths were put into oviposition jars (15×10-cm dia.) wrapped all around inside with wax paper, which served as an ovipositional substrate. Usually eight pairs per jar were kept on a water-based adult diet, as water has been reported to give better ovipositional results (Taneja & Nwanze, Reference Taneja and Nwanze1990). The oviposition jars were kept in the incubator at 22±1°C and 70% RH.
Oviposition preference tests
These tests were conducted under no-choice and dual-choice conditions in oviposition cages of 1×1×1 m, covered by fine nylon mesh netting (200 meshes per in2). The test plant material was thoroughly examined for the presence of eggs or larvae before using them for oviposition preference studies. In no-choice tests, two 20-day-old potted plants of each host were placed in the cage. Before the onset of the experiment, Napier millet plants were trimmed to leave only a single tiller of similar size to that of the maize plants. The cages were kept under natural light conditions of approximately L13:D11 h. A single moth pair, emerged during the preceding night, was allowed to oviposit for the next 48 h, when maximum egg laying has been reported (Pats, Reference Pats1991). Afterwards, the plants were removed and the number of egg batches and total number of eggs per plant were recorded on each plant using 10× magnifying lens. This arrangement was replicated ten times.
Dual-choice experiments were conducted to determine the oviposition preference of C. partellus moths, when presented with a choice between Napier millet or sorghum and maize. In these tests, two 20-day-old potted maize plants were placed into each cage. Twenty-day-old maize plants have been reported to be most susceptible to C. partellus damage (Ampofo, Reference Ampofo1985). Additionally, two whole plants of one of the Napier millet hybrids or sorghum of the same age as the maize were also introduced. The four plants were so placed in the oviposition cage that the leaves were intermingled, so that moths can choose any plant for oviposition regardless of their positioning. Two moth pairs, emerged during the preceding night, were introduced in the cage and allowed to lay eggs for 48 h. The number of egg batches and total number of eggs per plant were recorded on each plant. Each dual-choice test was replicated ten times.
Larval settling preference and feeding response
The experiment on larval settling preference was conducted using 6-cm long leaf cuts of 20-day-old test plants in dual-choice tests. Two leaf cuts of one of the Napier millet hybrids or sorghum and two leaf cuts of maize were arranged equidistantly along the edge of a Petri dish (15-cm dia.) lined with moist filter paper. Twenty neonates of C. partellus were released in the center of a Petri dish using a fine camel hair brush. The Petri dish was covered with a lid to avoid the larval escape and sealed with parafilm. It was placed in a dark room at 25±2°C and 70% RH. The number of larvae found on or under the leaf cuts were recorded twice at 1 h and 24 h after release. The experiment was replicated five times under controlled conditions.
In another experiment on leaf feeding, excised leaves (6-cm long) made from central whorl of 20-day-old plants were kept in plastic vials lined with moist filter paper. Five neonates were released in each vial, which were arranged randomly at 25±2°C and 70% RH in a dark room. After 24 h of feeding, leaf area consumed by the larvae was measured using graphical method. The experiment was replicated seven times. Similarly, this test was performed using two 15-day-old larvae in each vial.
Larval survival and development (laboratory studies)
In order to assess survival and development of C. partellus larvae on different host plants, an experiment was carried out under controlled conditions. Leaf whorls prepared from 20-day-old plants of different hosts (except sorghum), were introduced into glass jars (20-cm high, 15-cm dia.). The 15-cm long whorls consisting of stem, leaf and sheath regions provided a variety of material for larval feeding. Twenty neonate larvae were introduced into each jar using a fine camel hair brush and kept at 25±2°C and 70% RH in a dark room. Five replicates of 20 larvae per jar were kept for each test plant and arranged in a randomized manner. The larvae were allowed to feed for five days and then the leaf whorls were removed and dissected to recover the larvae. The surviving larvae were then placed into a similar jar containing fresh leaf whorl of the same host plant as that from which they were removed. They were then left to feed for another five days, following which the plants were dissected and reassessed for larvae. As the larvae reached the third instar, the stage at which they started boring into the stem, additional stem sections were introduced into the jars. Thus, larval mortality was recorded at five days interval. In addition, the larval weight was measured ten days after neonate introduction.
