Published online by Cambridge University Press: 25 November 2004
Acephalic cysticercus (Ac), a rarely developed multilobulated and nonencysted form of larval Taenia, causes hydrocephalus or adhesive arachnoiditis in the ventricles and subarachnoidal space that often lead to fatal outcome in affected patients. Ac has been proposed to originate from T. solium on the basis of morphological features, while no molecular data supporting the presumption have been available. In the present study, we investigated the immunological properties as well as molecular characteristics of Ac that was obtained surgically from 6 patients. Immunoblotting of the cyst fluid from Ac samples demonstrated the constitutive expression of a T. solium metacestode (TsM) 10 kDa protein. Specific antibodies against the truncated 10 kDa protein, which appears to be species specific for TsM cysticercosis, were detected in both serum and cerebrospinal fluid samples of Ac patients. Nucleotide sequences of mitochondrial cytochrome c oxidase subunit I (COI) and NADH dehydrogenase subunit 1 (ND1) genes of Ac were almost identical to those of T. solium but differed substantially from those of the other Taenia species. In phylogenetic analysis, Ac clustered with T. solium in a well-supported clade. Our results strongly suggest that Ac may have originated from T. solium.
Neurocysticercosis (NCC), a disease caused by the invasion of the central nervous system by Taenia solium metacestode (TsM), has been recognized as a major aetiology of neurological diseases in several countries of Latin America, Africa and Asia and in the United States (Del Brutto, Sotelo & Roman, 1998; White, 2000). A typical form of TsM is described as a fluid-filled cyst consisting of a cyst wall and an invaginated scolex with rostellar hooklets (Pawlowski, 2002). A unique form of larval Taenia worm that has no scolex was identified and is known as an acephalic cysticercus (Ac) or racemose cysticercus. It has been proposed that the term ‘cellulose form of T. solium cysticercosis and racemose form of T. solium cysticercosis’ differentiates Cysticercus cellulosae from C. racemosus (Flisser, 1994; Del Brutto, 2002). This particular form also results in NCC (Loo & Braude, 1982), which is presented as a bunch of grape-shaped irregular clusters and may enlarge up to 15 cm in the brain (Loo & Braude, 1982; McCormick, 1985). Ac is usually found in the ventricles and basal subarachnoid spaces, where the parasite causes severe adhesive arachnoiditis and often obstructive/non-obstructive hydrocephalus (McCormick, 1985; Del Brutto, 2002).
Despite its important association with human neurological diseases, the origin of Ac remains largely controversial to date. Previous studies have suggested that Ac might have originated from T. solium due to their similar ultrastructural and histological features (Slais, 1970; Voge & Brown, 1979; Rabiela, Rivas & Flisser, 1989; Valkounova, Zdarska & Slais, 1992). These observations are further supported by the identification of degenerated scolices in some surgical specimens (Slais, 1970; Del Brutto et al. 1988). Ac may be found simultaneously with TsM in the same patient. The coexistence of these two types has been reported in up to 10% of NCC cases in some countries (Rabiela et al. 1982). In contrast, other investigators have proposed that Ac originated from T. multiceps or T. serialis, because larval forms of these parasites disclosed similar histological characteristics including a large, cystic bladder wall without scolex or hooklets on the rostellum (Jung et al. 1981). It was also hypothesized that Ac might have originated from Echinococcus multilocularis since the gross and microscopic features of this parasite found in the human brain, heart and liver showed similar morphology to Ac (Thompson, Lymbery & Constantine, 1995). All of these results were based only on morphology of the different larval cestodes of the subject matter while no data have been described on the molecular identification.
Currently, identification and differentiation of different species of taeniid cestode are based largely on a combination of epidemiological, bio-ecological and morphological criteria. However, these approaches have limitations and are often unreliable (Thompson et al. 1995; McManus & Bowles, 1996). To overcome these shortcomings, a number of molecular phylogenetic studies have been performed (McManus & Bowles, 1996). Mitochondrial DNA (mtDNA) is considered one of the best molecular markers for demonstrating genetic relationships between genera, species and populations, due to its rapid evolution of sequence divergence (Bowles, Blair & McManus, 1992; Gasser, Zhu & McManus, 1999). Cytochrome c oxidase subunit I (COI) and NADH dehydrogenase subunit 1 (ND1) have been successfully used to reveal the phylogenetic relationships among Taenia tapeworms (De-Queiroz & Alkire, 1998; Gasser et al. 1999).
