Literature review
A general review of the literature on cholesteatoma was conducted. Literature searches of the Medline and PubMed databases included clinical and experimental work performed over the past three decades. Literature selection was limited to English-language articles.
The review process mainly focussed on the cellular processes involved, and aimed to provide information enabling stepwise explanation of acquired cholesteatoma pathogenesis.
Introduction
Cholesteatoma is a benign, gradually expanding, destructive epithelial lesion of the temporal bone which results in erosion of adjacent bony structures, leading to various complications. Bone resorption of the ossicular chain and otic capsule (i.e. the bony labyrinth) may result in subsequent hearing loss, vestibular dysfunction, facial paralysis and intracranial complications.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1–Reference Migirov, Bendet and Kronenberg5
In principle, it is accepted that cholesteatomas can be classified into two categories: congenital and acquired.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1 The pathogenesis of middle-ear acquired cholesteatoma, a subject of debate for decades, is addressed below. The diagnostic classification of acquired cholesteatoma proposed by Tos (1993),Reference Tos6 based on site of origin, is commonly accepted. However, although cholesteatomas may have different origins, they share similar cellular mechanisms.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1
This review re-evaluates the various theories of acquired cholesteatoma pathogenesis. Inflammation continues to be acknowledged as playing an important role in the development (i.e. onset, growth and expansion) and recurrence of the disease.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1, Reference Tos7–Reference Chang and Kim10 Cholesteatomas may have different growth patterns,Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1, Reference Dornelles, da Costa, Meurer and Scheiweger11–Reference Sudhoff and Tos15 as described below. A link has been found between abnormal wound healing and cholesteatoma pathogenesis, which may relate to enhanced perimatrix–matrix interactions, due specifically to collagen overproduction by fibroblasts and enhanced transforming growth factor-β activity.Reference Huisman, de Heer, Dijke and Grote16, Reference Louw17
This review gives stepwise explanations for acquired cholesteatoma pathogenesis, since it is imperative to understand the pathological mechanisms involved in order to instigate effective clinical interventions. Cellular processes are discussed, but molecular mechanisms fall outside the scope of the study. Inflammation plays an important role in the progression of pre-cholesteatomatous conditions to cholesteatoma. Bacterial otitis externa in the deep part of the external auditory canal may trigger cholesteatoma onset, while chronic inflammation of the tympanic membrane (with persistent perimatrix inflammation) and middle ear may play an important role in disease progression (i.e. growth and expansion) and recurrence.Reference Sudhoff and Tos15, Reference Chole and Faddis18–Reference Mustafa, Hysenaj, Latifi, Ukimeraj, Thaci and Heta23
Cholesteatoma overview
The term cholesteatoma, coined by Muller in 1838 and defined as a layered, pearly fat tumour, is considered a misnomer.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1 In general, the presence of epithelial cells within the tympanic cavity will trigger the formation of tumour-like growths, known as cholesteatomas. Diagnosis relies on careful otoscopic examination, aided by computed tomography, audiometric testing and surgical exploration. Clinically, acquired cholesteatomas commonly present with recurrent or chronic purulent discharge (i.e. otorrhoea), and crust formation often covers the mouth of an infected cholesteatoma.Reference Becker, Naumann and Pfaltz24 Olszewska and colleagues have proposed a classification system for cholesteatomas.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1 Of relevance to cholesteatoma pathogenesis are the classification systems based on site of origin and the presence or absence of tympanic membrane involvement.Reference Tos6, Reference Tos25
