Introduction
It has been nearly 50 years since the study of Pearson (Reference Pearson1968), which elucidated the first trematode life cycle from the Great Barrier Reef. Despite the great inherent value in understanding trematode life cycles (Thompson et al., Reference Thompson, Mouritsen and Poulin2005; Blasco-Costa & Poulin, Reference Blasco-Costa and Poulin2017) and significant advances in our understanding of the diversity, richness and host specificity of adult trematodes in this ecosystem since that time (e.g. Miller et al., Reference Miller, Bray and Cribb2011; Cribb et al., Reference Cribb, Bott, Bray, McNamara, Miller, Nolan and Cutmore2014, Reference Cribb, Bray, Diaz, Huston, Kudlai, Martin, Yong and Cutmore2016), relatively little life-cycle information has been added. The studies of Rohde (Reference Rohde1973, Reference Rohde1977) and Huston et al. (Reference Huston, Cutmore and Cribb2016) each elucidated a single trematode life cycle. Downie & Cribb (Reference Downie and Cribb2011) connected an adult trematode with a cercaria described by Lucas et al. (Reference Lucas, O'Brien, Cribb and Degnan2005), and a few studies have connected metacercariae from various hosts with adult trematodes (Cribb et al., Reference Cribb, Bray, Barker and Adlard1996, Reference Cribb, Adlard and Bray1998; Miller et al., Reference Miller, Downie and Cribb2009; Kudlai et al., Reference Kudlai, Cribb and Cutmore2016). Additionally, several studies have reported larval trematodes from molluscan hosts which remain unconnected with adults (Cannon, Reference Cannon1978; Rohde, Reference Rohde1981; Shelley et al., Reference Shelley, Glazebrook, Turak, Winsor and Denton1988; Beuret & Pearson, Reference Beuret and Pearson1994; Bott et al., Reference Bott, Healy and Cribb2005).
Cannon (Reference Cannon1978) found 11 distinct trematode species exploiting the cerithiid gastropod Clypeomorus batillariaeformis Habe & Kosuge from Heron Island, southern Great Barrier Reef, and provided high-quality descriptions of new cercariae, giving each a numerical placeholder name (i.e. Cercariae queenslandae I–X). Of these, Cannon (Reference Cannon1978) identified C. queenslandae II as a member of the Haploporidae Nicoll, 1914 and noted that these cercariae encysted in the environment about 12 h after emergence. Cannon (Reference Cannon1978) also mentioned a morphological similarity between this cercaria and a species considered a haploporid at the time, Atractotrema sigani Durio & Manter, 1969, a parasite of rabbitfishes (Siganidae). As siganids are common off the beachrock of Heron Island where C. battilariaeformis is found, Cannon (Reference Cannon1978) speculated that C. queenslandae II was likely the larva of A. sigani. No further study has explored this idea.
Current classification based upon morphological and molecular data recognizes two families, the Atractotrematidae Yamaguti, 1939 and Haploporidae, within the superfamily Haploporoidea Nicoll, 1914 (Jones Reference Jones, Jones, Bray and Gibson2005; Overstreet & Curran Reference Overstreet, Curran, Jones, Bray and Gibson2005a, Reference Overstreet, Curran, Jones, Bray and Gibsonb; Blasco-Costa et al., Reference Blasco-Costa, Balbuena, Kostadinova and Olson2009; Pulis & Overstreet, Reference Pulis and Overstreet2013; Andres et al., Reference Andres, Pulis, Cribb and Overstreet2014, 2016). Atractotrematids possess two symmetrical testes and utilize only estuarine and marine fishes as hosts, whereas haploporids usually have a single testis or, rarely, two tandem or oblique testes, and utilize marine, estuarine and freshwater fishes as hosts (Jones, Reference Jones, Jones, Bray and Gibson2005; Overstreet & Curran Reference Overstreet, Curran, Jones, Bray and Gibson2005a, b; Andres et al., Reference Andres, Pulis and Overstreet2016). While the Haploporidae includes many genera and species, the Atractotrematidae contains just 11 species in four genera (Overstreet & Curran, Reference Overstreet, Curran, Jones, Bray and Gibson2005b; Andres et al., Reference Andres, Pulis and Overstreet2016). Two of the four atractotrematid genera, Pseudisorchis Ahmad, 1985 and Pseudomegasolena Machida & Kamiya, 1976 are monotypic, Atractotrema Goto & Ozaki, 1929 includes three species and Isorchis Durio & Manter, 1969 includes six (Cribb, Reference Cribb2010). Andres et al. (Reference Andres, Pulis and Overstreet2016) described three new species of Isorchis from Australian waters, including the first known species from non-chanid hosts, and provided the first molecular data for the genus. Here, we describe an additional species of Isorchis from rabbitfish from the Great Barrier Reef, and using molecular data, demonstrate that C. queenslandae II of Cannon (Reference Cannon1978) is the larval form of this new species, elucidating the first confirmed life cycle for a species of the family Atractotrematidae. In addition, we provide a new record of Isorchis currani Andres, Pulis & Overstreet, 2016 collected from Moreton Bay in south-east Queensland.