In this study, we determined the partial sequences of COI and ND1 of Ac and provide molecular evidence that Ac may have originated from T. solium. We also observed the expression of a TsM 10 kDa protein (Chung et al. 1999, 2002) in cyst fluid (CF) of Ac samples obtained surgically from the patients, as well as the presence of specific antibodies against this protein in serum and cerebrospinal fluid (CSF) samples from these patients. We finally demonstrated phylogenetic relationships of the COI and ND1 of Ac samples and of other human-infecting Taenia tapeworms, i.e., T. solium, T. saginata and T. asiatica.
Ac samples were collected from 6 NCC patients who were diagnosed by typical neuroimaging findings together with positive antibody reactions in their serum and CSF by enzyme-linked immunosorbent assay (ELISA). Clinical background information of these patients is presented in Table 1 and an example of Ac specimens used in this study is shown in Figs 1 and 2. Intact cysts extracted from each Ac patient were extensively washed with physiological saline at 4 °C, after which CF was harvested by puncturing the cyst wall. All the samples from the patients including Ac specimens, CF, sera and CSFs were stored at −70 °C until use. Informed consent was obtained from each patient.

Gravid proglottids of T. saginata or T. asiatica were collected from each volunteer who was experimentally infected with the metacestodes from an experimental calf or pig (Chung et al. 1999; Eom et al. 2002). The viable eggs of T. saginata were a generous gift from Dr S. Geerts (Prince Leopold Institute of Tropical Medicine, Belgium). TsM was obtained from a naturally infected pig in an endemic area (Guangxi Autonomous Region, China). Parasites were identified based on their host/tissue origins and morphological, epidemiological and molecular characteristics (Eom et al. 2002). They were washed over 10 times in cold physiological saline and frozen at −70 °C until use.
To assess specific antibody responses in sera and CSFs of Ac patients against TsM-specific proteins, 2 deletion mutants of TsM 10-kDa protein were constructed as described previously (Chung et al. 1999, 2002). In brief, DNA fragments encoding a part of region (amino acids from 25 to 85; Δ1) and another part of region (amino acids from 30 to 85; Δ2) of CyDA (cyticercosis diagnostic antigen) were amplified with gene specific primers (Chung et al. 2002). Amplicons were cloned into the pGEX-4T-2 vector (Amersham Pharmacia, Piscataway, NJ, USA) and the expression fidelities were confirmed by DNA sequencing (Applied Biosystems model 373A; Perkin-Elmer). The recombinant proteins were expressed as a fusion with glutathione S-transferase (GST) after induction with 0·5 mM isopropyl-β-D-thiogalactoside (IPTG). Recombinant proteins were purified by a passage of glutathione-Sepharose 4B resin (Amersham Pharmacia).
Recombinant 10 kDa protein was separated by 10% sodium dodecyl sulfate (SDS)-PAGE under reducing conditions and transfer-blotted onto polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA). Blots were incubated overnight with patient sera (1[ratio ]200 dilution) and CSFs (1[ratio ]1 dilution). Peroxidase-conjugated anti-human IgG (Cappel, West Chester, PA, USA) was subsequently incubated for 3–4 h at a dilution of 1[ratio ]1000. The reaction was developed using 4-chloro-l-naphthol chromogen (4C1N, Sigma, St Louis, MO, USA). CFs collected from the Ac samples were also resolved by 10–15% SDS–PAGE and then electroblotted onto PVDF membrane and further processed with immunoblot using a monoclonal antibody against the 150 kDa protein or using a monospecific antibody against the 10 kDa protein (Chung et al. 1999, 2002).
Approximately 1 mg of each parasite was suspended in 3 ml of 20 mM Tris–HCl (pH 8·0), 100 mM EDTA, 1% SDS, 10 μg/ml RNase A supplemented with 20 μg/ml of proteinase K (Boehringer Mannheim, Indianapolis, IN, USA) and incubated overnight at 55 °C. The reaction solution was centrifuged at 10000 g for 3 min, after which the supernatant fraction was transferred to a fresh tube and extracted twice with phenol/chloroform/isoamyl alcohol (25[ratio ]24[ratio ]1). The genomic DNA was then precipitated with isopropanol and dissolved in 100 μl of deionized water. Mitochondrial DNA was not purified because the quality and quantity of the genomic DNAs extracted from the samples were suitable for PCR analysis.
