Bioactive peptides are specific protein fragments that, in addition to their nutritional value, have a positive effect on body functions or conditions and may ultimately influence health (Korhonen & Pihlanto, Reference Korhonen and Pihlanto2006). Food-derived bioactive peptides have been shown to display a wide range of physiological functions including antioxidative, antihypertensive, antimicrobial and antiproliferative effects, which are dependent on their inherent amino acid sequence and composition (Shimizu, Reference Shimizu2004). In general, biofunctional peptides are encrypted within the parent protein sequence and the peptides are inactive and thus must be released to exert an effect. To date, several food protein enzymatic peptides have been reported to have antioxidant activities, like those derived from soybean (Pena-Ramos & Xiong Reference Pena-Ramos and Xiong2002), rice bran (Parrado et al. Reference Parrado, Miramontes, Jover, Gutierrez, Collantes de Teran and Bautista2006), egg (Park et al. Reference Park, Jung, Nam, Shahidi and Kim2001), and canola (Cumby et al. Reference Cumby, Zhong, Naczk and Shahidi2008).
The term whey protein refers to milk-serum proteins. Whey represents a rich and varied mixture of secreted proteins with a wide range of chemical, nutritional and biological properties. Historically, whey has been overlooked as a source of physiologically functional protein, or only processed into low-value products such as whey powder and various grades of whey protein concentration. With the application of novel processing methods, new biological activities of dairy by-product have been developed; whey proteins have been described as possessing properties such as antimicrobial activity (Ramos et al. Reference Ramos, Santos, Leao, Pereira, Silva, Fernandes, Franco, Pintado and Malcata2012; Demers-Mathieu et al. Reference Demers-Mathieu, Gauthier, Britten, Fliss, Robitaille and Jean2013), antioxidant activity (Xu et al. Reference Xu, Liu, Xu and Kong2011), angiotensin-converting enzyme (ACE) inhibitory activity (Guo et al. Reference Guo, Pan and Tanokura2009; Unal & Akalin, Reference Unal and Akalin2012), and calcium-binding effect (Kim & Lim, Reference Kim and Lim2004; Rui, Reference Rui2009). With regards to antioxidant properties, previous studies have shown that whey protein hydrolysates contain a broad range of antioxidant activity as measured by electron spin resonance (Peng et al. Reference Peng, Xiong and Kong2009) or in an iron-catalysed liposome oxidation system (Pea-Ramos & Xiong, Reference Pea-Ramos and Xiong2001), depending on the protease used. The antioxidant property of peptides seems to be strongly correlated with their amino acid compositions and sequences. However, there are few reports on the purification of whey protein hydrolysates and the structural information of purified antioxidative peptides. Therefore, further study is needed to purify the enzymatic hydrolysates to obtain peptides with different antioxidative activities and identify the amino acid sequence of these antioxidative peptides so as to elucidate their structure-activity relationship.
The objective of this study was to compare the antioxidant activities of different peptides obtained from hydrolysed whey protein and to get structure-activity relationship information. Whey protein was first hydrolysed by various enzymes. The purification of the hydrolysates was performed using macroporous adsorption resins (MAR) and consecutive chromatography. The identification of the purified peptides was achieved by Quadrupole time-of-flight mass spectrometer (Q-TOF MS). In addition, the antioxidant activity was determined by lipid peroxidation inhibition assay and free radical scavenging activities.
Materials and methods
Materials
Bovine milk whey protein concentrate (NZMP alacen TM392, 80% protein) was a product of NZMP Ltd., New Zealand. Trypsin from porcine pancreas (EC. 3.4.21.4, 4×103 U/g) and papain enzymes (EC. 3.4.22.2, 8×105 U/g) were purchased from Wuxi Xuemei Enzyme Technology Co. Ltd. (Jiangsu, China). Alcalase® 2·4L (EC. 3.4.21.62, 5×105 U/g), a serine-protease from Bacillus licheniformis, promatex (EC. 3.4.24.28, 1×105 U/g) and flavourzyme (EC. 3.4.11.1, 2×104 U/g) were bought from Novo Nordisk Inc. (Bagsvard, Denmark), while protease N (2·5×105 U/g) was purchased from Amano Enzyme Company (Nagoya, Japan). Styrene-based MAR DA201-C was obtained from Jialian Resin Co. (Jiangsu, China). Linoleic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH) and Sephadex G-15 were products of Sigma. Other chemicals and reagents were of analytical grade obtained from chemical store of Jiangnan University.