Materials and methods
Specimen collection
Between 2015 and 2016, large numbers of the cerithiid gastropod C. batillariaeformis were collected from beach rock at Heron Island, southern Great Barrier Reef, Queensland, Australia (23°27′S, 151°55′E). Snails were isolated in a small amount of seawater in individual 10-ml wells and left for 24–48 h to observe natural emergence of cercariae. Snails from which no cercariae had emerged within 48 h were released at the site of capture. Upon discovery, emerged cercariae were fixed in near-boiling saline and preserved in 70% ethanol for parallel morphological and molecular analyses. Snails from which cercariae had emerged were either retained to acquire additional emerged cercariae and then released, or dissected for collection of intramolluscan trematode larvae, which were subsequently fixed and preserved as above. In 2016, as part of a larger parasitological survey and in collaboration with local commercial fishermen, specimens of the scat Selenotoca multifasciata (Richardson) were collected in Moreton Bay (27°28′S, 153°18′E) using a tunnel net. In 2017, the rabbitfishes Siganus fuscescens (Houttuyn) and Siganus lineatus (Valenciennes) were collected by spear off Heron Island. The gut of each fish was excised and examined following the recommendations of Cribb & Bray (Reference Cribb and Bray2010). Trematodes collected were fixed and preserved as above.
Morphological analyses
Trematode specimens used for morphological examination were washed in fresh water, overstained in Mayer's haematoxylin, destained in a solution of 1.0% hydrochloric acid and neutralized in 0.5% ammonium hydroxide solution. Specimens were then dehydrated in a graded ethanol series, cleared in methyl salicylate and mounted in Canada balsam. Drawings were made using an Olympus BX-53 compound microscope with attached drawing tube (Olympus, Notting Hill, Australia), and illustrations were digitized in Adobe Illustrator. Measurements were made with cellSens standard imaging software paired with an Olympus SC50 digital camera mounted on an Olympus BX-53 compound microscope. Measurements are provided in micrometres (μm) and given as the range followed by the mean in parentheses. Where length is followed by breadth, the two measurements are separated by ‘×’. All vouchers are lodged in the Queensland Museum (QM), Brisbane, Australia.
Molecular sequencing
Total genomic DNA was extracted from trematodes using phenol/chloroform extraction techniques (Sambrook & Russell, Reference Sambrook and Russell2001). Polymerase chain reaction (PCR) for the internal transcribed spacers ITS1 and ITS2, and 28S regions was performed using Bioline MyTaq™ DNA Polymerase and Reaction Buffer following the manufacturer's recommendations. The entire ITS2 rDNA region and partial 28S rDNA were amplified with the primers and cycling conditions used by Huston et al. (Reference Huston, Cutmore and Cribb2016). The ITS1 region was amplified using the primers S20T2 (5′-GGT AAG TGC AAG TCA TAA GC-3′) (Jousson et al., Reference Jousson, Bartoli and Pawlowski1999) and 5.8S1 (5′-GCT GCG CTC TTC ATC GAC A-3′) (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000), using the following cycling conditions: 40 cycles of 30 s at 94°C, 30 s at 53°C and 120 s at 72°C, then a 5 min final extension at 72°C.
Amplified DNA was purified using a Bioline ISOLATE II PCR and gel purification kit (Bioline, Alexandria, Australia), per the manufacturer's protocol. Cycle sequencing of purified DNA was carried out using ABI Big Dye™ v.3.1 chemistry (Thermo Fisher Scientific) following the manufacturer's recommendations at the Australian Genome Research Facility, using an AB3730 × 1 capillary sequencer. Primers used for sequencing the ITS1 region were those used in amplification. Primers used for sequencing the ITS2 and 28S regions are listed in Huston et al. (Reference Huston, Cutmore and Cribb2016). Sequencher™ version 4.5 (GeneCodes Corp., http://www.genecodes.com/sequencher) was used to assemble and edit contiguous sequences. For each newly generated sequence of ITS1 rDNA, the start and end of the ITS1 region was determined through comparison with previously published atractotrematid and haploporid sequences that include the ITS1 region. For each newly generated sequence of ITS2 rDNA, the start and end of the ITS2 region was determined by annotation using the ITS2 Database Metazoa model (Keller et al., Reference Keller, Schleicher, Schultz, Müller, Dandekar and Wolf2009; Ankenbrand et al., Reference Ankenbrand, Keller, Wolf, Schultz and Förster2015). Collection data and GenBank accession numbers for sequenced taxa are presented in the taxonomic section of this manuscript.