The primer set of JB3 and JB4.5, published elsewhere (Gasser et al. 1999), were used to amplify 444 nucleotides of the COI gene. The ND1 nucleotide sequence (approximately 530 bp) was amplified with primers JB11 and JB1, as described previously (Bowles & McManus, 1994). PCR was carried out on a Gene Amp 9700 (Perkin-Elmer) in a total volume of 20 μl consisting of a mixture of 2 μl (approximately 50–100 ng) genomic DNA, 20 mM each dNTP, 5 pM each primer, 10 mM Tris–HCl (pH 8·0), 50 mM KCl, 1·0 mM MgCl2 and 0·25 units of Taq polymerase (Takara Ex-Taq, Takara, Japan). The reaction involved 35 cycles of 35 sec at 94 °C, 35 sec at 56 °C and 45 sec at 72 °C, followed by final extension at 72 °C for 5 min. The amplicon was analysed by 1·5% agarose gel electrophoresis (Seakem LE agarose, FMC, Rockland, ME, USA).
Each amplicon was gel purified using a QIAEX II gel extraction kit (Qiagen, Valencia, CA, USA), ligated into pGEM-T easy vector (Promega, Madison, WI, USA) and transformed into E. coli cell (DH 5α). The plasmid DNA was extracted using QIAprep Spin Miniprep kit (Qiagen) and sequenced. T7 and SP6 primers were used in the pGEM-T easy vector for sequencing. Nucleotide sequences were determined by using a dideoxynucleotide chain termination sequenase kit (ABI PRISM Dye Terminator Cycle Sequencing Core Kit, Perkin Elmer) and an automated sequencer (Applied Biosystems M377, Perkin Elmer). To verify nucleotide substitutions, a pool containing 3 independent clones was sequenced.
Pairwise sequence alignment and comparisons were performed using GeneJockey II (Biosoft Co., Korea) and the NCBI database BLAST program (NIH, Bethesda, MD, USA). Multiple sequence alignment was carried out using Clustal-X Ver. 1.82 (Thompson et al. 1997). The amino acid sequences of COI and ND1 were predicted by known mitochondrial genetic code used in cestode parasites (Nakao et al. 2000). The unrooted phylogenetic tree was constructed by the neighbour-joining method (Saitou & Nei, 1987) using the Clustal-X program. Evolutionary distances were calculated using a Kimura's two-parameter method (Kimura, 1980). In total, 1000 bootstrapping replicates were used for the neighbour-joining analysis to observe relative support for internal nodes. Ac sequence data were compared with previously published sequences of Taenia spp. including T. multiceps (AJ239104), T. crassiceps (AF216699) and T. hydatigena (AJ277408).
Table 1 summarizes the clinical manifestation, and neuroimaging and surgical findings of the study subjects. All of the patients not only presented clinical manifestations compatible with NCC, such as seizure, symptoms due to increased intracranial pressure (IICP) and focal neurological deficits, but also revealed typical brain image findings of huge cystic mass together with hydrocephalus (panels a and b of Fig. 1A, case no. 5 in Table 1) or a hydrocephalus, except for one case that showed a cystic mass on the spinal canal. During surgery, viable or partially degenerated multi-lobulated cystic masses were extracted (panel c of Fig. 1A). All these cysts were confirmed to be Ac by histological examination with haematoxylin-eosin staining. Panel d of Fig. 1A demonstrates an example. The external cuticular layer with papillary-like budding warts, middle cellular layer and innermost reticular layer in which contained typical calcareous corpuscles are clearly observed. Fig. 1B shows another Ac specimen from the spinal cord (case no. 6) whose radiological examination revealed extramedullary leptomeningeal cystic masses on T2-L2 levels (panel a). During operation, 2 viable worms were removed on the lesions (panels b and c of Fig. 1B).

Fig. 1. Photograph of acephalic cysticercus (Ac) used in this study. (A) Ac case no. 5 in Table 1. (a) Hydrocephalus, noticed on computed tomographic scan. (b) Huge cystic mass observed on another view. (c) Multi-branched, multiple viable and partially degenerated cysts isolated from the 4th ventricle. (d) Microscopic examination revealed typical external cuticular layer of papillary like budding warts, middle cellular layer in which contained lymphocyte-like elements and innermost reticular layer with calcareous corpuscles. (B) Another Ac specimen extracted from the spinal cord (Ac case no. 6). (a) Magnetic resonance imaging scan showed 2 cystic masses (arrows) on the extramedullary leptomeningeal space on T2-L2 levels. (b) Huge cystic mass seen in operative field (arrow). (c) Three viable worms extracted from the lesions.