Plant damage and larval recovery (screen house experiments)
This experiment was conducted over a 20-day period under semi-controlled conditions. The different host plants, planted in earthen pots, were used for the experiment. When seedlings were ten days old, thinning was done so as to leave the best one tiller in each pot. Each pot represented one replication, and there were 15 pots for each test plant. The pots were placed approximately 80 cm apart in a randomized manner within the screen house. One egg batch (25–30 eggs) at black-head stage was pinned in the central leaf whorl of each 20-day-old plant. At 20 days after infestation, data were collected on number of dead hearts and larval/pupal recovery. The percentage of plants showing dead hearts was computed. All the plants were dissected to assess the larval/pupal recovery.
Dispersal of C. partellus larvae between Napier millet and maize
The neonate larvae of C. partellus have the tendency to disperse to the surrounding plants in the field (Berger, Reference Berger1989; Pats & Ekbom, Reference Pats and Ekbom1992). Unsuitability of Napier millet hybrids may cause dispersal of larvae from these hybrids to maize plants. To test this hypothesis, a field experiment was conducted. In each plot, a single row of Napier millet hybrid was planted adjacent to one row of maize (cv. PMH 1). The row length was kept one meter with row-row and plant-plant spacing as 45×20 cm, respectively. After planting, the plots were covered with nylon cages to prevent natural infestation. At the 3-week-old stage, 20 newly hatched larvae were introduced into the central whorl of each Napier millet plant. At the time of artificial infestation, the nylon cages were removed to expose the larvae to biotic and abiotic factors. All the plants were uprooted one day after larval infestation and carefully dissected to recover the larvae. This set of experiment was replicated 16 times for both the Napier millet hybrids.
Data analysis
Differences among different test plants with regards to egg laying under no-choice test, leaf area consumed, larval weight and larval recovery were analyzed using one-way analysis of variance (ANOVA). In dual-choice oviposition preference and larval settling tests, Students' t-test was used to determine differences between maize and other host plants tested with regards to number of egg batches, total number of eggs laid and larval settling preference. Means were separated at 5% level of significance using least significant difference test (LSD), and actual counts were presented in figures and tables.
Results
Oviposition preference tests
In no-choice tests, different host plants showed significant differences in respect of number of egg batches (F3,36=11.25) and total number of eggs laid (F3,36=7.53). Napier millet hybrids were preferred for oviposition when compared with maize or sorghum. These hybrids received about five times more eggs than maize (table 1). Results from dual-choice tests indicated that C. partellus females preferred to oviposit on Napier millet instead of maize. Significantly higher number of egg batches (t18=5.19) and total number of eggs (t18=4.52) were laid on Napier millet hybrid PBN 83 than on maize (table 2). Hybrid PBN 233 was also preferred over maize in respect of number of egg batches (t18=3.31) and total number of eggs laid (t18=2.69). However, the differences between sorghum and maize were non-significant in this respect.
Table 1. Ovipositional response of Chilo partellus to different host plants under no-choice test.
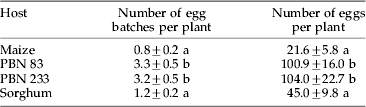
Mean±SE, the values in a column followed by the same letter are not significantly different (LSD test; p=0.05).
Table 2. Ovipositional preference of Chilo partellus to maize cultivar (PMH 1) and other test host plants in dual-choice tests.
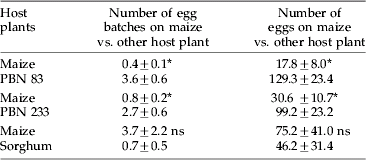
Mean±SE, the values in different treatment combinations in a column followed by an asterisk are significantly different (p=0.05); ns, non-significant by t-test.