The main aetiological factors in cholesteatoma development are: long-term eustachian tube dysfunction, leading to reduced middle-ear pressure, poor pneumatisation in the middle ear and mastoid process, and retraction pocket formation; and inflammatory conditions (e.g. chronic otitis media with effusion), resulting in negative middle-ear pressure and retraction pocket formation.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1, Reference Chole, Sudhoff and Cummings8, Reference Ruah, Schachem, Paparella and Zeltermann26–Reference Ohta, Sakagami, Suzuki and Mishiro30
A family cluster of acquired cholesteatoma has recently been reported (involving both parents and seven siblings), and a UK paper has described a genetic propensity for cholesteatoma in some individuals.Reference Homøe and Rosberg31, Reference Prinsley32 It has also been suggested that chromosomal imbalances (i.e. aneusomy of chromosomes 7, 8 and 17) may be associated with cholesteatoma growth and bone destruction.Reference Ecsedi, Rákosy, Vizkeleti, Juhász, Sziklai and Adány33
Cholesteatoma appears to have a male predominance, and there is a higher prevalence in underdeveloped countries. In Scandinavia, the cholesteatoma incidence has been found to be approximately three per 100 000 children and 9.2 per 100 000 Caucasian adults.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1, Reference Mustafa, Heta, Kastrai and Dreshaj22, Reference Mustafa, Hysenaj, Latifi, Ukimeraj, Thaci and Heta23, Reference Tos25, Reference Kemppainen, Puhakka, Laippala, Sipilä, Manninen and Karma34, Reference Osma, Cureoglu and Hosoglu35
Relevant anatomy and cell biology
Tympanic membrane and cavity
The tympanic membrane normally consists of an outer keratinising squamous epithelial layer, an intermediate fibrous connective tissue layer and an inner cuboidal epithelial layer, all of which participate in cholesteatoma pathogenesis. The outer epidermal layer is continuous with that of the external auditory canal, while the inner mucosal layer is continuous with that of the tympanic cavity. The presence of epidermal cells and keratin deposits within the tympanic cavity usually marks the onset of cholesteatoma. Trauma or disease of the tympanic membrane can play a central role in cholesteatoma pathogenesis, or may be secondarily involved. The pars flaccida region can present with perforations and retraction pockets, whilst the pars tensa region can present with invaginations (i.e. retraction pockets, atelectasis or adhesive otitis media).Reference Chole, Sudhoff and Cummings8, Reference Tos and Sanna36 Retraction pockets are regarded as the most common trigger for cholesteatoma formation. It is also postulated that diseased mucosa of the middle ear and mastoid air cells may lead to the development of cholesteatoma behind an intact tympanic membrane (i.e. occult cholesteatoma), with secondary tympanic membrane involvement.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1, Reference Tos6, Reference Chole, Sudhoff and Cummings8, Reference Sudhoff and Tos15, Reference Becker, Naumann and Pfaltz24, Reference Mills37
Under chronic inflammatory conditions in the tympanic cavity, the accumulation of mucus between mucosal folds and within pouches can impede ventilation and drainage, with consequent hypopneumatisation. It is plausible that hypoxic conditions in the tympanic cavity may also contribute to the formation of tympanic membrane invaginations (i.e. retraction pockets, atelectasis and adhesive otitis media), which may trigger cholesteatoma formationReference Chole, Sudhoff and Cummings8, Reference Sudhoff and Tos15, Reference Tos and Sanna36, Reference Sudhoff and Tos38, Reference Kultz and Brackmann39 or cholesteatoma growth or degeneration. Such hypoxic changes are marked by enhanced production of transforming growth factor-β and microvessel occlusion (observed during abnormal wound healing research).Reference Huisman, de Heer, Dijke and Grote16, Reference Louw17, Reference Adunka, Gstoettner, Knecht and Kiener40, Reference Olszewska, Chodynicki and Chyczewski41
Cell migration
It is important to note that the tympanic membrane epidermal layer does not regenerate by superficial desquamation, but rather by cell migration from the centre of the tympanic membrane (the umbo) to the periphery, and then upwards and downward into the external acoustic canal. This cell migration is an important part of the tympanic membrane self-cleaning mechanism. Loss of this normal mechanism, resulting in trapping of keratin within the tympanic cavity, is relevant to cholesteatoma formation.