Interestingly, one patient (case no. 2), who had experienced a past medical history of proglottid discharge was suggested by neuroimaging study to be infected concurrently with normal cysticercus and Ac, and was later confirmed to be infected with both types of the cysticercus during neurosurgery.
To determine the expression of the TsM specific 10 kDa protein in Ac, 4 CFs collected from the surgical specimens were analysed by immunoblotting using either monospecific antibody against the TsM 10 kDa protein purified from the NCC patients or monoclonal antibody against the TsM 150 kDa (Chung et al. 1999). As shown in Fig. 2A, the 10 kDa protein was clearly detected in all the CFs examined. A duplicate blot, probed with a monoclonal antibody against the 150 kDa produced a similar result (data not shown). In addition, proteins of approximately 66, 50 and 23 kDa appeared to react non-specifically with the secondary antibodies, suggesting that albumin and the heavy and light chains of IgG might have contaminated the samples.

Fig. 2. Immunoblot analysis of the CF proteins from Ac samples and of antibody responses in patients with Ac. (A) Blot containing CF proteins was probed with a monospecific antibody against the TsM 10 kDa protein. Specific reaction was observed for the 10 kDa protein (). Albumin (66 kDa; [bull ]) and the heavy (50 kDa; ) and light chains (23 kDa; ) of IgG also exhibited weak positive reactions, which were non-specifically bound to anti-human IgG antibody. (B) Strips containing the recombinant TsM 10 kDa protein (upper panel) and truncated protein that expressing amino acids 30–85 (lower panel) were simultaneously incubated with serum (A) and (B) CSF samples from the Ac cases. Specific antibody reactions against both proteins were observed.
The TsM 10 kDa protein was shown to have epitopes specific to larval-stage Taenia infections while the truncated form (expressing amino acids 30–85) was shown to be species specific for detecting TsM infections (Chung et al. 2002). Therefore, we examined whether these proteins recognized the specific antibodies present in the serum and CSF samples from the Ac cases. The immunoblot in Fig. 2B demonstrates that all of the serum and CSF samples from Ac cases recognized both TsM 10 kDa protein (upper panel) and truncated TsM 10 kDa protein (lower panel). These results showed not only that the CFs from Ac samples contained the 10 kDa protein but also that the TsM species-specific epitope was recognized by specific antibodies just as in the conventional NCC cases.
Two fresh Ac samples were subjected to DNA sequence analysis. The rest of the Ac samples were unreliable and irreproducible because of a long period of preservation for 10–15 years in formalin fixative solution. The nucleotide sequences of the COI and ND1 genes of 2 Ac samples (Ac5 from the brain and Ac6 from the spinal cord) as well as those of T. saginata, T. solium and T. asiatica were determined.
A 363 bp fragment for COI gene and a 461 bp fragment for ND1 gene were isolated from each of the Ac samples. The difference in the COI nucleotide sequence between Ac and other tapeworms varied from 11·02–15·70% (Table 2). The nucleotide sequences of COI genes of Ac5 and Ac6 samples have been registered at the GenBank database under Accession numbers AY395065 and AY395066, respectively. In the case of ND1 gene, the 461 bp-sized ND1 sequence of Ac5 was found to be identical to that of T. solium. The sequence of Ac6 differed only in 1 nucleotide at position 341, where guanidine (G) was replaced by adenine (A). This nucleotide change was seen in 3 clones and verified by an additional independent PCR analysis followed by direct sequencing. The ND1 sequences of Ac5 and Ac6 samples were registered at the GenBank database under Accession numbers AY395067 and AY395068, respectively. The ND1 nucleotide sequences of Ac5 and Ac6 differed by 11·93–25·6% from those of T. saginata, T. asiatica, T. crassiceps, T. multiceps and T. hydatigena (Table 2).
Table 2. Percentage nucleotide sequence differences in the COI and ND1 genes [COI (above the diagonal) and ND1 (below the diagonal) genes for Taenia solium (Tso), Ac from human brain (Ac5), Ac from human spinal cord (Ac6), T. saginata (Tsa), T. asiatica (Tas), T. multiceps (Tmu), T. hydatigena (Thy), T. crassiceps (Tcr). The sequence of Tmu published in the paper (Bowles & McManus, 1994) is not available from the GenBank.]