Preparation of whey protein hydrolysate (WPH)
Whey protein concentrate (30 mg/ml) in deionised water was preheated at 90 °C for 5 min (Zhang et al. Reference Zhang, Ling, Sun, Zhang, Yu, Kamau and Lu2012), and then hydrolysis was performed using various enzymes under optimal conditions: pH 8·0/50 °C for trypsin, pH 7·0/55 °C for papain, pH 8·5/65 °C for alcalase 2·4L, pH 7·0/50 °C for protamex, pH 7·0/50 °C for flavourzyme, and pH 7·0/55 °C for protease N. The enzyme/substrate ratio was 2% (w/w) in all cases. The mixture was incubated for 4 h at each optimal temperature with stirring and then boiled for 10 min to inactive the enzymes. After cooling, the hydrolysates were adjusted to pH 7·0, centrifuged at 8000 rpm for 30 min, and then lyophilised. The lyophilised hydrolysates were stored at −20 °C until use. The degree of hydrolysis (DH) was calculated according to Peng et al. (Reference Peng, Xiong and Kong2009)
Static desalination of WPH with macroporous adsorption resin (MAR)
WPH was treated by MAR as described by Cheison et al. with slight modification (Cheison et al. Reference Cheison, Zhang and Xu2007). MAR DA201-C was first pretreated with 100% ethanol and equilibrated with water for 24 h in a conical flask. Then 10 g wet resin was mixed with 50 ml WPH (10 mg/ml) and shaken at 25 °C for 24 h. The salt solution was removed by filtering and the absorbed peptides were desorbed with 60% ethanol. The adsorption yield was calculated as the percentage of protein desorbed from resin compared with the protein in the starting WPH solution. The eluted WPH peptides were pooled, concentrated by rotary evaporation and lyophilised as the fraction from MAR (WPM) for further purification.
Purification of antioxidative peptide
Gel filtration chromatograph
Two milligram of the freeze-dried WPM was suspended in 4 ml distilled water and loaded onto a Sephadex G-15 gel filtration column (φ1·6×100 cm), pre-equilibrated and eluted with distilled water. The flow rate was adjusted to 0·4 ml/min and eluted fractions (5·0 ml per tube) were collected. By using spectrophotometric detection at 220 nm, the eluted fractions showing antioxidant activity were pooled and freeze-dried.
Semi-preparative reversed-phase–HPLC
The fractions exhibiting antioxidant activity were purified by semi-preparative RP-HPLC on a Hedern ODS-2 C18 P/N 84176 (φ10×250 mm, Jiang Su Hanbang Co., China) with a linear gradient of acetonitrile (20–40% in 30 min) containing 0·05% trifluoroacetic acid (TFA) at 2 ml/min. Potent peaks detected at 220 nm were collected, evaluated for antioxidant activity, and then lyophilised.
Analytical reversed-phase-HPLC
The active fractions from semi-preparative column were further applied to a C18 S/N MT101 (φ4·6×150 mm) analytical column with different linear gradients of acetonitrile containing 0·05% TFA at 0·5 ml/min. The amino acid sequence of the finally purified peptides was analysed.
Determination of the antioxidative activity
DPPH free radical scavenging activity assay
The scavenging effect of WPH on DPPH free radical scavenging activities was measured based on methods described in Lu et al. (2010). A 2 ml test sample (dissolved in deionised water) was mixed with 2 ml of 0·2 mm DPPH solution in ethanol (freshly prepared). The mixture was kept under dark condition at room temperature for 30 min before monitoring for absorbance at 517 nm. The blank was conducted in the same manner using deionised water instead of test sample. Radical scavenging activity was determined as the decrease in absorbance at 517 nm between the blank and test sample. The IC50 value was defined as the concentration required to scavenge 50% of the initial radical. Therefore, lower IC50 value indicates higher free radical-scavenging ability.
