INTRODUCTION
All species of Echinococcus require 2 mammalian hosts for completion of their life-cycles. A variety of non-carnivorous hosts can support development of the metacestode whereas the adult tapeworm can only develop in relatively few species of carnivore host (Thompson, 1995). Most of what is known about the development of Echinococcus has come from studies in vitro, particularly those of J. D. Smyth and colleagues (reviewed by Howell, 1995), which have demonstrated the plasticity and complexity of developmental processes. To some extent, observations in vitro have been complemented by studies in vivo although these have principally been confined to the metacestode stage. Studies on strobilar development of the adult parasite in vitro have demonstrated that extrinsic factors can have a profound influence on development, particularly with E. multilocularis, and differences in how the strobilar stages of E. granulosus and E. multilocularis respond to environmental triggers (Smyth and Davies, 1975; Thompson, Deplazes and Eckert, 1990; Constantine et al. 1998). In this respect, it was predicted that the definitive host would have a marked effect on both the induction of development and sequential processes (Smyth, 1969; Thompson, 1995).
There is no widely available laboratory host for maintaining infections with the adult parasite and the difficulties associated with maintaining infections in natural definitive hosts of Echinococcus spp. have limited in vivo investigations. Those that have been conducted have been of short duration, up to the initial onset of egg production, and little is known of subsequent development. Most comparative studies in the definitive host have concentrated on E. granulosus because of the longer pre-patent period available, up to 35 days, whereas with E. multilocularis, worms can commence the production of infective eggs as early as 25 days post-infection (Howell, 1995).
There is much to be gained from a better understanding of the development of E. multilocularis in the definitive host. This species has a lower definitive host specificity than E. granulosus, and from an epidemiological perspective, E. multilocularis is extending both its geographical and host range, particularly in Europe (Eckert, Conraths and Tackmann, 2000; Eckert and Deplazes, 2004; Deplazes et al. 2004; Sreter et al. 2003). In addition to the fox, dog and cat, the raccoon dog (Nyctereutes procyonoides), a known definitive host for E. multilocularis in Japan (Yimam et al. 2002), has recently been identified as a definitive host for E. multilocularis in Europe (Thiess et al. 2001). This may be of additional significance since the distribution of the raccoon dog in Europe is rapidly expanding and animals infected with E. multilocularis have been reported from as far east as Germany and Switzerland (Thiess et al. 2001; Deplazes, personal communication).
It has been difficult to compare and extrapolate results of previous experimental infections of definitive hosts with E. multilocularis from separate studies due to differences in age of the animals, the duration of infections, the parasite isolate and numbers of protoscoleces used. The only previous study to have compared development of E. multilocularis in the fox, dog and cat was that of Vogel in 1957. He demonstrated a ‘gradient’ of host suitability in terms of parasite development and maturation with the fox better than the dog, and both better than the cat. Subsequent studies have compared development of E. multilocularis in dogs (Yamashita, Ohbayashi and Kitamura, 1958), dogs and cats (Crellin, Marchiondo and Andersen, 1981; Thompson and Eckert, 1983; Kamiya et al. 1985; Kamiya, Ooi and Ohbayashi, 1986; Thompson, Deplazes and Eckert, 2003), foxes and dogs (Yagi, Ito and Ishige, 1996), foxes (Nonaka et al. 1996) and cats (Jenkins and Romig, 2000), and have reinforced the fox as representing the optimum host for E. multilocularis in terms of worm burden and development. However, few of these studies have allowed infections to reach patency, and questions also remain regarding the suitability of the dog and cat, and now, more recently, the raccoon dog.
The present study was thus undertaken in order better to understand the development of E. multilocularis in its definitive hosts beyond the pre-patent period.
MATERIALS AND METHODS
All experimental infections were undertaken at the Danish Centre for Experimental Parasitology where all animals used in this study (cats, dogs, foxes, raccoon dogs), were maintained. Fifteen red foxes (Vulpes vulpes, 7 male, 8 female) and 15 raccoon dogs (N. procyonoides, 8 male, 7 female) obtained from a large-scale Danish fur farm (Møldrup minkfarm), in addition to 15 cats (European short hair, 7 male, 8 female) and 15 dogs (FBI hounds, 7 male, 8 female) bred for experimental purposes by Harland (Cats: Harland Nederland, Horst, Netherlands; Dogs: Harland Sprague Dawley, Madison, Wisconsin, USA) were used for the study. Further details on husbandry can be found in Kapel et al. (2006). All animals were 13–15 weeks of age at the time of infection when each animal received 20000 protoscoleces of the same isolate of Echinococcus multilocularis orally, on the same day, via a stomach tube. The E. multilocularis isolate (IM280) used in this study was originally obtained from naturally infected water voles (Arvicola terrestris) from Zurich and subsequently passaged in the laboratory in jirds (Meriones unguiculatus) and field vole (Microtus arvalis) for less than 6 months.
Five of each species of definitive host were necropsied at each of 35, 63 and 90 days post- inoculation (PI). Worm counts were performed using the dilution technique (Eckert et al. 2001), and morphological features of the worms were determined on 50 randomly selected specimens, if sufficient worms were available, 10% formalin-fixed and stained worms per animal as previously described (Eckert et al. 1989).
Growth was assessed by measuring total length; segmentation by noting the number of segments in addition to the scolex, and maturation by determining the number of worms exhibiting the following stages: (i) testes containing spermatozoa, ovary, uterine streak and other female genitalia (T+Fg); (ii) dilating uterus (U); (iii) developing eggs in the dilating uterus (U+C); (iv) ‘thin-shelled’ (partly developed embryophore) eggs with a fully formed oncosphere in the uterus (U+E); (v) ‘thick-shelled’ (fully developed embryophore) eggs with a fully formed oncosphere in the uterus (U+TE). A formula was developed to express the degree of maturation, which we have called the Maturation Index. It was calculated as M=1/4(U+2C+3E+4T) where U is the proportion of worms with a uterus only, C is the proportion with a uterus with cells, E the proportion with thin-shelled eggs and T the proportion with thick-shelled eggs. A sample of worms, which includes only those not even containing a uterus would score 0. There would be a linear increase in score up to a value of 1 for a sample with all worms containing thick-shelled eggs. Differences between host species and infection periods were tested for significance using factorial analyses of variance followed by Newman-Keuls multiple range tests where appropriate (Zar, 1984).
RESULTS
Worm burdens declined in all hosts apart from dogs, and dogs maintained higher, and more consistent worm burdens compared to other hosts. The highest worm burdens were seen in foxes and raccoon dogs at 35 days PI (Fig. 1).