Results
Molecular results
ITS1, ITS2 and partial 28S rDNA sequence data were generated for the specimens of adult Isorchis collected from S. lineatus and S. fuscescens from Heron Island and S. multifasciata from Moreton Bay. Additionally, ITS1 and ITS2 sequences were generated for C. queenslandae II from infected C. batillariaeformis collected at Heron Island. The ITS1 and ITS2 sequences from infected C. batillariaeformis were identical to those of adult Isorchis from Heron Island siganids. Comparison of these new sequence data with that available on GenBank indicated that the Heron Island material represented an uncharacterized genotype, which, with the inclusion of morphological data, is described here as Isorchis cannoni n. sp. Sequences of adult Isorchis from Moreton Bay S. multifasciata matched those of I. currani reported by Andres et al. (Reference Andres, Pulis and Overstreet2016) from Darwin, Northern Territory.
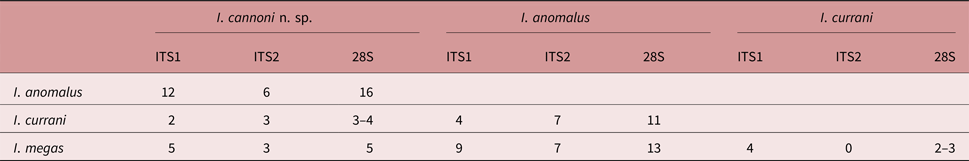
All ITS1 sequences were 703 bp long, comprising 156 bp of flanking 18S rDNA, 513 bp of ITS1 rDNA and 34 bp of flanking 5.8S rDNA. The ITS2 sequences generated for I. cannoni n. sp. were 437 bp long, comprising 123 bp flanking 5.8S rDNA, 265 bp ITS2 rDNA and 49 bp flanking 28S rDNA. The ITS2 sequences generated for I. currani from Moreton Bay were 439 bp long, comprising 123 bp flanking 5.8S rDNA, 267 bp ITS2 rDNA and 49 bp flanking 28S rDNA. The ITS1 and ITS2 sequences of I. cannoni n. sp. differed from those of Isorchis anomalus Andres, Pulis & Overstreet, 2016, I. currani and Isorchis megas Andres, Pulis & Overstreet, 2016 (the only other species of Isorchis for which molecular data are available) by 12 and 6, 2 and 3, and 5 and 3 base positions, respectively (table 1). The ITS1 and ITS2 sequences of I. currani from Moreton Bay were identical to those of I. currani provided by Andres et al. (Reference Andres, Pulis and Overstreet2016) from Darwin, Northern Territory, whereas the 28S sequence from Moreton Bay differed from those of Darwin by one base. The 28S sequences of I. cannoni n. sp. differed from those of I. anomalus, I. currani isolate 1, I. currani isolate 2 and I. megas by 16, 4, 3 and 5 base positions, respectively (table 1).
Table 1. Base pair differences between the ITS1, ITS2 and 28S rDNA regions for all species of Isorchis for which molecular data are available.
Family Atractotrematidae Yamaguti, 1939; Genus Isorchis Durio & Manter, 1969; Isorchis cannoni n. sp.
Taxonomic summary
Type host. Siganus lineatus (Valenciennes), golden-lined spinefoot (Perciformes: Siganidae).
Other definitive hosts. Siganus fuscescens (Houttuyn), mottled spinefoot (Perciformes: Siganidae).
Intermediate host. Clypeomorus batillariaeformis Habe & Kosuge (Cerithiidae).
Type locality. Off Heron Island, southern Great Barrier Reef, Queensland, Australia (23°27′S, 151°55′E).
Prevalence. Three of six S. lineatus and six of six S. fuscescens.
Type material. Holotype (QM G236318) and 19 paratypes (QM G236319–236337) including two hologenophores (QM G236338–236339).
Larval voucher material. Excised rediae and cercariae, five slides (QM G236340–236344).
Site in definitive host. Intestine.
Site in intermediate host. Digestive gland.
Representative DNA sequences. For both ITS1 and ITS2 rDNA, nine identical replicates, three from adult worms ex S. lineatus, three from adults ex S. fuscescens and three from intramolluscan stages ex C. batillariaeformis (one replicate of ITS1 and ITS2 submitted to GenBank, ITS1: MF803155; ITS2: MF803156). For 28S rDNA, three identical replicates, two from adult worms ex S. lineatus, and one from an adult ex S. fuscescens (one submitted to GenBank: MF803154).
Zoobank registration. The Life Science Identifier for Isorchis cannoni n. sp. is urn:lsid:zoobank.org:act:121143A3-D7CF-4F31-AFA3-4B83A26DAB1C.
Etymology. This species is named for Dr Lester R.G. Cannon, in recognition of his contributions to marine parasitology and knowledge of the larval digenean fauna of the Great Barrier Reef.
