Introduction
Maternal infection during pregnancy with Toxoplasma gondii, rubella, cytomegalovirus (CMV), herpes simplex virus (HSV) and other microbes (i.e. TORCH infections) have long been known to be associated with mental retardation, cerebral hypoplasia, ventriculomegaly and other brain and behavioural abnormalities in the offspring (Remington et al. Reference Remington, Klein, Wilson and Baker2006). The neurodevelopmental theory of schizophrenia postulates abnormalities in early neurodevelopment as a possible cause of the disorder (Murray & Lewis, Reference Murray and Lewis1987; Weinberger, Reference Weinberger1987). Empirical evidence for this theory comes from birth cohort studies demonstrating delays in motor milestone, and deficits in pre-morbid cognitive function in future cases of schizophrenia (Jones et al. Reference Jones, Rodgers, Murray and Marmot1994; Khandaker et al. Reference Khandaker, Barnett, White and Jones2011). Consequently, several studies have investigated links between maternal infection during pregnancy and risk of schizophrenia in adult offspring.
Many of the early investigations on this topic were based on data from influenza epidemics (Mednick et al. Reference Mednick, Machon, Huttunen and Bonett1988), which yielded conflicting results (Selten et al. Reference Selten, Frissen, Lensvelt-Mulders and Morgan2010). These studies defined maternal exposure to influenza as being pregnant at the time of an epidemic rather than direct measurement of exposure at the individual level. Thus, the ecological fallacy could be a reason for their discrepant findings (Selten et al. Reference Selten, Frissen, Lensvelt-Mulders and Morgan2010). More recent studies based on general population cohorts have overcome this issue. These have used objective measures to determine exposure to infection during the prenatal period at the individual level (Susser, Reference Susser1999; Susser et al. Reference Susser, Schaefer, Brown, Begg and Wyatt2000; Buka et al. Reference Buka, Tsuang, Torrey, Klebanoff, Bernstein and Yolken2001a; Mortensen et al. Reference Mortensen, Norgaard-Pedersen, Waltoft, Sorensen, Hougaard, Torrey and Yolken2007; Susser et al. Reference Susser, Buka, Schaefer, Andrews, Cirillo, Factor-Litvak, Gillman, Goldstein, Ivey Henry, Lumey, McKeague, Michels, Terry and Cohn2011). Findings from these studies merit further attention, as they may provide a better understanding of the true nature of this association.
A previous review implicated several prenatal maternal infections with increased risk of schizophrenia in adult offspring (Brown & Derkits, Reference Brown and Derkits2010). However, fundamental questions have yet to be settled. These include the timing of prenatal infection or any links between prenatal infection and structural and functional brain abnormalities associated with schizophrenia. These issues are important because they would provide important clues to the relevance of prenatal infections in the causation of adult psychotic disorders. Therefore, associations between prenatal infection and neurodevelopmental outcomes in offspring are of much interest in current epidemiological and preclinical research (Meyer & Feldon, Reference Meyer and Feldon2010; Brown et al. Reference Brown, Vinogradov, Kremen, Poole, Bao, Kern and McKeague2011). We have carried out a systematic review of robust population-based studies to address the following issues: (i) association between adult psychotic illness and specific types of prenatal maternal infection, (ii) association between risk and timing of prenatal infection, and (iii) effects of prenatal infection on neurodevelopment. We have included three new studies published on this topic since the most recent review, contributing evidence on a further 4000 cases and more than 1.1 million controls (Xiao et al. Reference Xiao, Buka, Cannon, Suzuki, Viscidi, Torrey and Yolken2009; Mortensen et al. Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010; Nielsen et al. Reference Nielsen, Laursen and Mortensen2011). Findings from these studies provide a new insight into the nature of the association between prenatal maternal infection and psychosis in offspring. We also discuss the biological plausibility of such associations in view of recent human and preclinical research.
Method
Search strategy
We searched Medline, PubMed and EMBASE databases from their respective inceptions to November 2011 for studies of prenatal infection that were based on human samples and published in the English language. Search terms included indexing terms (e.g. MeSH) and free texts: [(prenatal OR in utero OR foetal OR maternal) AND (infection OR inflammation OR illness) AND (schizophrenia OR psychotic disorder)]. We also identified potential studies in the reference lists of included studies and wrote to prominent authors in this field for any further relevant studies.
Study selection
Included studies (i) were based on general population datasets, (ii) cohort or nested case–control in design, (iii) used serological assays or clinical examination to determine exposure to infection during the prenatal period at the individual level and (iv) used contemporary ICD or DSM directions to define the outcome of schizophrenia, non-affective psychosis, affective psychosis and other psychotic disorders. Studies that used self-report of exposure to infections instead of clinical examination or serology were excluded. Genetic high-risk studies and studies that used aggregate data to define exposure, for example pregnancy at the time of an epidemic or incidence or prevalence of certain infection during pregnancy rather than direct measurement of infection at the individual level, were also excluded.
Data extraction
Electronic search and study selection were carried out by two researchers working independently (G.M.K. and J.Z.). They examined all titles and abstracts, obtained full texts of potentially relevant papers and applied inclusion criteria. Any differences in opinion were resolved through discussions with the wider study team. All studies that met inclusion criteria were critically appraised using the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) checklists for observational studies (von Elm et al. Reference von Elm, Altman, Egger, Pocock, Gøtzsche and Vandenbroucke2007), with focus on sampling, measurement of exposure and outcome, follow-up, attrition, analytical strategy, bias, confounding and other methodological issues. At this stage, studies with serious methodological concerns that might have biased their findings were identified and excluded from the review.
Data synthesis and rationale for not proceeding to meta-analysis
There was considerable variation among selected studies with regard to design, measurement of prenatal infection, definition of outcome and methods of case ascertainment. For example, three studies investigated links between schizophrenia and maternal exposure to HSV type 2 (HSV-2) during the prenatal period. To define exposure, one study used the ratio of anti-HSV-2 immunoglobulin (Ig)G concentration between cases and controls (Buka et al. Reference Buka, Cannon, Torrey and Yolken2008). The rest of the studies used absolute concentration of IgG in maternal serum (Brown et al. Reference Brown, Schaefer, Quesenberry, Shen and Susser2006; Mortensen et al. Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010). Again, the cut-off values used to define exposure were not the same between these two studies (issues around exposure measurement are considered in detail in the discussion section). Definitions of outcomes were also different. For example, two studies used non-affective psychosis by DSM-IV (Brown et al. Reference Brown, Schaefer, Quesenberry, Shen and Susser2006; Buka et al. Reference Buka, Cannon, Torrey and Yolken2008) whereas one used schizophrenia by ICD-10 (Mortensen et al. Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010). Such differences in exposure and outcome measurements were also observed for other infections. Because of this heterogeneity the results of meta-analyses would be difficult to interpret and we therefore present a descriptive review of the studies.