Fig. 1. Mean worm burden of Echinococcus multilocularis in each host individual at each of the 3 infection durations, and upper 95% confidence limits. At each infection duration, hosts are shown in ascending order of worm burden. A solid line connecting bars indicates a lack of significant difference between host means.
Apart from cats, most worms were found in the medial regions of the small intestine (Fig. 2). At 35 days post-infection in cats, the majority of worms were recovered from the distal regions of the small intestine whereas at 63 and 90 days they were recovered from the proximal and medial regions respectively.

Fig. 2. Comparison of intestinal distribution of Echinococcus multilocularis between proximal ([squf ]), medial () and distal (□) regions, in cats, dogs, raccoon dogs and foxes at 35, 63 and 90 days post-infection.
Worm growth, as reflected by total worm length, in foxes was initially more advanced than in other hosts. However, by day 63 worms in raccoon dogs were significantly longer than in other hosts. By day 90, growth in all hosts except cats was similar (Fig. 3). Worms from raccoon dogs were consistently more advanced in terms of segmentation throughout the infection period, with that in foxes and dogs showing more variability but similarly advanced compared to worms in cats whose rate of segmentation was less than that in the other 3 host species (Fig. 4).

Fig. 3. Mean worm length of Echinococcus multilocularis at 35, 63 and 90 days post-infection for each of the 4 host species. At each infection duration, hosts are shown in ascending order of worm burden. A solid line connecting bars indicates a lack of significant difference between host means.

Fig. 4. Mean number of segments per worm of Echinococcus multilocularis at 35, 63 and 90 days post-infection for each of the 4 host species. Results of statistical tests are illustrated in Fig. 2.
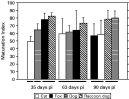
The maturation of worms at 35, 63 and 90 days PI, and the proportion of worms with thick-shelled eggs, are shown in Figs 5, 6 and 7. When worms at the different stages of maturation were compared (Fig. 5), egg production was found to be a continuous process throughout the 90-day period as demonstrated by the presence of a large proportion of worms at the early stages of egg production (U+C/U+E). Worms recovered from foxes and raccoon dogs exhibited rapid maturation, but whereas this was sustained in raccoon dogs it declined in foxes at 63 and 90 days (Figs 5, 6 and 7). Worms in dogs were not as mature as those in raccoon dogs and foxes at 35 days PI, but at 90 days, maturation in dogs and raccoon dogs was more advanced than in foxes and cats. The maturation and proportion of worms with thick-shelled eggs recovered from cats was consistently less advanced than worms in other hosts (Figs 5, 6 and 7).