Description
Based on 20 gravid whole mounts, ten from S. lineatus, ten from S. fuscescens (fig. 1A, B). Body fusiform, 378–492 × 180–251 (436 × 223). Body length/width 1.7–2.2 (1.9). Tegument finely spinose throughout. Forebody 144–189 (170), occupying 35–43 (39)% of body length; hindbody 139–244 (187), occupying 37–46 (43)% of body length. Eyespot pigment mostly restricted to forebody, profuse, most prominent dorsally. Oral sucker terminal, subglobular, 57–74 × 74–94 (64 × 84). Ventral sucker in midbody, globular, 72–94 × 73–96 (80 × 84); aperture oval. Ventral sucker length/oral sucker length 1.14–1.45 (1.3); ventral sucker width/oral sucker width 0.91–1.10 (1.0). Prepharynx 9–23 (15) long; pharynx ovoid, 42–58 × 46–60 (48 × 54). Pharynx width/oral sucker width 0.56–0.72 (0.64). Oesophagus relatively straight, 34–65 (50) long. Intestinal bifurcation occurring between region just anterior to ventral sucker and mid-level of ventral sucker. Caeca blind, 136–197 × 26–60 (168 × 37), terminating 79–128 (99) from posterior extremity; post-caecal space representing 18–27 (23)% of body length.
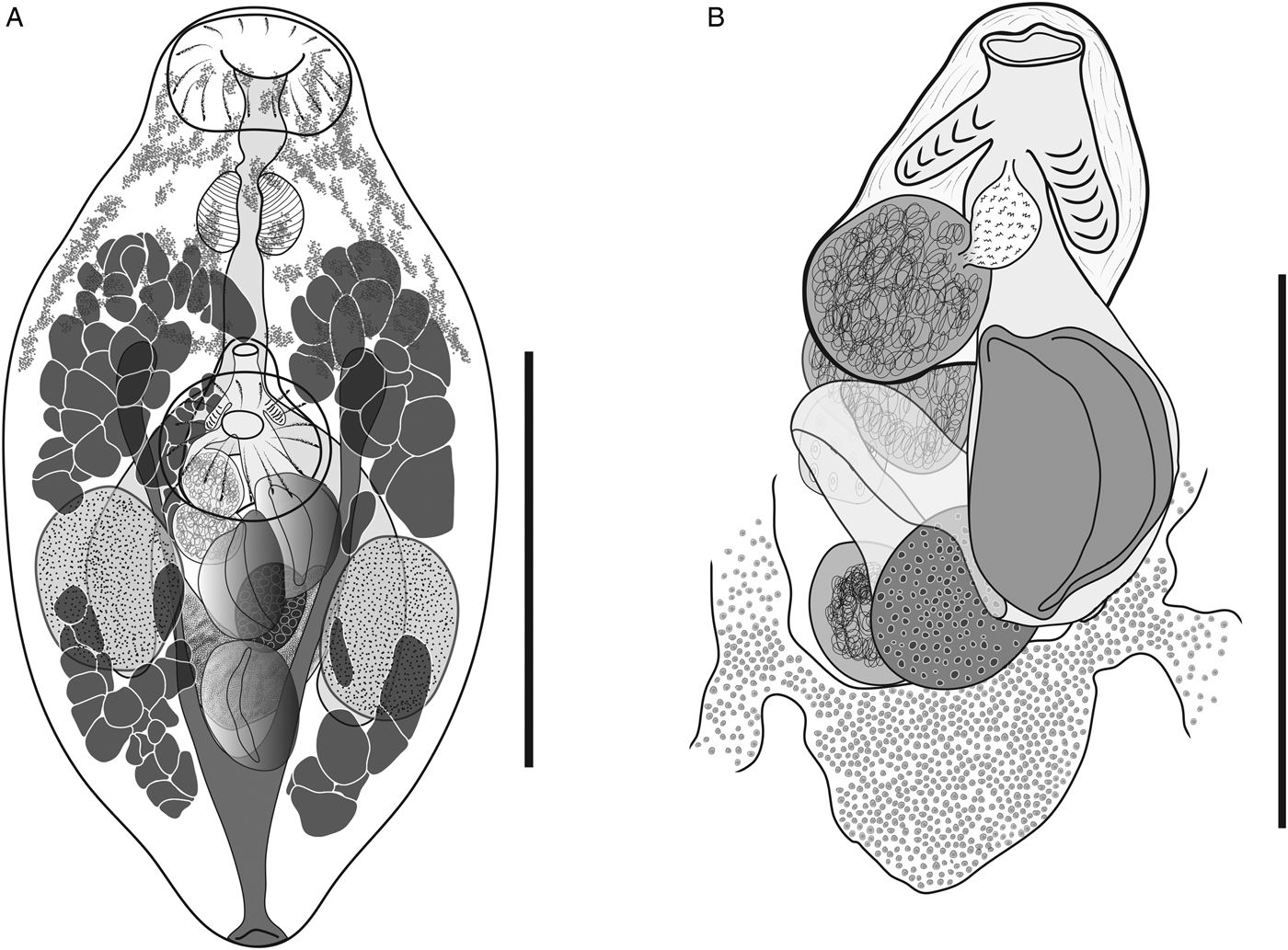
Fig. 1. (A) Isorchis cannoni n. sp., ventral view, holotype, from Siganus lineatus, Heron Island, Great Barrier Reef. (B) Isorchis cannoni n. sp., ventral view, hermaphroditic sac and ovarian complex, paratype, from Siganus fuscescens, Heron Island. Scale bars: (A) 200 μm; (B) 100 μm.