Results
Twenty-one studies were included in the review (Fig. 1). There were 16 studies concerning risk of adult psychotic illness. One of these studies also examined childhood IQ in relation to exposure to influenza in the prenatal period. Five studies looked exclusively into structural and functional brain abnormalities related to schizophrenia. Excluded studies with reasons for exclusion are presented as online supplementary material in Table S1.
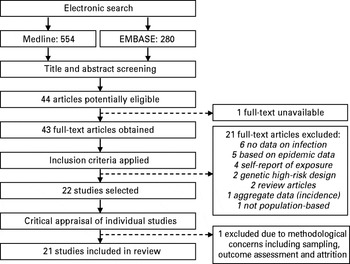
Fig. 1. Study selection process for the systematic review.
Prenatal maternal infection and schizophrenia and other psychoses in adult offspring
We reviewed 16 studies on this topic (Brown et al. Reference Brown, Schaefer, Wyatt, Goetz, Begg, Gorman and Susser2000, Reference Brown, Begg, Gravenstein, Schaefer, Wyatt, Bresnahan, Babulas and Susser2004a, Reference Brown, Hooton, Schaefer, Zhang, Petkova, Babulas, Perrin, Gorman and Susserb, Reference Brown, Schaefer, Quesenberry, Liu, Babulas and Susser2005, Reference Brown, Schaefer, Quesenberry, Shen and Susser2006; Buka et al. Reference Buka, Tsuang, Torrey, Klebanoff, Bernstein and Yolken2001a, Reference Buka, Tsuang, Torrey, Klebanoff, Wagner and Yolkenb, Reference Buka, Cannon, Torrey and Yolken2008; Babulas et al. Reference Babulas, Factor-Litvak, Goetz, Schaefer and Brown2006; Mortensen et al. Reference Mortensen, Norgaard-Pedersen, Waltoft, Sorensen, Hougaard, Torrey and Yolken2007; Clarke et al. Reference Clarke, Tanskanen, Huttunen, Whittaker and Cannon2009; Ellman et al. Reference Ellman, Yolken, Buka, Torrey and Cannon2009; Sorensen et al. Reference Sorensen, Mortensen, Reinisch and Mednick2009; Xiao et al. Reference Xiao, Buka, Cannon, Suzuki, Viscidi, Torrey and Yolken2009; Mortensen et al. Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010; Nielsen et al. Reference Nielsen, Laursen and Mortensen2011). Eleven of these studies were based on two US birth cohorts, the National Collaborative Perinatal Project (NCPP) cohort (Susser, Reference Susser1999; Susser et al. Reference Susser, Buka, Schaefer, Andrews, Cirillo, Factor-Litvak, Gillman, Goldstein, Ivey Henry, Lumey, McKeague, Michels, Terry and Cohn2011) and the Prenatal Determinants of Schizophrenia (PDS) cohort (Susser et al. Reference Susser, Schaefer, Brown, Begg and Wyatt2000). The PDS cohort (Susser et al. Reference Susser, Schaefer, Brown, Begg and Wyatt2000) was derived from the Child Health and Development Study (CHDS; van dan Berg et al. Reference van dan Berg, Christianson and Oeschsli1988), a prospective birth cohort study of factors affecting outcomes of pregnancy and child development. The NCPP is a multicentre prospective cohort study involving 13 sites. Studies included in the present review are all based on the samples from Providence, Boston and Philadelphia; together, the first two sites are known as the New England Family Study (Susser, Reference Susser1999; Susser et al. Reference Susser, Buka, Schaefer, Andrews, Cirillo, Factor-Litvak, Gillman, Goldstein, Ivey Henry, Lumey, McKeague, Michels, Terry and Cohn2011). The remaining five studies were based on general population cohorts from Denmark and Finland. Characteristics of these cohorts and assessment of exposure and outcome in studies based on these cohorts are presented in Table 1.
Table 1. Cohort characteristics and assessment of exposure and outcome in studies of prenatal infection

NCPP, National Collaborative Perinatal Project; PDS, Prenatal Determinants of Schizophrenia; DIGS, Diagnostic Interview for Genetic Studies; SSD, schizophrenia spectrum disorder.
a Includes schizophrenia, schizo-affective, other non-affective psychosis, schizo-affective bipolar type, bipolar disorder with psychotic features, major depressive disorder with psychotic features and psychosis not otherwise specified (NOS).
b Includes schizophrenia, delusional disorder, psychotic disorder NOS, schizo-affective disorder and schizotypal personality disorder.
To define exposure, five studies used prospectively collected records of clinically diagnosed infection. Nine studies used serological assays for various antimicrobial antibodies in maternal blood samples obtained during pregnancy, and a further two used inflammatory cytokines as a proxy of exposure to infections. Serological studies were all nested case–control in design whereas the studies of clinically diagnosed infection involved analyses of entire cohorts. We have summarized the results of ‘serological’ and ‘clinically diagnosed’ studies separately, including effect of adjustment for confounding factors in Table 2 (for an extended version of this table see Table S2 in the online supplementary material). Strengths and weaknesses of individual studies are presented in Table 3.
Table 2. Population-based studies of prenatal infection and schizophrenia and other psychosis

NCPP, National Collaborative Perinatal Project; PDS, Prenatal Determinants of Schizophrenia; SSD, schizophrenia spectrum disorder; CMV, cytomegalovirus; Ig, immunoglobulin; HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2; IL, interleukin; TNF, tumour necrosis factor; OR, odds ratio; CI, confidence interval.
a Maternal respiratory infections included tuberculosis, influenza, influenza with pneumonia, bronchopneumonia, atypical pneumonia, pleu-risy, emphysema/viral respiratory infections, acute bronchitis, and upper respiratory infections.
b Genital/reproductive infections included endometritis, cervicitis, pelvic inflammatory disease, vaginitis, syphilis, condylomata, ‘venereal disease’ and gonorrhoea. Periconceptional period = 30 days before and after last menstrual period.
c Bacterial infections included sinusitis, tonsillitis, pneumonia, cystitis, pyelonephritis, bacterial venereal infection, and any other bacterial infection. A viral infection was judged to be present if (1) a medical diagnosis had been made (commonly by the general practitioner) or (2) symptoms consistent with minor respiratory illnesses or influenza had been present and the mother had been confined to bed and had a rise in temperature to at least 38 °C.
d Infections include general infections, skin infections, respiratory infections, infections related to puerperium (mothers), genital and other infections.
Table 3. Strengths and weaknesses of individual studies

NCPP, National Collaborative Perinatal Project; PDS, Prenatal Determinants of Schizophrenia; IgG, immunoglobulin G; TNF-α, tumour necrosis factor-α.