Superoxide anion radical scavenging activity assay
The assay is based on the inhibition of non-enzymatically generated (β-NADH/PMS (Phenazine methosulphonate)) superoxide radical by measuring the reduction of nitro-blue tetrazolium (NBT). The assay of Moure et al. (Reference Moure, Dominguez and Parajo2006) was used with minor modification. The reaction mixture contained 0·5 ml of test sample, 0·5 ml of NBT (2·52 mm), 0·5 ml of NADH (624 μm) and 0·5 ml of PMS (120 μm). NBT, NADH and PMS solutions were prepared in 0·1 m phosphate buffer (pH 7·4) and kept on ice during the experiment. The reaction mixture was incubated at 25 °C for 5 min and absorbance at 560 nm was measured. Percentage inhibition of NBT was calculated at steady state.
Lipid peroxidation inhibition assay
The antioxidant activity was measured in a linoleic acid model system according to the methods of Osawa & Namiki (Reference Osawa and Namiki1985) with slight modifications. A 1·3 mg sample was dissolved in 10 ml 50 mm phosphate buffer (pH 7·0), and added into a mixture of 0·13 ml linoleic acid and 10 ml 99·5% ethanol in which the final volume was adjusted to 25 ml with distilled water. The mixed solution was incubated in a conical flask with a screw cap at 40 °C under dark condition. The degree of oxidation was evaluated by measuring the ferric thiocyanate values (Mitsuta et al. Reference Mitsuta, Yasumoto and Iwami1996). Aliquot (100 μl) of reaction mixture in the linoleic acid model system was mixed with 75% ethanol (4·7 ml), 30% ammonium thiocyanate (0·1 ml), and 20 mm ferrous chloride (0·1 ml) in 3·5% HCl. After 3 min, the thiocyanate value represented linoleic acid oxidation was measured spectrophotometrically at 500 nm during the incubation period at 40 °C.
Amino acid content analysis
Amino acid compositions were determined using the method described by Reference Lu, Qian, Sun, Zhou, Chen, He, Zhang and WuLu et al. (2010). Lyophilised hydrolysate sample was digested with 6 m HCl at 110 °C for 24 h followed by chromatographic separation. Degradation of Met and Cys was prevented by oxidation with performic acid. The method is further referred to as the reference mentioned.
Determination of amino acid sequence by Q-TOF MS
The purified peptides with high antioxidant activity were analysed by a Quadrupole time-of-flight mass spectrometer (Q-TOF MS) (Waters Synapt, USA) equipped with an electrospray ionisation (ESI) source. The purified peptides were separately infused into the electrospray source after being dissolved in methanol/water (1 : 1, v/v). The molecular mass was determined by the double charged (M+2H)2+ state in the mass spectrum. Automated Edman sequencing was performed by standard procedures using a 477-A protein sequencer chromatogram (Biolynx, Waters, USA).
Statistical analysis
All the experiments were conducted at least in triplicate. One-way ANOVA using SPSS software was used to compare the mean values of each treatment. Significant differences (P<0·05) among the means were determined by using Duncan's multiple range test.
Results and discussion
Antioxidant activities of whey protein hydrolysates
To obtain peptides with different length and amino acid sequence, six proteases (trypsin, papain, alcalase 2·4L, protamex, flavourzyme, and protease N) were used to hydrolyse whey protein. The extent of protein degradation by proteolytic enzymes was estimated by assessing the degree of hydrolysis (DH). As shown in Table 1, the DH of whey protein was observed to be 22·02 and 20·17% for alcalase and trypsin, respectively. While the DH for other proteolytic enzymes was less than 20%. The high DH of alcalase maybe due to its endopeptidase property. Many previous studies reported that alcalase is capable of producing bioactive peptides. When compared with other specific and non-specific proteases, it showed higher yields in the production of antioxidative peptides (Li et al. Reference Li, Chen, Wang, Ji and Wu2007; Elias et al. Reference Elias, Kellerby and Decker2008).