Testes two, opposite, in anterior to mid-hindbody, ventral to caeca, generally slightly asymmetrical, ovoid; sinistral testis 62–100 × 48–76 (85 × 63); dextral testis 65–102 × 50–78 (85 × 62). Post-testicular space 86–160 (121), representing 23–33 (28)% of body length. External seminal vesicle dorsal to ventral sucker, ovoid, 35–45 × 36–49 (41 × 39). Hermaphroditic sac dorsal to ventral sucker, ventral to caeca, 53–77 × 47–74 (68 × 57), occupying 13–19 (16)% of body length. Internal seminal vesicle 28–37 × 29–36 (33 × 33); prostatic bulb elongate to ovoid with male duct a short tube uniting with female duct at about mid-level of hermaphroditic sac; hermaphroditic duct occupying approximately half the length of sac; diverticula two, joined to anterior portion of hermaphroditic duct. Genital pore ventral, just anterior to ventral sucker.
Ovary in anterior hindbody, intercaecal and intertesticular, with anterior margin dorsal to posterior margin of ventral sucker or nearly so, subglobular, 32–49 × 30–44 (40 × 36). Seminal receptacle subglobular, dorsal to and slightly smaller than ovary. Ootype anterior to seminal receptacle and ovary. Mehlis’ gland indistinct. Laurer's canal not observed. Vitellarium follicular, arranged in two fields confluent dorsally or not, interrupted ventrally at level of testes; anterior field wrapping from dorsal longitudinal median bilaterally to sinistral and dextral body regions, not confluent ventrally, 68–119 (97) from anterior body extremity, representing 17–29 (22)% of body length; posterior field not confluent dorsally, wrapping bilaterally from dorsal sinistral and dextral body regions to ventral sinistral and dextral body regions, 47–74 (58) from posterior body extremity, representing 10–19 (13)% of body length. Vitelline reservoir crescentiform to elliptical, with anterior margin contiguous with posterior margin of ovary; arms of collecting ducts passing anteriorly from reservoir bilaterally around ovary. Uterus between posterior margins of hermaphroditic sac and vitelline reservoir. Eggs 1–5 in number, 62–87 × 40–61 (79 × 49); egg length representing 13–22 (18)% of body length.
Excretory vesicle Y-shaped, extending 64–74 (69)% of body length, bifurcating 108–123 (96) from posterior extremity; arms extending into mid-forebody to 108–165 (140) from anterior extremity. Excretory pore terminal.
Measurements of the holotype
Body 450 × 237. Body length/width 1.8. Forebody 176, occupying 39% of body length; hindbody 202, occupying 45% of body length. Oral sucker 63 × 87. Ventral sucker 72 × 79. Ventral sucker length/oral sucker length 1.14; ventral sucker width/oral sucker width 0.91. Prepharynx 19 long; pharynx 42 × 52. Pharynx width/oral sucker width 0.60. Oesophagus 65 long. Caeca 171 × 33, terminating 85 from posterior extremity; post-caecal space representing 19% of body length. Sinistral testis 90 × 69; dextral testis 87 × 61. Post-testicular space 106, representing 24% of body length. External seminal vesicle 39 × 38. Hermaphroditic sac 76 × 57–74, occupying 17% of body length. Internal seminal vesicle 35 × 29. Ovary 41 × 34–44. Vitellarium 111 from anterior body extremity, representing 24% of body length; 48 from posterior body extremity, representing 11% of body length. Eggs 4 in number, 66–81 × 56–44 (74 × 50); egg length representing 15–18 (17)% of body length. Excretory vesicle extending 64% of body length, bifurcating 90 from posterior extremity; arms extending to 161 from anterior extremity.
Remarks
All ITS1 and ITS2 rDNA sequences generated from larval digeneans from three separate C. batillariaeformis were identical to those ITS1 and ITS2 sequences generated for I. cannoni n. sp. from S. lineatus and S. fuscescens. All these hosts were collected in the same area of Heron Reef. Cannon (Reference Cannon1978) noted that this cercaria encysts in the environment, and would thus readily encyst upon algae growing on the beachrock found along Heron Island. Siganids are a major group of grazers along this beachrock (Stephenson & Searles, Reference Stephenson and Searles1960), therefore this is undoubtedly the route of metacercarial ingestion in the life cycle of I. cannoni n. sp.