Serologically determined prenatal maternal infection
HSV
Three out of five studies reported a statistically significant increased risk of schizophrenic psychosis in the adult offspring for exposure to HSV-2 infection during pregnancy (Buka et al. Reference Buka, Tsuang, Torrey, Klebanoff, Bernstein and Yolken2001a, Reference Buka, Cannon, Torrey and Yolken2008; Mortensen et al. Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010). These studies used elevated levels of IgG antibodies to HSV-2 to define exposure, that is seropositivity. A recent Danish study involving 602 cases reported a statistically significant 50% increased risk of adult schizophrenia for prenatal exposure to HSV-2 (Table 2). Risk was slightly attenuated (but still statistically significant) only after adjustment for family history of mental illness (Mortensen et al. Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010). This risk estimate is in line with a previous report from the NCPP birth cohort (Buka et al. Reference Buka, Cannon, Torrey and Yolken2008).
One study from the PDS cohort reported a 30% increase in risk of schizophrenia spectrum disorder (SSD) for maternal HSV-2 seropositivity during late pregnancy (Brown et al. Reference Brown, Schaefer, Quesenberry, Shen and Susser2006), but this was not statistically significant. The study also reported similar results for exposures to HSV-1 and CMV. A Danish study also reported no increase in risk of schizophrenia for prenatal exposure to HSV-1 and -2 (Mortensen et al. Reference Mortensen, Norgaard-Pedersen, Waltoft, Sorensen, Hougaard, Torrey and Yolken2007).
T. gondii
Elevated levels of maternal IgG antibodies to T. gondii were reported to be associated with increased risk of schizophrenic psychosis from two cohorts (Brown et al. Reference Brown, Schaefer, Quesenberry, Liu, Babulas and Susser2005; Mortensen et al. Reference Mortensen, Norgaard-Pedersen, Waltoft, Sorensen, Hougaard, Torrey and Yolken2007). One study from the PDS cohort (Brown et al. Reference Brown, Schaefer, Quesenberry, Liu, Babulas and Susser2005) found a more than twofold increase in risk of SSD for maternal ‘high’ IgG antibody titre (⩾1:128) during late pregnancy, compared to ‘low’ titre (<1:16), with an odds ratio (OR) of 2.61 and 95% confidence interval (CI) 1.00–6.82. Similar findings (OR 1.79) were observed in the Danish study (Mortensen et al. Reference Mortensen, Norgaard-Pedersen, Waltoft, Sorensen, Hougaard, Torrey and Yolken2007), which also suggested that elevated maternal IgG to T. gondii was associated specifically with risk of schizophrenia, out of all four outcomes included in the analysis.
One study involving the largest number of cases (n = 219) and controls to date investigated prenatal exposure to three specific serotypes of T. gondii in relation to affective and non-psychosis in adult offspring (Xiao et al. Reference Xiao, Buka, Cannon, Suzuki, Viscidi, Torrey and Yolken2009). Infection with T. gondii Type I (out of all three serotypes) was reported to be associated with increased risk of affective psychosis (more than fivefold) but not non-affective psychosis (Table 2). However, analysis of non-affective psychosis was based on an insufficient sample size of 11 cases and 29 controls.
Influenza
Two studies were available. One was based on the PDS birth cohort and reported a sevenfold increased risk of SSD for exposure during the first trimester and a threefold increased risk for exposure during the first half of pregnancy (Brown et al. Reference Brown, Begg, Gravenstein, Schaefer, Wyatt, Bresnahan, Babulas and Susser2004a). However, the sample size was relatively small and none of the risk estimates were statistically significant (Table 2). There was no increase in risk for exposure during late pregnancy. The second study was from the NCPP birth cohort and it assayed maternal blood samples collected at delivery for IgG antibodies to influenza B virus and reported a non-significant 70% increase in risk of schizophrenia in the adult offspring (Ellman et al. Reference Ellman, Yolken, Buka, Torrey and Cannon2009).
Inflammatory cytokines
One study from the NCPP cohort suggested that inflammatory cytokines may mediate risk of psychosis related to prenatal infection (Buka et al. Reference Buka, Tsuang, Torrey, Klebanoff, Wagner and Yolken2001b). Among 27 cases of affective and non-affective psychosis and 54 matched controls, Buka et al. (Reference Buka, Tsuang, Torrey, Klebanoff, Wagner and Yolken2001b) found that mean tumour necrosis factor (TNF)-α level was significantly higher in case mothers. Furthermore, adult offspring of women with both clinical report of third trimester infection and elevated TNF-α level were eight times more likely to develop psychosis. However, this analysis was based on a small sample. A similar study based on the PDS cohort reported a nearly twofold increase in mean and median values of interleukin (IL)-8 during mid- to late pregnancy in SSD case mothers (Brown et al. Reference Brown, Hooton, Schaefer, Zhang, Petkova, Babulas, Perrin, Gorman and Susser2004b). This association was unchanged in analysis restricted to cases of schizophrenia only.
Clinically diagnosed prenatal maternal infection
General infections
In a large Danish cohort maternal hospitalization for infection during pregnancy was associated with increased risk of schizophrenia in offspring [risk ratio (RR) 1.39, 95% CI 1.18–1.62; Nielsen et al. Reference Nielsen, Laursen and Mortensen2011]. Only second-trimester infection was statistically significant (RR 1.72, 95% CI 1.15–2.46). Of note, maternal infection any time in the past (before, during or after pregnancy combined) was also associated with increased risk of schizophrenia (RR 1.24, 95% CI 1.16–1.33). Maternal infection during pregnancy was not significantly different from that before or after pregnancy in terms of offspring's risk of schizophrenia (Nielsen et al. Reference Nielsen, Laursen and Mortensen2011).
In the Copenhagen Perinatal Cohort bacterial infection during the first trimester was reported to be associated with a nearly twofold increased risk of schizophrenia at follow-up at both age 34 and 47 years (Sorensen et al. Reference Sorensen, Mortensen, Reinisch and Mednick2009) (Table 1). Risk was also increased, but not statistically significant, for second-trimester exposure to bacterial infections. There was no significant increase in risk for viral infections. However, broad definitions of bacterial and viral infection were used in this cohort.
Respiratory infection
In an analysis of the entire PDS cohort, maternal respiratory infection during the second trimester was reported to be associated with a twofold increased risk of SSD in adult offspring (Brown et al. Reference Brown, Schaefer, Wyatt, Goetz, Begg, Gorman and Susser2000). A similar increase in risk was observed for schizophrenia. Most exposed cases suffered from an upper respiratory tract infection. Similarly, in the Copenhagen Perinatal Cohort maternal upper respiratory infection during any time in pregnancy was associated with a threefold increased risk of schizophrenia in the offspring by age 47 years.