Fig. 5. Diagram summarizing proportions of worms of Echinococcus multilocularis at different stages of maturation at 35, 63 and 90 days post-infection for each of the 4 host species.

Fig. 6. Mean values of the Maturation Index for Echinococcus multilocularis calculated for each infected host individual at 35, 63 and 90 days post-infection. Results of statistical tests are illustrated in Fig. 2.

Fig. 7. Mean percentage of Echinococcus multilocularis worms with eggs calculated for each infected host individual at 35, 63 and 90 days post-infection for each of the 4 host species. Results of statistical tests are illustrated as in Fig. 2.
DISCUSSION
This study has confirmed that the fox, dog and cat are susceptible to infection with E. multilocularis, and has demonstrated that the raccoon dog is also highly susceptible to infection with this parasite. All hosts, apart from cats, were capable of supporting substantial worm burdens, but these populations were sustained for longer in raccoon dogs and dogs compared to foxes. No worms were recovered from 1, 2 and 3 cats at 35, 63 and 90 days PI respectively, but it is not known whether worms initially established in these cats and were subsequently expelled (Kapel et al. 2006).
From an epidemiological perspective, the present results demonstrate that the fox and dog, in contrast to the cat, are both likely to play a significant role in the epidemiology of E. multilocularis infections in endemic regions in Europe. These results thus largely substantiate conclusions based on previous experimental studies in foxes, dogs and cats (Vogel, 1957; Crellin et al. 1981; Thompson and Eckert, 1983; Kamiya et al. 1985, 1986; Nonaka et al. 1996; Yagi et al. 1996; Jenkins and Romig, 2000; Thompson et al. 2003). The results also demonstrate the potential significance of the raccoon dog in the epidemiology of alveolar echinococcosis.
Results of the mean worm burden showed that numbers of worms were initially high in foxes but subsequently declined compared to dogs and raccoon dogs. A more detailed analysis by Kapel et al. (2006) found that foxes initially (35 days PI) harboured the highest numbers of gravid worms (40% of the total), but this was not sustained compared to raccoon dogs, and overall, the potential impact of raccoon dogs was equally as great when assessed over 90 days (8% of the total in foxes compared to 54% in raccoon dogs). This observation requires further study and comparative observations on infections of longer duration than 90 days are required. If validated by further investigation, the difference may be related to the ecological interactions between the fox and its short-lived rodent intermediate hosts, in terms of the availability of infected rodents. However, recent ecological studies in Switzerland suggest that this may not be the case in terms of either fluctuations in seasonal availability or levels of infection (Hofer et al. 2000; Stieger et al. 2002). Alternatively, there is the possibility that foxes may ‘self-cure’ one infection to facilitate establishment of a subsequent infection without competition between different populations of adult worms. The generation of immunity may impose a barrier to subsequent infections and data from field studies suggest that exposure to infection in young, sub-adult foxes may generate a partial immunity in adults (Hofer et al. 2000). However, there is little evidence that such responses are likely to be effective in terms of sterilizing immunity, and competitive interactions may play a more important role. In the case of concurrent infections with E. granulosus and E. multilocularis, they occupy different sites within the small intestine and thus separate physiological niches that limit competition (Thompson and Eckert, 1983; Howell, 1995). In contrast, an established infection with mature E. multilocularis may impose a competitive barrier to ‘incoming’ infections with the same species that has the same site specificity but possibly more demanding nutritional requirements during the early stages of establishment and maturation.
The raccoon dog, which has only recently (not in Japan) been identified as a definitive host for E. multilocularis in Europe, as in Japan (Yimam et al. 2002), is likely to exacerbate the already worsening problem caused by the spread of the fox in Europe. Although the raccoon dog occupies a similar ecological niche to that of foxes, particularly in terms of diet and urbanization, competition has not been observed (Kauhala, 1994). However, the raccoon dog has a greater reproductive potential than the fox and this may increase with the effects of global warming. This is because climate is likely to have a marked effect on the productivity of the raccoon dog (Kauhala, 1994; Mustonen et al. 2004), especially on the proportion of reproducing females and annual birth rate. There will thus be greater opportunities for the raccoon dog to increase its numbers and continue to extend its range in Europe.
The intestinal distribution of adult E. multilocularis in foxes, dogs and raccoon dogs was reasonably similar with decreasing proportions in the posterior segment over time. This pattern may be indicative of resource competition in the more proximal, high intensity regions of the small intestine, which were maintained in the early stages of the infection when development needs are greatest, resulting in displacement of worms to the distal less favoured region of the small intestine.