Larval settling preference and feeding response
In larval settling tests, a higher number of larvae settled on the leaf cuts of maize than hybrid PBN 83 after one hour of release (t8=5.51) (table 3). Twenty-four hours after release, the pattern of the larval settling remained almost the same as for one hour after release (t8=4.36). The differences between maize and hybrid PBN 233, however, were non-significant in this respect. After 24 h of release, a significantly higher number of larvae settled on leaf cuts of maize than on hybrid PBN 233 (t8=6.73). In dual-choice tests between maize and sorghum, greater number of larvae settled on sorghum both one hour (t8=2.63) and 24 h (t8=2.68) after larval release.
Table 3. Comparison of settling preference of Chilo partellus neonates on leaf cuts of maize and other host plants in dual-choice tests.
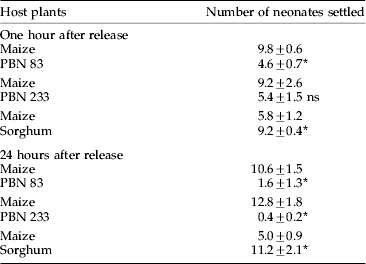
Mean±SE, the values in different treatment combinations in a column followed by an asterisk are significantly different (p=0.05); ns, non-significant by t-test.
The results from leaf feeding experiments showed that maize and sorghum were more suitable as larval hosts than Napier millet hybrids (table 4). The larvae consumed greater leaf area in maize than in Napier millet hybrids and the results were significant for both newly hatched (F3,24=6.81) as well as 15-day-old larvae (F3,24=9.41). Sorghum was equally suitable as maize for larval feeding.
Table 4. Leaf area consumed by Chilo partellus larvae on different host plants.

Mean±SE, the values in a column followed by the same letter are not significantly different (LSD test; p=0.05).
Larval survival and development (laboratory studies)
Larval survival on Napier millet hybrids over a 30-day period indicated that greater mortality (approximately 60%) occurred within the first five days after egg hatching (fig. 1). None of the C. partellus larvae survived to pupation in case of Napier millet hybrids. In maize, 28% of the initially introduced larvae reached pupation. The larvae reared on Napier millet plants were weak, and larval weight was significantly less than those reared on maize (F2,12=8.66) (fig. 2).

Fig. 1. Larval survival (%) of Chilo partellus on Napier millet and maize at different times following egg hatching under laboratory conditions (bars indicate ±SE) (–■–, Maize; –•–, PBN 83;![]() , PBN 233).
, PBN 233).

Fig. 2. Average larval weight (mg)±SE of Chilo partellus larvae on Napier millet and maize ten days after egg hatching. Bars marked with different letters are significantly different (LSD test; p=0.05).
Plant damage and larval recovery (screen house experiments)
Notably, all the sorghum plants were transformed into dead hearts 20 days after infestation. In the case of maize cv. PMH 1, the respective value was 66.6%. However, no plant showed dead heart injury in the case of Napier millet hybrids. A greater number of larvae/pupae were recovered from maize cv. PMH 1 than all other test plants (F3,56=30.59) 20 days after infestation (fig. 3), while no larval recovery was observed in the case of Napier millet hybrids. Sorghum was also inferior in terms of larval/pupal recovery when compared to maize.

Fig. 3. Average (±SE) larval recovery of Chilo partellus on different host plants after 20 days of infestation. Bars marked with different letters are significantly different (LSD test; p=0.05).
Dispersal of C. partellus larvae between Napier millet and maize
Significantly greater number of larvae were recovered from Napier millet hybrids than maize cv. PMH 1 (PBN 83: F1,30=14.96; PBN 233: F1,30=15.88) after 24 hoursof infestation (table 5). Only 8% of the introduced larvae settled on maize as compared to 24–26% in the case of Napier millet hybrids. However, the majority of the larvae died or was lost during the observation period.
Table 5. Recovery of Chilo partellus larvae from Napier millet hybrids and maize one day after infestation.