Based on COI and ND1 sequence alignments, phylogenetic trees were constructed by a use of the neighbour-joining method (Fig. 3). In both trees, Ac isolates used in this study clustered with T. solium in a well-supported clade. Since the sequence of the ND1 gene of Ac6 sample was different from that of T. solium by only 1 nucleotide, they were tightly clustered as a same group by unrooted neighbour-joining tree. A similar topology was found using the maximum parsimony method (not shown). The placement of T. asiatica and T. saginata in the same clade was in accord with a previous observation that these 2 tapeworms are more closely related to each other than any other species (Zarlenga et al. 1991; De-Queiroz & Alkire, 1998).

Fig. 3. Phylogenetic trees constructed by unrooted neighbour-joining method using the nucleotide sequences of the COI and ND1 genes from 2 Ac samples and 6 Taenia spp. including T. solium (Tso), T. saginata (Tsa), T. asiatica (Tas), T. multiceps (Tmu, AJ239104 for ND1), T. hydatigena (Thy, AB33410 for COI and AJ277408 for ND1) and T. crassiceps (Tcr, AB033411 for COI and AF216699 for ND1, respectively). The nucleotide sequence of Tmu COI has not been deposited at GenBank, but has been published (Bowles & McManus, 1994). The scale bar represents the estimated number of nucleotide substitutions per nucleotide site. The bootstrap values based on the 1000 bootstrap replicates are indicated on each node.
It is generally accepted that NCC due to Ac is caused by an unusual form of larval Taenia, which is rarely developed and contains grape-like multilobulated cysts without a scolex (Loo & Braude, 1982; McCormick, 1985). However, the origin of Ac remains largely unclear since its first description by Zenker in 1882 (Henneberg, 1912). Previous studies on Ac have relied primarily on morphological and histological observations and no molecular information on Ac is available to date. To clarify the nature and origin of Ac, we employed specific protein markers (TsM 10-kDa protein and its mutant), and 2 mtDNA markers (COI and ND1 genes). Immunoblot analysis demonstrated that the 10 kDa protein was consistently expressed in CF collected from Ac samples. We also observed the presence of specific antibodies against both TsM 10 kDa and truncated 10 kDa proteins in sera and CSFs of these patients. Cloning and sequencing of the COI and ND1 genes revealed that the nucleotide sequences of both genes of Ac were almost identical to those of T. solium but differed substantially from those of other Taenia members. Phylogenetic analysis based on these two genes established that Ac clustered well with T. solium. All of these results strongly suggest that our samples of Ac have originated from T. solium.
In this study, we determined partial sequences of the mitochondrial COI and ND1 genes from 2 Ac samples because these sequences are frequently used to differentiate Taenia species (Gasser et al. 1999; Bowles & McManus, 1994). Analysis of the nucleotide sequences of the COI and ND1 genes of both samples revealed that COI gene was identical to that of T. solium while a single nucleotide substitution from G to A was observed in the ND1 sequence in one Ac sample (case no. 5). This Ac specimen was obtained from a patient's brain. The nucleotide change appeared to be non-synonymous, leading to a code change from AGG to AGA. Given the fact that it occurred in a third position of the codon, it is likely that both AGG and AGA codons encode serine in the cestode COI genes (Bowles et al. 1992; Nakao et al. 2000) as well as in invertebrate mitochondrial systems (Okimoto et al. 1992). This finding also reinforced the hypothesis that the AGR codon occurred in each of 3 highly conserved sites of serine (Nakao et al. 2000). Based on phylogenetic study of the nucleotide sequences of COI and cytochrome b genes, it has been proposed that T. solium could be classified to 2 groups such as an Asian group and an Latin American plus African group (Nakao et al. 2002). The COI sequence of the 2 Ac samples characterized in this study appeared to be more closely related to the Asian group.
Previous investigations suggested that Ac might have originated from either a sterile coenurus of T. multiceps or T. serialis or from some other Taenia spp., or alternatively from an aberrant form of TsM due to their lack of scolices and rostellar hooks on histological examination (Jung et al. 1981). In addition, histological characteristics of the cyst wall of cysticercus were hardly distinguishable from those of coenurus, in terms of a rim of hair-like projections and a wart-like appearance (Slais, 1970). The major differential morphological characteristics of Taenia spp. included the subtegumental muscles, excretory vessels and tegumental microvilli (Slais, 1970). However, identification of such characteristics requires a well-skilled and broadly experienced specialist.