Genital, reproductive and urinary infection
Maternal genital or reproductive infection during the periconceptional period (i.e. 30 days before and after the last menstrual period) was associated with a fivefold increased risk of SSD in adult offspring in the PDS cohort (Babulas et al. Reference Babulas, Factor-Litvak, Goetz, Schaefer and Brown2006). However, there was no increase in risk for such infections during pregnancy (first, second or third trimester). In another study, maternal hospital admission for genital infection during pregnancy was associated with a twofold increased risk of schizophrenia in adult offspring (Nielsen et al. Reference Nielsen, Laursen and Mortensen2011). Similarly, in the Copenhagen Perinatal Cohort maternal gonococcal infection during any time in pregnancy was associated with a more than threefold increased risk of schizophrenia by age 47 years (Sorensen et al. Reference Sorensen, Mortensen, Reinisch and Mednick2009). One Finnish study did not find any significant association between maternal hospitalization with pyelonephritis during pregnancy and risk of schizophrenia in the offspring (Clarke et al. Reference Clarke, Tanskanen, Huttunen, Whittaker and Cannon2009).
Timing of prenatal maternal infection and risk of adult schizophrenia
Most studies lacked power to address the issue of a ‘sensitive period’, that is maternal infection during which stage of pregnancy may be most harmful. However, in general we observed a trend for greater risk of adult psychotic illness for exposure to infections during early stages of gestation (Table 4). This needs to be interpreted in the context of significant variation among studies in design, exposure and outcome measurement.
Table 4. Timing of prenatal infection and risk of schizophrenia and other psychotic disorders in adult offspring

NCPP, National Collaborative Perinatal Project; PDS, Prenatal Determinants of Schizophrenia; HSV-2, herpes simplex virus type 2.
a Only studies that reported an increase in risk in relation to prenatal infection were included in this table; approximate point estimates shown for guide purpose only; heterogeneity in exposure measurement, case definition, analysis and measure of risk exists between individual studies, refer to Table 1 for individual study details and results.
b Early second-trimester exposure.
Interaction between prenatal maternal infection and other risk factors
Family history of psychosis
Using an additive model, a Finnish study reported evidence of an interaction between family history of psychosis and maternal pyelonephritis during pregnancy (Clarke et al. Reference Clarke, Tanskanen, Huttunen, Whittaker and Cannon2009). In a stratified analysis, the risk difference between those exposed and those unexposed to prenatal infection in the group with a family history of psychosis was five times larger than the risk difference in those without such history.
Preterm birth
In the largest study of HSV-2, involving 602 cases, there was an indication that the incidence rate ratio (IRR) for schizophrenia is slightly higher among exposed individuals born before 36 weeks of gestation (IRR 3.68, 95% CI 0.76–17.79) compared to those born at term (IRR 1.52, 95% CI 1.13–2.03). However, this estimate was based on small numbers: only seven exposed cases and two exposed controls were born preterm (Mortensen et al. Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010).
Maternal sexual and reproductive behaviour
One study from the NCPP birth cohort reported that the mother's sexual behaviour prior to and during pregnancy was relevant to HSV-2 seropositivity and subsequent risk of psychotic illness in the offspring (Buka et al. Reference Buka, Cannon, Torrey and Yolken2008). Rates of HSV-2 seropositivity at delivery were similar among case and control mothers who reported use of contraception or lower frequency of intercourse during pregnancy. By contrast, case mothers who reported frequent intercourse or no use of contraception were twice as likely to be seropositive than control mothers.
Prenatal maternal infection and structural and functional brain abnormalities in schizophrenia
We identified six population-based studies, mostly from the PDS and NCPP birth cohorts (Table 5) (Brown et al. Reference Brown, Cohen, Harkavy-Friedman, Babulas, Malaspina, Gorman and Susser2001, Reference Brown, Deicken, Vinogradov, Kremen, Poole, Penner, Kochetkova, Kern and Schaefer2009a, Reference Brown, Vinogradov, Kremen, Poole, Deicken, Penner, McKeague, Kochetkova, Kern and Schaeferb, Reference Brown, Vinogradov, Kremen, Poole, Bao, Kern and McKeague2011; Ellman et al. Reference Ellman, Yolken, Buka, Torrey and Cannon2009, Reference Ellman, Deicken, Vinogradov, Kremen, Poole, Kern, Tsai, Schaefer and Brown2010). Maternal infection or increased inflammatory cytokines during pregnancy were reported to be associated with both structural and functional brain phenotypes relevant to schizophrenia in the offspring.
Table 5. Prenatal infection and structural and functional brain abnormalities relevant to schizophrenia

PDS, Prenatal Determinants of Schizophrenia; SSD, schizophrenia and spectrum disorder; NCPP, National Collaborative Perinatal Project; WISC, Wechsler Intelligence Scale for Children; IL-8, interleukin 8; IgG, immunoglobulin G; CSP, cavum septum pallucidum; s.d., standard deviation; SMD, standardized mean difference.
Exposed cases were reported to show significant deficit in childhood and adult verbal IQ, and greater IQ decline during the pre-morbid period. Exposed cases were also reported to show increased ventricular volume, increased length of the cavum septum pallucidum, reduced cortical volume and deficits in executive function in adulthood. However, prenatal infections did not seem to affect some of these parameters, such as childhood verbal IQ or adult ventricular and cortical volume in exposed healthy controls (Ellman et al. Reference Ellman, Yolken, Buka, Torrey and Cannon2009, Reference Ellman, Deicken, Vinogradov, Kremen, Poole, Kern, Tsai, Schaefer and Brown2010). Some of these studies did not include a healthy comparison group (Brown et al. Reference Brown, Deicken, Vinogradov, Kremen, Poole, Penner, Kochetkova, Kern and Schaefer2009a, Reference Brown, Vinogradov, Kremen, Poole, Bao, Kern and McKeague2011).
Discussion
A possible role of infection and immunity in the aetiology of psychotic illness has been in the focus of neuropsychiatric research for more than a century (Menninger, Reference Menninger1994). Potential disruption of foetal development from prenatal maternal infections is in keeping with the neurodevelopmental theory of schizophrenia. Our review of robust epidemiological evidence suggests that exposure to infectious agents or inflammatory response during pregnancy may be associated with schizophrenic psychosis in the adult offspring.
Risk of schizophrenia in adult offspring was examined in relation to maternal non-specific bacterial, respiratory or genital and reproductive infection during pregnancy. The results differed between studies, within and across infections, with an upper range of a two- to fivefold increased risk in all of the reports reviewed. With regard to particular infectious agents, evidence for HSV-2 and T. gondii was mixed, some studies reporting up to a twofold increased risk of schizophrenic psychosis. There was some indication that exposure to influenza, and other infections in general, during early stages of gestation may be more harmful. No evidence of an association was found between prenatal HSV-1 or CMV infection and adult psychotic illness. There was some suggestion that inflammatory cytokines may mediate risk of psychotic illness associated with prenatal infection. With regard to neurodevelopment, structural and functional brain abnormalities relevant to schizophrenia were reported in exposed cases.