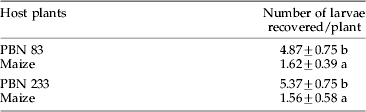
Mean±SE, the values in different treatment combinations in a column followed by an asterisk are significantly different (LSD test; p=0.05).
Discussion
The results of the current study showed that Napier millet hybrids were more preferred for oviposition by C. partellus females than maize cv. PMH 1. The preference was demonstrated under both no-choice and dual-choice tests. Sorghum, however, was not preferred for egg laying when compared with maize. This study corroborates the findings of Khan et al. (Reference Khan, Midega, Hutter, Wilkins and Wadhams2006) and van den Berg (Reference van den Berg2006), who reported a significant preference for Napier grass over maize for oviposition by C. partellus.
The choice for hairy leaf surfaces of Napier millet hybrids instead of maize was most likely influenced by plant volatiles since egg laying was negatively correlated to leaf hairiness (van den Berg, Reference van den Berg2006). Six plant volatiles, namely octanal, nonanal, linalool, naphthalene, allylanisole and eugenol, were reported to mediate host location and oviposition by gravid C. partellus females (Khan et al., Reference Khan, Pickett, van den Berg, Wadhams and Woodcock2000). Later, Birkett et al. (Reference Birkett, Chamberlain, Khan, Pickett, Toshova, Wadhams and Woodcock2006) detected octanal, nonanal and linalool in the volatiles emitted by Z. mays, S. bicolor, P. purpureum and Hyparrhenia tamba (Steud.); but electrophysiologically significant levels of 4-allylanisole and eugenol were found only in P. purpureum, H. tamba and S. bicolor. In the current theory of host location, it has been emphasized that recognition of a host plant by olfactory signals occurs through reception of specific blend of ubiquitous compounds rather than a single compound (Bruce et al., Reference Bruce, Wadhams and Woodcock2005). Differential release of plant volatiles from P. purpureum and H. tamba was related with their greater preference. These plants also produced higher levels of green leaf volatiles, particularly hexanal, (E)-2-hexenal, (Z)-3-hexen-1-ol, (Z)-3-hexenyl acetate and indole than Z. mays and S. bicolor, possibly suggesting their role in host location (Birkett et al., Reference Birkett, Chamberlain, Khan, Pickett, Toshova, Wadhams and Woodcock2006). Although the above-mentioned studies were based on Napier grass, the hybrids between Napier grass and pearl millet might also have a similar volatile profile to attract C. partellus females for oviposition. This aspect needs to be investigated in future research studies.
A greater number of larvae settled on leaf cuts of maize than on Napier millet hybrids. However, Sorghum was preferred over maize in this respect. Plant chemical characteristics are likely to condition the plants for acceptance or rejection by the larvae (Dethier, Reference Dethier1982). Low levels of larval establishment and high larval dispersal were recorded from resistant maize cultivars and were linked with the presence of certain plant chemicals (Ampofo, Reference Ampofo1986). The higher preference of C. partellus for sorghum over maize has been documented in earlier reports (Kfir, Reference Kfir1992; Rebe et al., Reference Rebe, van den Berg and McGeoch2004). Further, C. partellus larvae fed more on maize and sorghum, when compared with Napier millet hybrids. This is in agreement with the earlier report of Mohamed et al. (Reference Mohamed, Khan, Overholt and Elizabeth2004). The reduced feeding on the leaves of Napier millet hybrids suggests that they may have antibiotic properties or be physically less suitable for feeding or lack nutrients necessary for optimum larval growth.
Our results indicate that Napier millet hybrids were less conducive for C. partellus larval feeding relative to maize, leading to their low survival. Rapid mortality of the early instars had occurred within five days after egg hatching and none of the larvae survived to pupation on these hybrids. However, a significantly greater number of larvae survived on maize. Napier millet hybrids also suffered less plant damage as compared to maize and sorghum, possibly suggesting their unsuitability for larval feeding. Sorghum and maize were highly susceptible to C. partellus damage. van den Berg et al. (Reference van den Berg, Midega, Wadhams, Khan and Grimshawk2003) reported up to 97% C. partellus larval mortality on Napier millet in South Africa. Khan et al. (Reference Khan, Pickett, van den Berg, Wadhams and Woodcock2000) ascribed the high mortality of borer larvae on Napier grass to sticky sap produced in response to penetration by first and second instar larvae.