Another piece of epidemiological evidence further supports our observation. In Korea, no authenticated case of T. multiceps infection either in man or in animal has ever been reported. In addition, as shown in Table 1, neurosurgery confirmed that one of our patients (Ac case no. 2) was infected with both types of cysticercus. However, it cannot be completely ruled out whether Ac originated from a single sp. of T. solium or from several different Taenia spp. To accurately elucidate the origin of Ac, further investigations of the Ac in other endemic areas are required, because Ac often results in serious and even fatal clinical manifestation in affected patients.
In conclusion, the present study demonstrates that the CFs of Ac samples contain a TsM-specific 10 kDa protein. The specific antibodies in these patients recognized this 10 kDa protein and its truncated form. In addition, the COI and ND1 DNA sequences of the Ac samples were found to be almost identical to those of T. solium while they differed substantially from the other Taenia species. Since no effective drug for Ac NCC is currently available, identification of the biological nature of Ac should aid in the development of effective chemotherapeutic in the future.
J. Y. Chung and W. G. Kho contributed equally to the work. The authors are grateful to Dr S Geerts, Prince Leopold Institute of Tropical Medicine, Belgium who kindly provided viable eggs of T. saginata. Drs L. Ma (Louisiana State University Health Science Center, Louisiana), and J. Traicoff (National Institute of Health, Bethesda) are greatly acknowledged for their critical reading of the manuscript. This work was supported by an Anti-Communicable Diseases Control Program of the National Institute of Health (NIH 348-6111-215), Ministry of Health and Welfare, Republic of Korea.

Table 1. Summary of clinical information of the 6 patients with acephalic neurocysticercosis

Fig. 1. Photograph of acephalic cysticercus (Ac) used in this study. (A) Ac case no. 5 in Table 1. (a) Hydrocephalus, noticed on computed tomographic scan. (b) Huge cystic mass observed on another view. (c) Multi-branched, multiple viable and partially degenerated cysts isolated from the 4th ventricle. (d) Microscopic examination revealed typical external cuticular layer of papillary like budding warts, middle cellular layer in which contained lymphocyte-like elements and innermost reticular layer with calcareous corpuscles. (B) Another Ac specimen extracted from the spinal cord (Ac case no. 6). (a) Magnetic resonance imaging scan showed 2 cystic masses (arrows) on the extramedullary leptomeningeal space on T2-L2 levels. (b) Huge cystic mass seen in operative field (arrow). (c) Three viable worms extracted from the lesions.

Fig. 2. Immunoblot analysis of the CF proteins from Ac samples and of antibody responses in patients with Ac. (A) Blot containing CF proteins was probed with a monospecific antibody against the TsM 10 kDa protein. Specific reaction was observed for the 10 kDa protein (). Albumin (66 kDa; [bull ]) and the heavy (50 kDa; ) and light chains (23 kDa; ) of IgG also exhibited weak positive reactions, which were non-specifically bound to anti-human IgG antibody. (B) Strips containing the recombinant TsM 10 kDa protein (upper panel) and truncated protein that expressing amino acids 30–85 (lower panel) were simultaneously incubated with serum (A) and (B) CSF samples from the Ac cases. Specific antibody reactions against both proteins were observed.
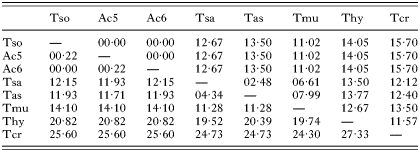
Table 2. Percentage nucleotide sequence differences in the COI and ND1 genes
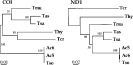
Fig. 3. Phylogenetic trees constructed by unrooted neighbour-joining method using the nucleotide sequences of the COI and ND1 genes from 2 Ac samples and 6 Taenia spp. including T. solium (Tso), T. saginata (Tsa), T. asiatica (Tas), T. multiceps (Tmu, AJ239104 for ND1), T. hydatigena (Thy, AB33410 for COI and AJ277408 for ND1) and T. crassiceps (Tcr, AB033411 for COI and AF216699 for ND1, respectively). The nucleotide sequence of Tmu COI has not been deposited at GenBank, but has been published (Bowles & McManus, 1994). The scale bar represents the estimated number of nucleotide substitutions per nucleotide site. The bootstrap values based on the 1000 bootstrap replicates are indicated on each node.