Methodological issues that might have influenced the findings are discussed in the following sections.
Study populations
Much of the evidence on this topic comes from two US birth cohorts from which 15 reports were included (10 from the PDS cohort and five from the NCPP cohort). We observed overlap of participants between the reports of individual infectious exposures within each cohort. Five studies that reported associations with T. gondii, influenza, respiratory infection, genital/reproductive infection and IL-8 are based on the same 71 cases of SSD from the PDS cohort (Brown et al. Reference Brown, Schaefer, Wyatt, Goetz, Begg, Gorman and Susser2000, Reference Brown, Begg, Gravenstein, Schaefer, Wyatt, Bresnahan, Babulas and Susser2004a, Reference Brown, Hooton, Schaefer, Zhang, Petkova, Babulas, Perrin, Gorman and Susserb, Reference Brown, Schaefer, Quesenberry, Liu, Babulas and Susser2005; Babulas et al. Reference Babulas, Factor-Litvak, Goetz, Schaefer and Brown2006). Cases in four studies of structural and functional brain abnormalities related to prenatal infection also came from the same pool (Brown et al. Reference Brown, Deicken, Vinogradov, Kremen, Poole, Penner, Kochetkova, Kern and Schaefer2009a, Reference Brown, Vinogradov, Kremen, Poole, Deicken, Penner, McKeague, Kochetkova, Kern and Schaeferb, Reference Brown, Vinogradov, Kremen, Poole, Bao, Kern and McKeague2011; Ellman et al. Reference Ellman, Deicken, Vinogradov, Kremen, Poole, Kern, Tsai, Schaefer and Brown2010). A similar overlap of cases was noted in some studies from the NCPP cohort (Buka et al. Reference Buka, Tsuang, Torrey, Klebanoff, Bernstein and Yolken2001a,Reference Buka, Tsuang, Torrey, Klebanoff, Wagner and Yolkenb, Reference Buka, Cannon, Torrey and Yolken2008; Ellman et al. Reference Ellman, Yolken, Buka, Torrey and Cannon2009; Xiao et al. Reference Xiao, Buka, Cannon, Suzuki, Viscidi, Torrey and Yolken2009).
It is possible that the individual infections were allocated randomly among the mothers. Alternatively, exposure to several infections in the same group of case mothers would suggest that related factors, such as lifestyle, may also be important. Indeed, in the NCPP cohort, the mother's sexual behaviour during pregnancy was found to be associated with HSV-2 infection and subsequent risk of schizophrenia in the adult offspring (Buka et al. Reference Buka, Cannon, Torrey and Yolken2008). Alternatively, or in addition, clusters of infections in a few mothers might suggest an innate susceptibility to infection that may itself be related to schizophrenia risk (discussed in biological plausibility). Regardless of the precise explanation for the associations, the validity of some the findings from the NCPP and PDS cohorts has been assisted by independent replications from large Danish datasets.
Because of the small sample size most studies could not address the issue of a sensitive period, with infection being harmful only during a particular stage of gestation. Sample sizes in studies of structural and functional brain abnormalities were also often small. None of them included any mediation model to properly explore the effects of prenatal infection on other indicators of schizophrenia.
Measurement of exposure
Selecting the criteria for exposure in serological studies can be difficult. For example, before deciding on cut-off values for antibodies to infectious agents, such as T. gondii or HSV, the prevalence of that infection in the population at the time and geographical region needs to be considered. Such considerations were given in some of the US studies and the Danish register-based studies.
IgG and IgM antibodies are both generated in response to infection (Janeway et al. Reference Janeway, Travers, Walport and Shlomchik2001). IgM immunoglobulins are usually generated within a few days following systemic infection and are detectable for several months whereas IgG immunoglobulins are generated 1–3 weeks after initial infection and are detectable for several years (Buka et al. Reference Buka, Tsuang, Torrey, Klebanoff, Bernstein and Yolken2001a; Janeway et al. Reference Janeway, Travers, Walport and Shlomchik2001). None of the studies of HSV-2 or T. gondii reported an increase in specific IgM antibodies, suggesting acute infections with these agents during pregnancy were unlikely.
Misclassification of exposure may be an issue in some studies. For example, in the Copenhagen Perinatal Cohort relatively broad categories of exposure were used (clinically diagnosed viral and bacterial infection) (Sorensen et al. Reference Sorensen, Mortensen, Reinisch and Mednick2009). Random misclassification would minimize the effect size by introducing bias results towards null.
The source of the prenatal sample may also be an issue. Studies from the NCPP and PDS cohorts used maternal serum samples that were stored at −20 °C for >20 years. Degradation of antibodies and cytokines over time is possible; however, the authors reported that there was no evidence of freeze thawing (Buka et al. Reference Buka, Tsuang, Torrey, Klebanoff, Bernstein and Yolken2001a; Brown et al. Reference Brown, Begg, Gravenstein, Schaefer, Wyatt, Bresnahan, Babulas and Susser2004a). The Danish register-based studies used blood samples collected from neonates 5–7 days post-partum (Mortensen et al. Reference Mortensen, Norgaard-Pedersen, Waltoft, Sorensen, Hougaard, Torrey and Yolken2007, Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010). However, it is highly likely that antibodies against HSV-2 or T. gondii in these samples represent foetal exposure to maternal infection rather than newly acquired infection after birth. Therefore, these can be regarded as valid measures of prenatal exposure to these infections.
Measurement of outcome
Broad outcomes were used in some cohorts (Table 2). Although studies from the NCPP cohort included both affective and non-affective psychosis, the outcome in the PDS cohort was non-affective psychosis only. This made examination of any links between an infection and specific diagnostic categories difficult. Differences in outcome definition may also account for discrepant findings between some of the studies from these two cohorts. The criteria for defining outcome also varied between studies. Typically, DSM was used in the US and ICD in the European studies.
Differences in methods of case ascertainment may have introduced bias in some studies. For example, in a study of maternal genital or reproductive infection, Babulas et al. (Reference Babulas, Factor-Litvak, Goetz, Schaefer and Brown2006) reported that risk of SSD was higher in those where diagnosis was based on review of clinical notes as opposed to face-to-face interview.
Confounding and other methodological issues
Observed associations between schizophrenia in the adult offspring and maternal infections before and after pregnancy (Nielsen et al. Reference Nielsen, Laursen and Mortensen2011) and exposure to several infections in the same group of case mothers (PDS and NCPP cohort) may indicate possible confounding by other factors. Individuals who get one infection may be more likely to get others, so the infections might confound each other or all be markers for a third factor.