Immediately after hatching, C. partellus larvae move from the egg batch laid on the leaf and climb on the plant whorl. During this period, they disperse to the surrounding plants (Berger, Reference Berger1989). Thus, post hatching dispersal of larvae is an important aspect, which can affect infestation levels on a crop. As the Napier millet is unsuitable for C. partellus larvae, these may disperse to the adjoining maize crop to increase the infestation levels. This aspect will be an important consideration in the success of Napier millet trap crop against C. partellus under field conditions. On the contrary, our results showed that the larvae do not shift from Napier millet to maize. Only 8% of the released larvae climbed to adjoining maize plants, which is considered a normal dispersal rate under field conditions (Pats & Ekbom, Reference Pats and Ekbom1992). About 25% of the released larvae were recovered from Napier millet hybrids after one day of infestation. Although natural abiotic and biotic stresses might have some role in larval mortality, unsuitability of Napier millet plants appears to be the major factor involved in their low survival.
Once oviposition has taken place, the suitability of the host plant for larval feeding and development is one of the most important aspects of a trap crop (Hokkanen, Reference Hokkanen1991). Poor larval survival and/or development are essential for a successful trap crop (Shelton & Nault, Reference Shelton and Nault2004). The high preference of C. partellus moths for Napier millet hybrids evaluated in this study indicates that moths select the most suitable sites for egg survival but not necessarily for larval development. Larval feeding on these hybrids resulted in significantly reduced larval survival in spite of oviposition preference for Napier millet. Our results, therefore, suggest the potential use of Napier millet as a trap plant, which is highly attractive for C. partellus oviposition but unsuitable for its larval survival. Sorghum is, however, unsuitable for use as a trap crop as it is not preferred for egg laying and has shown susceptible reaction towards larval infestation. For that reason, it was not included in the laboratory and field experiments on larval survival and dispersal.
The present observations were based on laboratory and screen house experimentation. However, field evaluation of Napier millet hybrids is needed to examine their effect on stem borer colonization and yield levels. Midega et al. (Reference Midega, Khan, van den Berg and Ogol2005) evaluated Napier grass around maize fields under push-pull systems and found that plant damage, oviposition preference and stem borer incidence were significantly lower in push-pull plots than maize mono crop plots. Similarly, Ogol et al. (Reference Ogol, Spence and Keddie1999) observed significantly greater oviposition of C. partellus in a maize mono crop compared to maize-leucaena (Leucaena leucocephala L.) intercrops. Recently, Koji et al. (Reference Koji, Khan and Midega2007) determined that Guinea grass, Panicum maximum Jacq. is a good agent of habitat management to selectively enhance arthropod predators of C. partellus and acts as a sink for the pest.
The push-pull strategy is a powerful and effective integrated pest management tool, and this strategy against C. partellus has contributed to increased crop yields and livestock production in Africa, resulting in a significant impact on food security in the region (Midega et al., Reference Midega, Khan, van den Berg and Ogol2005; Cook et al., Reference Cook, Khan and Pickett2007; Koji et al., Reference Koji, Khan and Midega2007). Wild host plants that are highly attractive for oviposition had been used in habitat management programmes against C. partellus in Africa (Khan et al., Reference Khan, Pickett, Wadhams and Muyekho2001). However, region specific evaluation of different plant species is needed to develop sound push-pull systems to combat this pest in diverse agro ecosystems. Like Napier grass, the hybrids between Napier grass and pearl millet are also potent candidates for use as trap crop and could be utilized in managing C. partellus on maize.