Table 1. Degree of hydrolysis and antioxidant activities of the hydrolysates by different protease treatment

† Expressed as means±sd of triplicates
‡ Radical scavenging activities (%) were tested at a concentration of 10 mg/ml
§ Inhibition of lipid peroxidation in the linoleic acid model system was determined as described in the text after 7 d
Different superscript characters (a, b, c, d and e) indicate the significant difference at P<0·05 level within a same column
As the antioxidative mechanisms are diverse, the radical scavenging activities of the six whey protein hydrolysates were tested using three different systems (Table 1). Results revealed that the hydrolysate obtained by treatment with alcalase from whey protein exerted the highest DPPH radical- (72·06%), superoxide radical-scavenging activity (60·68%) and lipid peroxidation inhibition ability (41·28%), which suggests that WPH could convert free radicals to more stable products, hence terminating the radical chain reaction. Li et al. (2007) pointed out that DH can highly affect the antioxidant properties of peptides. Our results also proved that higher DH exhibited higher antioxidant activities.
Thus, WPH treated by alcalase dominated in the highest DH and activities in the antioxidative assay when compared with the other hydrolysates. Therefore, alcalase was chosen to further hydrolyse whey protein for antioxidative peptides.
The static desalination effect on amino acid compositon of WPH
The purification effect of WPH may be affected by salt introduced to the mixture using pH-state method. Recently, there has been an increasing interest in employing hydrophobic MAR to isolate the bioactive compounds from crude extracts (Cheison et al. Reference Cheison, Zhang and Xu2007). MAR has good recovery characteristics of peptides because of their unique binding properties and other advantages, such as easy regeneration, low expense, ideal pore structure and various surface functional groups (Hogan et al. Reference Hogan, Zhang, Li, Wang and Zhou2009). In this study, MAR DA201-C was employed for static desalting the enzymatic hydrolysates. After that, the antioxidant activities of WPH, which was named WPM, were detected. Table 2 showed that the IC 50 values of DPPH and superoxide radical scavenging activities of WPM were significantly (P<0·05) lower than those of WPH, representing a decrease of 6·89 and 38·88% in MAR-treated peptides, respectively. All the results above indicated that MAR processing WPH could significantly improve the antioxidant activities.
Table 2. The effects of macroporous resin separation on amino acid composition and antioxidant activities of WPH

† Expressed as means±sd of triplicates
‡ IC 50, mg/ml
§ Inhibition of lipid peroxidation in the linoleic acid model system was determined as described in the text after 7 d
¶ g/100 g protein, inculding Tyr, Val, Met, Phe, Ile, Leu and Pro
∥ g/100 g protein, inculding Cys, His, Trp, Lys, Arg, Leu, Tyr, Asp and Val
†† Whey protein isolate hydrolysate
‡‡ Hydrolysate fraction separated using macroporous resin (MAR)
Different superscript characters (a and b) indicate the significant difference at P<0·05 level within a same column
In order to clarify the mechanism by which WPM had higher antioxidant activities than WPH, the amino acid compositions of WPH and WPM were determined. As shown in Table 2, the amount of hydrophobic amino acids (Tyr, Val, Met, Phe, Ile, Leu and Pro) was increased in the fractions eluted by MAR compared with WPH. Many natural peptides derived from protein sources with high hydrophobicity have been reported to relate with strong antioxidative properties (Rajapakse et al. Reference Rajapakse, Mendis, Jung, Je and Kim2005; Ren et al. Reference Ren, Zhao, Shi, Wang, Jiang, Cui, Kakuda and Xue2008). Therefore, it was assumed that hydrophobic amino acids present in the WPM's sequence contributed to its radical scavenging properties.