The characteristics of women who develop infections may be different from those who do not. Paternal infection was also reported to be associated with increased risk of schizophrenia in offspring (Nielsen et al. Reference Nielsen, Laursen and Mortensen2011). This indicates that poor living conditions and social adversity may be important confounding factors. However, most studies adjusted for social class in their analysis. In two large Danish studies the risk of schizophrenia in offspring associated with prenatal maternal HSV-2 or general infections was attenuated (although it remained statistically significant) after taking into account family history of psychosis (Mortensen et al. Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010; Nielsen et al. Reference Nielsen, Laursen and Mortensen2011). Nearly half of the studies were unable to adjust for this factor. It is possible that observed associations between various prenatal infections and schizophrenia in these studies may be partly confounded by family history of psychosis. However, as only 10–15% of patients with schizophrenia have a positive family history (Gottesman & Shields, Reference Gottesman and Shields1982), confounding by this factor is unlikely to be the sole explanation for the observed associations. Attenuation of the effect size after adjusting for family history of psychosis in the Danish studies might reflect interaction. Indeed, one study reported evidence of interaction between infection and family history of psychosis using an additive model (Clarke et al. Reference Clarke, Tanskanen, Huttunen, Whittaker and Cannon2009). However, unlike the multiplicative model this approach is often more likely to yield a significant interaction in a large enough sample, and may therefore be less informative (Zammit et al. Reference Zammit, Lewis, Dalman and Allebeck2010). Therefore, these findings require replication.
Other methodological issues include the duration of follow-up and over-matching. The duration of follow-up was short in the Danish register-based studies and therefore their findings can be generalized to only early-onset cases of schizophrenia (Mortensen et al. Reference Mortensen, Norgaard-Pedersen, Waltoft, Sorensen, Hougaard, Torrey and Yolken2007, Reference Mortensen, Pedersen, Hougaard, Norgaard-Petersen, Mors, Borglum and Yolken2010). As most studies matched their cases and controls on gender, they were not able to explore any gender difference in the effects of prenatal infection.
Biological plausibly of association
Mechanisms by which prenatal infections interfere with foetal development and contribute to risk of adult schizophrenia may include (i) direct interference with neurodevelopment by infectious agents or antibodies, (ii) induction of autoimmunity, (iii) the involvement of mediators of acute infection, (iv) reprogramming of the hypothalamic–pituitary–adrenal (HPA) axis, (v) activation of the innate immune system and effects of inflammatory cytokines, and (vi) interaction between host and microbe genome and/or environmental factors.
Maternal infection during pregnancy with neurotrophic agents, such as T. gondii, HSV and rubella, can interfere directly with foetal neurodevelopment. An extensive body of literature suggests that congenital infections with these agents can lead to mental retardation, cerebral hypoplasia, ventriculomegaly and other brain and behavioural abnormalities in the offspring (Remington et al. Reference Remington, Klein, Wilson and Baker2006). This is in line with findings from birth cohort studies reviewed here that reported structural and functional brain abnormalities relevant to schizophrenia in exposed cases. This is supported by evidence from animal model studies. Studies of prenatal maternal influenza (or immune activation) using experimental mouse models have reported increased ventricular and reduced brain volume, deficits in working memory, and reduced social and exploratory behaviour in offspring (Shi et al. Reference Shi, Fatemi, Sidwell and Patterson2003; Meyer & Feldon, Reference Meyer and Feldon2010). In rhesus monkeys, prenatal maternal influenza has been reported to affect neural development in the offspring, reducing grey matter throughout most of the cortex and decreasing white matter in the parietal cortex (Short et al. Reference Short, Lubach, Karasin, Olsen, Styner, Knickmeyer, Gilmore and Coe2010). It has been suggested that these brain alterations are likely to be permanent, given that they were still present at the monkey equivalent of older childhood, and thus might increase the likelihood of later behavioural pathology (Short et al. Reference Short, Lubach, Karasin, Olsen, Styner, Knickmeyer, Gilmore and Coe2010).
None of the studies of T. gondii or HSV reviewed here used microbial culture, and the absence of IgM antibodies argues against acute infections with these agents during pregnancy. However, there is evidence that maternal IgG antibodies can cross the placenta and directly affect the foetal brain through molecular mimicry (Wright et al. Reference Wright, Takei, Murray, Sham, Susser, Brown and Gorman1999).
Infections can also induce autoimmunity, which may lead to further central nervous system (CNS) damage (Albert & Inman, Reference Albert and Inman1999). It has been proposed that certain prenatal infections such as influenza may increase the risk of adult psychotic illness through induction of autoimmunity (Wright & Murray, Reference Wright and Murray1993). Maternal neuronal autoantibodies against cerebellar Purkinje cells have been linked with classic neurodevelopmental disorders, such as autism (Dalton et al. Reference Dalton, Deacon, Blamire, Pike, McKinlay, Stein, Styles and Vincent2003) and dyslexia (Vincent et al. Reference Vincent, Deacon, Dalton, Salmond, Blamire, Pendlebury, Johansen-Berg, Rajogopalan, Styles and Stein2002), in offspring. Increased autoimmunity including autoantibodies against ion channels, such as the voltage-gated potassium channel (VGKC), or the N-methyl-d-aspartate (NMDA) receptor is well documented in schizophrenia (Parthasarathi et al. Reference Parthasarathi, Harrower, Tempest, Hodges, Walsh, McKenna and Fletcher2006; Dalmau et al. Reference Dalmau, Lancaster, Martinez-Hernandez, Rosenfeld and Balice-Gordon2011; Zandi et al. Reference Zandi, Irani, Lang, Waters, Jones, McKenna, Coles, Vincent and Lennox2011). Search for other autoantibodies, in addition to microbe-specific antibodies, in prenatal maternal blood samples should help to elucidate to what extent prenatal infections are associated with autoimmunity.
Developmental effects of acute infections such as influenza may be mediated by hyperthermia and foetal hypoxia (Edwards, Reference Edwards1968; Milunsky et al. Reference Milunsky, Ulcickas, Rothman, Willett, Jick and Jick1992; Cannon et al. Reference Cannon, Jones and Murray2002). The finding of increased risk of schizophrenia for maternal analgesic use during pregnancy in the Copenhagen cohort is in line with such mechanisms (Sorensen et al. Reference Sorensen, Mortensen, Reinisch and Mednick2009). Maternal analgesic use during pregnancy has also been linked with increased risk of childhood non-clinical psychotic symptoms (Gunawardana et al. Reference Gunawardana, Zammit, Lewis, Gunnell, Hollis, Wolke and Harrison2011). However, it is yet to be established whether these are direct effect of the analgesics or of the infection that led to the use of analgesics.