Isorchis cannoni n. sp. is morphologically distinct from all other species of Isorchis. From I. anomalus, I. cannoni n. sp. differs in having a genital pore margin that is ovoid without folds or creases, a smaller body (378–492 × 180–251 vs. 523–709 × 232–394), smaller testes (sinistral 65–100 × 48–76, dextral 65–102 × 50–78 vs. sinistral 122–203 × 91–144, dextral 127–189 × 90–116), smaller hermaphroditic sac, both absolutely (53–77 × 47–74 vs. 93–151 × 50–134) and relative to body length (13–18 vs. 17–23%), and a 12–20% larger oral sucker to pharynx width ratio. In addition, the eggs of I. cannoni n. sp. are shorter but broader than those of I. anomalus (62–87 × 40–61 vs. 63–101 × 37–55). From Isorchis chanosi (Zhukov, 1972) Ahmad, 1985, I. cannoni n. sp. differs in having tegumental spines that extend to the posterior end of the body rather than to only the mid-hindbody, shorter testes (sinistral 65–100 × 48–76, dextral 65–102 × 50–78 vs. 100–167 × 41–83), caeca that terminate posterior to the testes rather than mid-testes, a vitellarium that does not extend to the posterior end of the body and by possessing a uterus that does not extend to the posterior end of the body. From I. currani, I. cannoni n. sp. differs in having a smaller body (378–492 × 180–251 vs. 591–695 × 192–353), testes (sinistral 65–100 × 48–76, dextral 65–102 × 50–78 vs. sinistral 134–200 × 70–125, dextral 118–198 × 70–125), shorter post-caecal space (17–29 vs. 27–36% of body length), an 18–36% larger oral sucker to pharynx width ratio and shorter, broader eggs (62–87 × 40–61 vs. 75–94 × 32–47). Furthermore, I. currani possesses a larger oral sucker than ventral sucker, whereas I. cannoni n. sp. possesses a ventral sucker that is slightly larger than the oral sucker (longer and of nearly equal width). From I. megas, I. cannoni n. sp. differs in having a larger ovary (32–49 × 30–44 vs. 19–35 × 15–35), a vitellarium that extends anteriorly to the level of the pharynx rather than the posterior forebody, an oral sucker that is smaller than the ventral sucker and a ~40% greater oral sucker to ventral sucker width ratio. Additionally, I. cannoni n. sp. has more eggs, which are shorter and broader than those of I. megas (62–87 × 40–61 vs. 83–92 × 37–44), and are less than 20% of body length. From Isorchis parvus Durio & Manter, 1969, I. cannoni n. sp. differs in having a shorter body length (378–492 vs. 567–912), lacking radial muscles surrounding the genital pore, and gut bifurcation in the region of the ventral sucker rather than mid-way between the oral and ventral suckers. Additionally, the eggs of I. cannoni n. sp. are smaller than those of I. parvus (62–87 × 40–61 vs. 72–88 × 43–51), but larger relative to body size (13–22 vs. ~12% body length). From Isorchis skrjabini Ahmad, 1985, I. cannoni n. sp. differs in having a smaller body (378–492 × 180–251 vs. 665–1065 × 370–600), a terminal, subglobular oral sucker rather than a subterminal cup-shaped oral sucker, an oral sucker smaller than the ventral sucker, vitellarium extending anteriorly to the level of the pharynx rather than to the level of caecal bifurcation, a ventral sucker posterior to the caecal bifurcation, an ovary that is oval rather than triangular, and longer excretory vesicle arms that extend into the forebody rather than terminating in the hindbody.
Isorchis cannoni n. sp. is further distinguished from all other species of Isorchis by utilization of a siganid as a definitive host, rather than a chanid or scatophagid. Molecular data also demonstrate that I. cannoni n. sp. is distinct from those other species for which such data are available: I. anomalus, I. currani and I. megas.
Isorchis currani Andres, Pulis & Overstreet, 2016
Taxonomic summary
Type host. Selenotoca multifasciata (Richardson), spotbanded scat (Perciformes: Scatophagidae).
Type locality. Fannie Bay, Northern Territory, Australia (12°26′S, 130°49′E).
New material: taxonomic summary
Host. Selenotoca multifasciata.
Locality. Moreton Bay, Queensland, Australia (27°28′S, 153°18′E).
Prevalence. Two of 28 S. multifasciata.
Voucher material. Twelve whole mounts (QM G236345–236356).
Site in host. Intestine.
Representative DNA sequences. For both ITS1 and ITS2 rDNA, two identical replicates (one of each submitted to GenBank, ITS1: MF803158; ITS2: MF803159). For 28S rDNA, one replicate (MF803157).