Purification and identification of antioxidative peptides
To elucidate the peptides responsible for antioxidant activity, WPM was initially subjected to size exclusion chromatography on a Sephadex G-15 column and six fractions (A-F) were obtained. Fraction D was found to possess the highest radical scavenging ability (38·49%) at 1 mg/ml (Fig. 1a). Thus, fraction D was subjected to semi-preparative RP-HPLC and 11 portions were obtained. Among them, fractions D4, D5, D6, and D9 exhibited relatively higher antioxidative activity (Fig. 1b). Then they were further fractionated by analytical RP-HPLC. Seven main peaks (D4a, D4b, D5a, D5b, D6a, D6b and D9a) were obtained (Fig. 2). All the peaks were collected separately through repeated chromatograph using analytical RP-HPLC column and concentrated in vacuum.

Fig. 1. Consecutive chromatography profiles (lower panel) and antioxidant activities of the fractions measured by the DPPH scavenging (upper panel) for the purification of antioxidant peptides from WPM. (a) Sephadex G-15 gel filtration chromatography, (b) Semi-preparation RP-HPLC analysis.

Fig. 2. Analytical RP-HPLC profiles of the D4, D5, D6 and D9 fractions obtained from Hedern ODS-2 C18 P/N 84176. (a) D4, (b) D5, (c) D6, (d) D9.
Peptides in the seven fractions were further analysed by Q-TOF MS for amino acid sequence determination (Table 3). Among them peptide Trp–Tyr–Ser–Leu displayed the highest DPPH radical- (56·87%) and superoxide radical-scavenging activity (87·90%). In fact, Davalos et al. (Reference Davalos, Miguel, Bartolome and Lopez-Fandino2004) reported that among the amino acids, Trp, Tyr and Met showed the highest antioxidant activity, followed by Cys, His and Phe. The high antioxidant activity of Trp and Tyr may be due to the special capacity of the indolic and phenolic groups as hydrogen donors. Also, Trp–Tyr–Ser–Leu contains hydrophobic amino acid Leu at C-terminal. Saiga et al. found that the hydrophobic amino acids in peptides help to strengthen their antioxidant activity. They can also react with non-peptide antioxidants, and could act as effective scavengers of various radical species (Saiga et al. Reference Saiga, Tanabe and Nishimura2003).
Table 3. Peptides identified by Q-TOF MS in fractions D4a, D4b, D5a, D5b, D6a, D6b and D9a separated by RP-HPLC and their radical-scavenging activity
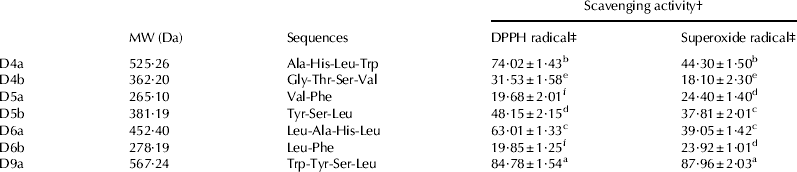
† Expressed as means±sd of triplicates
‡ Scavenging effects were tested at 500 μg/ml
Different superscript characters (a, b, c, d, e and f) indicate the significant difference at P<0·05 level within a same column
In addition, Ala–His–Leu–Trp also showed higher antioxidant activity. It is reported that the antioxidant activity of His-containing peptides was attributed to the proton-donation ability of the His imidazole group (Li et al. 2007). Therefore, it can be confirmed that the presence of amino acids associated with antioxidant activity and their suitable position in the parent sequence contributed to the high radical scavenging potency of the WPH peptides.
Conclusion
WPH obtained by alcalase 2·4L was found to possess the highest antioxidant activity among six proteolytic preparations. Seven different peptides showing antioxidant activity were isolated and their amino acid sequences were determined. The peptide Trp–Tyr–Ser–Leu displayed the highest radical-scavenging activity. The bioactive peptides developed from whey proteins could be used as natural antioxidants in enhancing antioxidant properties of functional foods and in preventing oxidation reactions in food processing.
This study was supported by the grants from the State Natural Sciences Foundation of China (No 31071491), National Key Technology R&D Program of China (2012BAD33B05, 2011BAD09B03), the 863 project (2013AA102207), and the Priority Academic Programme Development of Jiangsu Education Institutions.