Infection can increase foetal exposure to maternal glucocorticoids by inhibiting the placental enzyme 11-beta-hydroxysteroid dehydrogenase type 2 (Edwards et al. Reference Edwards, Benediktsson, Lindsay and Seckl1993; Johnstone et al. Reference Johnstone, Bocking, Unlugedik and Challis2005; Seckl & Holmes, Reference Seckl and Holmes2007; Meyer & Feldon, Reference Meyer and Feldon2010). Excess glucocorticoids can reprogramme the HPA axis in the offspring, leading to either increased basal secretion or enhanced stress-related secretion of glucocorticoids later in life (Owen et al. Reference Owen, Andrews and Matthews2005; Weinstock, Reference Weinstock2008). Thus, increased basal cortisol levels observed in some cases of schizophrenia (Bradley & Dinan, Reference Bradley and Dinan2010) may be related to maternal infections during pregnancy.
Prenatal maternal infection may explain common links between chronic physical and neuropsychiatric diseases of adult life. It has been suggested that people exposed to excess levels of glucocorticoids during pregnancy may be more likely to develop hypertension, hyperglycaemia, hyperinsulinaemia and hyperactivity of the HPA axis as adults (Barker et al. Reference Barker, Gluckman, Godfrey, Harding, Owens and Robinson1993; Seckl & Holmes, Reference Seckl and Holmes2007). Similarly, activation of the HPA axis has been reported to be associated with increased blood pressure, insulin resistance, glucose intolerance and hyperlipidaemia (Reynolds et al. Reference Reynolds, Walker, Syddall, Andrew, Wood, Whorwood and Phillips2001). This might explain why schizophrenia patients have a higher risk of cardiovascular disease, diabetes and impaired glucose tolerance, which persists even after taking into account effects of antipsychotic drugs and lifestyle factors (Bushe & Holt, Reference Bushe and Holt2004; Curkendall et al. Reference Curkendall, Mo, Glasser, Rose Stang and Jones2004).
As increased risk of psychosis has been observed for a diverse range of prenatal infections, it has been proposed that they may share a common pathway to exert pathogenic effects on the foetal brain. One such mechanism involves activation of the innate immune system and release of proinflammatory cytokines (Meyer et al. Reference Meyer, Feldon and Yee2009). Reports from the NCPP and PDS cohorts suggest inflammatory cytokines may mediate risk of psychotic illness associated with prenatal infection (Buka et al. Reference Buka, Tsuang, Torrey, Klebanoff, Wagner and Yolken2001b; Brown et al. Reference Brown, Hooton, Schaefer, Zhang, Petkova, Babulas, Perrin, Gorman and Susser2004b).
A role of prenatal immune activation in schizophrenia is also supported by evidence from animal model studies (reviewed by Meyer & Feldon, Reference Meyer and Feldon2010). Mouse models using pregnant females have included simulated viral or bacterial infection or direct injection with a cytokine (IL-6). These have been reported to produce intermediate phenotypes related to schizophrenia in the adult offspring. Some of these phenotypes, such as deficits in sensory gating and abnormal latent inhibition, have been shown to be reversible by treatment with clozapine, an antipsychotic (Smith et al. Reference Smith, Li, Garbett, Mirnics and Patterson2007). Cytokines can be neurotrophic and also neurotoxic depending on the developmental stage and condition (Garver et al. Reference Garver, Tamas and Holcomb2003). Elevated levels of cytokines during pregnancy have been reported to be associated with reduced cortical neuronal survival and reduced grey- and white-matter volume in animal studies (Meyer & Feldon, Reference Meyer and Feldon2010; Short et al. Reference Short, Lubach, Karasin, Olsen, Styner, Knickmeyer, Gilmore and Coe2010). Prenatal immune activation in animals has also been linked with structural and functional alterations in the mesocorticolimbic dopaminergic system long before the onset of the full spectrum of psychosis-associated behavioural and cognitive abnormalities in adulthood (Meyer & Feldon, Reference Meyer and Feldon2009).
Characteristics of both the microbial and human genome are likely to determine the pathological response to an infection to a large extent (Yolken & Torrey, Reference Yolken and Torrey2008). For example, one study reported that infection with T. gondii type I, specifically, out of all three genotypes, was associated with increased risk of psychosis (Xiao et al. Reference Xiao, Buka, Cannon, Suzuki, Viscidi, Torrey and Yolken2009). However, genetic determinants of human (host) response to infection include receptors, transcription factors, cytokines and other components of innate immunity (Yolken & Torrey, Reference Yolken and Torrey2008).
It has been suggested that some genetic vulnerability to schizophrenia may be explained by genetic vulnerability to infection (Shi et al. Reference Shi, Levinson, Duan, Sanders, Zheng, Pe'er, Dudbridge, Holmans, Whittemore, Mowry, Olincy, Amin, Cloninger, Silverman, Buccola, Byerley, Black, Crowe, Oksenberg, Mirel, Kendler, Freedman and Gejman2009; Stefansson et al. Reference Stefansson, Ophoff, Steinberg, Andreassen, Cichon, Rujescu, Werge, Pietilainen, Mors, Mortensen, Sigurdsson, Gustafsson, Nyegaard, Tuulio-Henriksson, Ingason, Hansen, Suvisaari, Lonnqvist, Paunio, Borglum, Hartmann, Fink-Jensen, Nordentoft, Hougaard, Norgaard-Pedersen, Bottcher, Olesen, Breuer, Moller, Giegling, Rasmussen, Timm, Mattheisen, Bitter, Rethelyi, Magnusdottir, Sigmundsson, Olason, Masson, Gulcher, Haraldsson, Fossdal, Thorgeirsson, Thorsteinsdottir, Ruggeri, Tosato, Franke, Strengman, Kiemeney, Melle, Djurovic, Abramova, Kaleda, Sanjuan, de Frutos, Bramon, Vassos, Fraser, Ettinger, Picchioni, Walker, Toulopoulou, Need, Ge, Yoon, Shianna, Freimer, Cantor, Murray, Kong, Golimbet, Carracedo, Arango, Costas, Jonsson, Terenius, Agartz, Petursson, Nothen, Rietschel, Matthews, Muglia, Peltonen, St Clair, Goldstein, Stefansson and Collier2009; Nielsen et al. Reference Nielsen, Laursen and Mortensen2011). Variations in the gene encoding IL-10 are reported to be associated with susceptibility to both CMV infection and schizophrenia (Bocchio Chiavetto et al. Reference Bocchio Chiavetto, Boin, Zanardini, Popoli, Michelato, Bignotti, Tura and Gennarelli2002; Hurme et al. Reference Hurme, Haanpaa, Nurmikko, Wang, Virta, Pessi, Kilpinen, Hulkkonen and Helminen2003). More recently, a genome-wide association study (GWAS) of schizophrenia reported an association with a cytokine receptor gene, colony stimulating factor, receptor 2 alpha (CSF2Rα; Lencz et al. Reference Lencz, Morgan, Athanasiou, Dain, Reed, Kane, Kucherlapati and Malhotra2007). This gene is responsible for regulating important components of the innate immune system such as granulocytes and macrophages, which are the primary source of proinflammatory cytokines.