New measurements
Based on 12 gravid whole mounts, all from S. multifasciata from Moreton Bay. Body 564–727 × 250–364 (673 × 316). Body length/width 1.9–2.4 (2.2). Forebody 220–305 (267), occupying 36–44 (40)% of body length; hindbody 266–337 (307), occupying 41–51 (46)% of body length. Oral sucker 88–134 × 125–149 (98 × 140). Ventral sucker 85–131 × 80–120 (105 × 102). Ventral sucker length/oral sucker length 0.79–1.26 (1.1); ventral sucker width/oral sucker width 0.64–0.81 (0.73). Prepharynx 8–18 (12) long; pharynx 40–67 × 53–68 (51 × 64). Pharynx width/oral sucker width 0.42–0.48 (0.46). Oesophagus 56–106 (88) long. Caeca 196–307 × 48–64 (247 × 53), terminating 134–224 (185) from posterior extremity; post-caecal space representing 24–32 (27)% of body length. Sinistral testis 132–186 × 80–122 (162 × 95); dextral testis 127–192 × 76–153 (164 × 103). Post-testicular space 126–230 (180), representing 18–35 (27)% of body length. Hermaphroditic sac 82–116 × 70–97 (92 × 83), occupying 12–17 (14)% of body length. Ovary 44–70 × 45–67 (58 × 53). Vitellarium 125–175 (149) from anterior body extremity, 35–92 (66) from posterior body extremity. Eggs 4–16 in number, 75–86 × 50–67 (81 × 57); egg length representing 11–15 (12)% of body length. Excretory vesicle extending 72–77 (75)% of body length, bifurcating 109–216 (166) from posterior extremity, arms extending into mid-forebody to 156–201 (172) from anterior extremity.
Remarks
These specimens are morphologically indistinguishable from those representing I. currani described by Andres et al. (Reference Andres, Pulis and Overstreet2016) and are from the same host species. Furthermore, ITS1 and ITS2 sequences generated for the new material are identical to sequences of I. currani provided by Andres et al. (Reference Andres, Pulis and Overstreet2016), and the 28S sequences generated for the new material differ by only one base. There is little doubt that the Isorchis specimens recovered from Moreton Bay are I. currani. This new report represents a significant range extension for the species.
Discussion
The molecular data generated in this study demonstrate that I. cannoni n. sp. is a member of the Atractotrematidae and is closely related to existing species in the genus Isorchis. The differences between I. cannoni n. sp. and those other species of Isorchis for which molecular data are available are slight, but these differences are sufficient to consistently distinguish I. cannoni n. sp. from I. anomalus, I. currani and I. megas. Sequences generated for I. cannoni n. sp. differ from those of I. currani and I. megas at only a few base positions. Sequence variation increases when comparing I. cannoni with I. anomalus, a species that utilizes Chanos chanos (Forsskål) as a definitive host. Isorchis anomalus was resolved as basal to I. currani and I. megas in the molecular phylogeny of Andres et al. (Reference Andres, Pulis and Overstreet2016). This may provide some insight into the relationship between I. cannoni and other species of Isorchis that utilize C. chanos, and might lend support to the speculation of Andres et al. (Reference Andres, Pulis and Overstreet2016) that chanids are the ancestral host group for species of Isorchis. However, we face the same limitation as Andres et al. (Reference Andres, Pulis and Overstreet2016) in that additional molecular evidence from Isorchis species exploiting C. chanos will be required to test this theory.
Isorchis cannoni n. sp. is the first species of Isorchis known from the fish family Siganidae. Of the other known species of Isorchis, I. anomalus, I. chanosi, I. parvus and I. skrjabini utilize C. chanos (Chanidae) as a definitive host, whereas I. currani and I. megas utilize S. multifasciata (Scatophagidae). Species of the monotypic genera Pseudisorchis and Pseudomegasolena utilize species of Mugilidae and Scaridae, respectively. All species of Atractotrema utilize siganids as definitive hosts. In the molecular phylogeny of the Haploporoidea Nicoll, 1914 of Andres et al. (Reference Andres, Pulis and Overstreet2016), Atractotrema is resolved as the most basal lineage of the Atractotrematidae. Thus, although chanids might represent the ancestral host group of Isorchis, siganids might represent the ancestral host group of all atractotrematids. If this were the case, then the utilization of a siganid host by I. cannoni n. sp. would represent a recolonization host-switch. Although theories on the ancestral state of atractotrematids remain strictly speculative, further molecular exploration in this family may reveal an interesting evolutionary narrative.
All our Isorchis specimens from S. multifasciata from Moreton Bay were consistent with I. currani; no specimens consistent with I. megas were found. Andres et al. (Reference Andres, Pulis and Overstreet2016) found only I. megas in Western Australia, whereas they found I. currani and I. megas occurring sympatrically in the Northern Territory. Considering the molecular similarities between I. currani and I. megas, and utilization of the same definitive host in both these species, perhaps our collecting only I. currani in Moreton Bay is consistent with the existence of two species that have diverged based on geography, or have geographically restricted intermediate hosts. This seems plausible, as phylogenetic differentiation has been observed between eastern and western Australia for multiple animal groups (Benzie et al., Reference Benzie, Frusher and Ballment1992; Chenoweth et al., Reference Chenoweth, Hughes, Keenan and Lavery1998; Gopurenko & Hughes, Reference Gopurenko and Hughes2002; Reid et al., Reference Reid, Lal, Mackenzie-Dodds, Kaligis, Littlewood and Williams2006). This differentiation is thought to be a result of lowering sea levels exposing the Sahul Shelf during the Pleistocene, breaking the connection between the Indian and Pacific Oceans (Voris, Reference Voris2000). Much of the Sahul Shelf is now under the shallow Timor and Arafour Seas, a distinct ecoregion, which includes the coastal region of the Northern Territory, and separates the marine regions of Western Australia and Queensland (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdana, Finlayson, Halpern, Jorge, Lombana and Lourie2007).