The cat clearly has little potential to play any significant epidemiological role in maintaining the life-cycle of E. multilocularis in endemic areas, but since worms in cats are capable of producing embryonated thick-shelled eggs they could be a risk to public health as a source of E. multilocularis infection in domestic environments. However, total egg excretion in cats was around 600–1000 times lower than in the other three species and eggs isolated from gravid worms of the cats were not infective to mice (Kapel et al. 2006).
The differences in development of E. multilocularis in foxes compared to dogs complement results on egg production reported by Kapel et al. (2006). Compared to worm establishment and survival in foxes, the mean worm number was initially reduced in dogs but persisted without significant reduction up to 90 days PI. Despite an approximately 7-fold lower mean worm burden in dogs, only a reduction of around 30% of the total egg number excreted was found in dogs compared to foxes. Furthermore, the time to excrete 95% of the total egg biomass was longer in dogs than in foxes which again fits with the results of worm development in dogs.
The results presented here have also provided valuable data on the developmental processes of E. multilocularis in vivo. The development of adult Echinococcus involves germinal and somatic differentiation and can be divided into the following 4 processes: growth, segmentation, proglottization, and maturation (Thompson, 1995). Germinal differentiation comprises proglottization, which refers to the sequential formation of new reproductive units (proglottids), and the maturation of the proglottids. Somatic differentiation consists of growth, comprising increase in size, and the somatic delineation of each proglottid by segmentation (strobilization). Studies on the strobilar development of E. granulosus and E. multilocularis in vitro in which worms grew and/or segmented without exhibiting signs of maturation, or exhibited proglottization and maturation without segmentation, have demonstrated that these four developmental processes can take place independently (Smyth, 1971; Smyth and Davies, 1975; Smyth and Barrett, 1979; Thompson et al. 1990). This has been supported by the results of the present study.
By comparing developmental processes in different hosts and examining germinal and somatic differentiation, our results have confirmed that these processes can be influenced by their environment; in this case the small intestine of different carnivore host species. In cats, the investment by worms in somatic processes, growth and segmentation, was not complemented in terms of maturation. Similarly, in foxes, growth and segmentation were very similar to that of worms in dogs and raccoon dogs throughout the period of infection whereas maturation declined markedly by 90 days PI in foxes. The fact that the parasite will put more effort into somatic (growth and segmentation) compared to germinal (maturation) differentiation in one host than another is intriguing. From the parasite's perspective, this would seem to be a waste of resources in terms of perpetuating the species. It is tempting to suggest that this may reflect a less balanced host/parasite relationship and that in situations where worms expend more resources on somatic than germinal differentiation, which is related to transmission potential, it may reflect the fact that the host parasite relationship is still evolving, as with the case of the dog and raccoon dog. However, in foxes, the decline in maturation by 90 days may be related to senescence and enhancing opportunities for subsequent infections. Alternatively, ‘crowding’ factors may come in to play and such effects may not be evident in infections of lower worm burdens.
The present results support the hypothesis that developmental processes in Echinococcus are independent and as such open the way to trying to determine the nature of the complex regulatory switches that may be involved in controlling development and differentiation in Echinococcus. It has been over 30 years since such a proposal was advocated on the basis of in vitro observations (Smyth, 1969), but it is only relatively recently that appropriate molecular ‘tools’ have become available that offer the potential to identify and characterize the genetic mechanisms involved. Research over the last 10 years has identified a diversity of genes that appear to be involved in development, differentiation and the control of gene expression in Echinococcus (Ferreira and Zaha, 1990; Oliver et al. 1992; da Silva et al. 1993; Martinez et al. 1997; Esperon et al. 2000). Signalling proteins associated with growth factor regulation, that are thought to be involved in developmental processes, have also been identified in E. multilocularis (Zavala-Góngora et al. 2003). However, it is only recently that a comparative approach has been taken. Stage-specific differential gene expression has been demonstrated in E. multilocularis and evidence provided indicating an involvement of parasite-determined, epidermal growth factor-like signal transduction systems in metacestode proliferation and development (Brehm et al. 2003).
Recent research has also demonstrated differences in mRNA expression between immature and mature adult worms of E. granulosus (Zhang et al. 2003). Although seemingly involved in the regulation of egg development, the functional significance of these observations remain to be determined but certainly offer promise in providing a better understanding of the regulation of development in Echinococcus.