GWAS have also reported significant associations between schizophrenia and markers close to the major histocompatibility complex (MHC) region on chromosome 6 (Shi et al. Reference Shi, Levinson, Duan, Sanders, Zheng, Pe'er, Dudbridge, Holmans, Whittemore, Mowry, Olincy, Amin, Cloninger, Silverman, Buccola, Byerley, Black, Crowe, Oksenberg, Mirel, Kendler, Freedman and Gejman2009; Stefansson et al. Reference Stefansson, Ophoff, Steinberg, Andreassen, Cichon, Rujescu, Werge, Pietilainen, Mors, Mortensen, Sigurdsson, Gustafsson, Nyegaard, Tuulio-Henriksson, Ingason, Hansen, Suvisaari, Lonnqvist, Paunio, Borglum, Hartmann, Fink-Jensen, Nordentoft, Hougaard, Norgaard-Pedersen, Bottcher, Olesen, Breuer, Moller, Giegling, Rasmussen, Timm, Mattheisen, Bitter, Rethelyi, Magnusdottir, Sigmundsson, Olason, Masson, Gulcher, Haraldsson, Fossdal, Thorgeirsson, Thorsteinsdottir, Ruggeri, Tosato, Franke, Strengman, Kiemeney, Melle, Djurovic, Abramova, Kaleda, Sanjuan, de Frutos, Bramon, Vassos, Fraser, Ettinger, Picchioni, Walker, Toulopoulou, Need, Ge, Yoon, Shianna, Freimer, Cantor, Murray, Kong, Golimbet, Carracedo, Arango, Costas, Jonsson, Terenius, Agartz, Petursson, Nothen, Rietschel, Matthews, Muglia, Peltonen, St Clair, Goldstein, Stefansson and Collier2009). This region includes several immunity-related genes and a histone gene cluster relevant to gene expression. This region contains genes involved in brain development, memory and cognition (Stefansson et al. Reference Stefansson, Ophoff, Steinberg, Andreassen, Cichon, Rujescu, Werge, Pietilainen, Mors, Mortensen, Sigurdsson, Gustafsson, Nyegaard, Tuulio-Henriksson, Ingason, Hansen, Suvisaari, Lonnqvist, Paunio, Borglum, Hartmann, Fink-Jensen, Nordentoft, Hougaard, Norgaard-Pedersen, Bottcher, Olesen, Breuer, Moller, Giegling, Rasmussen, Timm, Mattheisen, Bitter, Rethelyi, Magnusdottir, Sigmundsson, Olason, Masson, Gulcher, Haraldsson, Fossdal, Thorgeirsson, Thorsteinsdottir, Ruggeri, Tosato, Franke, Strengman, Kiemeney, Melle, Djurovic, Abramova, Kaleda, Sanjuan, de Frutos, Bramon, Vassos, Fraser, Ettinger, Picchioni, Walker, Toulopoulou, Need, Ge, Yoon, Shianna, Freimer, Cantor, Murray, Kong, Golimbet, Carracedo, Arango, Costas, Jonsson, Terenius, Agartz, Petursson, Nothen, Rietschel, Matthews, Muglia, Peltonen, St Clair, Goldstein, Stefansson and Collier2009). Thus, increased risk of adult schizophrenia in the offspring for both paternal and maternal infection, as observed in a Danish sample (Nielsen et al. Reference Nielsen, Laursen and Mortensen2011), may reflect high genetic susceptibility to severe infection in future cases of schizophrenia. It is also possible that prenatal infection, by affecting gene expression or in the presence of pre-existing genetic vulnerabilities, can lead to a distinct or pathological immune response. This, in turn, may lead to CNS alterations, making these individuals susceptible to developing psychotic illness later in life.
Directions for future research
Studies using animal models could address whether or not there is a ‘sensitive period’ for vulnerability, an issue that must be resolved to understand the underlying biological mechanisms and to consider therapeutic interventions.
Duration of follow-up should cover at least the age of peak incidence of schizophrenia to make findings more generalizable. Effects of prenatal infections need to be examined in relation to specific psychopathology rather than broad diagnostic categories of schizophrenia or non-affective psychosis. Relationships between prenatal infection and well-established intermediate phenotypes of adult schizophrenia, such as childhood motor and cognitive development, should be explored using larger samples. The use of mediation models should be used to elucidate potential effects of prenatal infection on other antecedents of schizophrenia. Future studies should use triangulation of genetic information in addition to inflammatory cytokines and other immunological markers to elucidate effects of prenatal and early life infections on neurodevelopment and adult psychotic illness. Future investigations should also address to what extent genetic vulnerability to schizophrenia may be explained by genetic vulnerability to infection. Indeed, a new generation of general population birth cohorts, including the Norwegian Mother and Child Cohort Study (MoBa; Magnus et al. Reference Magnus, Irgens, Haug, Nystad, Skjaerven and Stoltenberg2006), the Danish National Birth cohort (Olsen et al. Reference Olsen, Melbye, Olsen, Sorensen, Aaby, Andersen, Taxbol, Hansen, Juhl, Schow, Sorensen, Andresen, Mortensen, Olesen and Sondergaard2001) and the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort (Golding et al. Reference Golding, Pembrey and Jones2001) are currently conducting similar studies of neurodevelopmental outcomes. These cohorts have in-depth biological and other data and (for Norway and Denmark) large enough sample sizes to address issues such as mediation and mechanisms.
Strengths and limitations of this review
Our review is based on rigorous examination of robust population-based studies. Included studies reliably measured exposure at the individual level and defined outcome by contemporary ICD or DSM directives. Cases of psychosis were collected from general population registers or by a two-stage process (screening followed by a diagnostic interview). Individual studies also accounted for several confounding factors in design and analysis. These measures should increase both internal and external validity of the evidence. We included a variety of studies, for example those examining serological and clinically diagnosed infections, that used various epidemiological designs. We were not able to combine results of individual studies in a meta-analysis or carry out formal tests for publication bias. Meta-analyses were deemed unsuitable because of significant variation among studies on several important parameters. Nevertheless, to our knowledge this is the most rigorously conducted independent appraisal of the population-based studies published on this topic to date.
Note
For supplementary material accompanying this paper, visit http://dx.doi.org/10.1017/S0033291712000736.
Acknowledgements
We are grateful to Dr C. Dalman for valuable comments on an earlier draft of this article and to Professors E. Susser and A. Brown for helpful discussions.
G.M.K. is supported by a grant from the Wellcome Trust (Clinical PhD Programme, grant no. 094790/Z/10/Z). P.B.J. is supported by the Wellcome Trust (095844/Z/11/Z and 088869/Z/09/Z) and the National Institute for Health Research (NIHR; RP-PG-0606-1335). The funding bodies had no further role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Declaration of Interest
None.