Our molecular pairing of Cannon's (Reference Cannon1978) C. queenslandae II with I. cannoni n. sp. has provided the first confirmed atractotrematid cercaria and intermediate host. Cannon (Reference Cannon1978) recognized the strong similarities between his cercaria and those of haploporids. Haploporid cercariae are all ocellate, of the gymnocephalus type and have long, simple tails (for a list of known haploporid cercariae, see Overstreet & Curran, Reference Overstreet, Curran, Jones, Bray and Gibson2005a). The cercaria of I. cannoni n. sp. agrees with haploporid cercariae in all these respects. However, the cercariae of I. cannoni n. sp. can be differentiated from those of the Haploporidae much in the same manner as adults of atractotrematids and haploporids are differentiated. The cercariae of haploporids and those of I. cannoni n. sp. have well-developed reproductive organs. Thus, the presence of two symmetrical testes is indicative of the Atracotrematidae, whereas a single testis, or two tandem or oblique testes, would suggest the Haploporidae. These testes are easily detectible in our stained and mounted specimens of I. cannoni n. sp. cercariae, and the reproductive organs are well characterized in most descriptions of haploporid cercariae. Although not explicitly stated, Cannon (Reference Cannon1978) must have based his suggestion that C. queenslandae II was the larva of A. sigani partially on the symmetrical testes present in both. Although we cannot know with certainty if this morphological differentiation will hold as more atractotrematid and haploporid cercariae are described, it seems likely.
The first intermediate hosts of atractotrematids had been predicted to be marine gastropods in the superfamilies Rissooidea or Truncatelloidea, as these groups serve as intermediate hosts for haploporids (Andres et al., Reference Andres, Pulis and Overstreet2016). The discovery of C. batillariaeformis as the intermediate host in the life cycle of I. cannoni n. sp. has defied prediction, as this gastropod is a member of the superfamily Cerithioidea. In the taxonomy of the Gastropoda of Bouchet & Rocroi (Reference Bouchet and Rocroi2005), the superfamilies Rissooidea and Truncatelloidea are related Hypsogastropoda lineages within the greater clade Caenogastropoda. Although the Cerithioidea is also within the Caenogastropoda clade, it is outside the Hypsogastropoda. Thus, the intermediate host lineages utilized by atractotrematids and haploporids are distantly related. Such a pattern might at first seem improbable, but parallels are known from the Lepocreadioidea Odhner, 1905 lineages. Most known intermediate hosts for lepocreadioid species are within the Hypsogastropoda, and yet the only known intermediate host for the Gyliauchenidae Fukui, 1929 is a cerithiid, and intermediate hosts of the Deropristidae Cable & Hunnien, 1942 are reported from both cerithiid and truncatelloid gastropods (Huston et al., Reference Huston, Cutmore and Cribb2016). Additional evidence of broad molluscan host utilization can be found in the Schistosomatidae Poche, 1907, species of which are known from caenogastropods, pulmonates and opisthobranchs (Blair et al., Reference Blair, Davis and Wu2001).
Because of these precedents observed for the Lepocreadioidea and Schistosomatidae, a single known atractotrematid intermediate host is insufficient to draw deeper conclusions about the evolutionary history of the Haploporoidea. However, efforts should be made to discover additional atractotrematid intermediate hosts as these data could provide profound insights. Considering that species of the Cerithiidae are exclusively marine (Bouchet & Rocroi, Reference Bouchet and Rocroi2005), atractotrematid intermediate host utilization may restrict definitive host availability. Furthermore, if additional cerithiid species are found as hosts of atractotrematids, then it may be possible to use the geological timing of the diversification of the Caenogastropoda lineages to determine the timing of the split between the Atractotrematidae and the Haploporidae. In addition, as atractotrematids and haploporids use both phylogenetically and ecologically related hosts, further life-cycle data would be useful in determining whether intermediate, definitive or both host groups are major drivers of speciation in the Haploporoidea.
Acknowledgements
The authors thank the staff of the Heron Island and Moreton Bay (University of Queensland) research stations for their ongoing support of our field expeditions. We thank John Page and Dave Thompson for assistance with specimen collection in Moreton Bay. We would also like to thank our anonymous reviewers for constructive criticisms which greatly improved the content of this manuscript.
Financial support
D.C.H. is funded through grants from the PADI Foundation, Linnean Society of New South Wales, Systematics Research Fund (Systematics Society in partnership with the Linnean Society of London), and a Moreton Bay Research Station Scholarship in partnership with SIBELCO Australia. Collections in Moreton Bay were part of a larger project funded by an ABRS National Taxonomy Research Grant RF215–40 awarded to S.C.C. and T.H.C.
Conflict of interest
None.
Ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.