Following tympanic membrane perforation, epidermal cells proliferate and migrate through the perforation to make direct contact with the mucous epithelium of the tympanic membrane or cavity, creating mucocutaneous junctions (as observed in experimental and clinical studies).Reference Oktay, Cureoglu, Schachern, Paparella, Kariya and Fukushima13, Reference Becker, Naumann and Pfaltz24, Reference Ong, Narayanan, Godbole and Raman42 Extensive research on cytokeratins and, specifically, the Ki-67 protein (a keratinocyte proliferation marker) has helped to elucidate the role of epidermal migration in cholesteatoma pathogenesis. If infection intervenes, this migration process is arrested or terminated and hyperplasia commences, marking the onset of cholesteatoma formation.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1, Reference Chole, Sudhoff and Cummings8, Reference Kim, Tinling and Chole43–Reference Park, Min, Min, Jun, Seo and Kim48 Migration of epidermal epithelium can mark the onset of cholesteatoma formation, as noted in: human temporal bones with cholesteatoma and tympanic membrane perforation; experimentally induced tympanic membrane perforation in animal models; and surgically encountered tympanic membrane perforation or displacement.Reference Oktay, Cureoglu, Schachern, Paparella, Kariya and Fukushima13, Reference Murphy and March49 Perforations are not often associated with cholesteatoma, except in iatrogenic cases. In addition, the previously popular concept of a ‘marginal perforation’ is, rather, a mistaken description of a retraction pocket.
Cell hyperplasia and metaplasia
Hyperplasia (i.e. overproduction of the same cell type) is evident in cholesteatomas. It is known that lipid-driven mitogenetic and anti-apoptotic pathways contribute to hyperplasia during tumorigenesis.Reference Huisman, de Heer, Dijke and Grote16, Reference Louw and Claassen50 The mucosal layer of middle-ear spaces can undergo metaplasia (i.e. overproduction of transformed cells) when subject to infection. In this way, it has been suggested that cuboidal epithelial cells become lightly keratinising squamous epithelial cells. The assumption exists that nests (also known as colonies) of these cells in middle-ear spaces eventually contribute to the formation of occult cholesteatoma.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1, Reference Dornelles, Meurer, de Costa and Schweiger14 There is indirect clinical and experimental evidence for the pluripotential properties of simple squamous or cuboidal epithelium, but this concept still lacks sound proof.Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1, Reference Chole, Sudhoff and Cummings8 Nevertheless, tympanic membrane mucosa is often seen embedded in cholesteatoma perimatrix (forming enlarged, gland-like structures with mucous secretions), and the suggestion that such mucosa is involved in chronic inflammation, epidermal proliferation and bone destruction needs further investigation.Reference Nagai, Suganuma, Ide, Shimoda and Kato51
Cholesteatoma histopathology
Acquired cholesteatoma comprises a sac containing keratin, a surrounding keratinising squamous epithelial layer (i.e. a matrix) and an adjacent subepithelial connective tissue layer with a bounding mucous cuboidal epithelial layer (i.e. a perimatrix).Reference Dornelles, da Costa, Meurer and Scheiweger11, Reference Dornelles, da Costa, Meurer and Schweiger12, Reference Dornelles, Meurer, de Costa and Schweiger14 Different growth patterns have been documented for acquired cholesteatoma, depending on the aetiology, stage of development and extent of inflammation. In the case of attic and sinus cholesteatomas, Sudhoff and TosReference Sudhoff and Tos15, Reference Sudhoff and Tos38 have described a diagnostic growth pattern marked by the presence of papillary cones and keratin deposits within small lakes (termed lacunae) before cone fusion, or small spaces (termed cysts) after cone fusion, with eventual formation of an expanded cholesteatoma with a keratin sac. It is thought that the more severe or chronic the inflammation, the greater the impact on perimatrix–matrix interactions and layer thickness. Chronic and recurrent inflammations determine the aggressiveness and extent of cholesteatomas, and eruption of the mucosal layer is often evident. Cholesteatomas may also display haphazard growth with dispersed keratin deposits. It seems feasible that enhanced perimatrix–matrix interactions may eventually lead to such chaotic growth, in cases with evident mucocutaneous junctions (i.e. between the epidermal and mucosal layers of the tympanic membrane or tympanic cavity) or diseased tympanic cavity mucosa affecting the tympanic membrane. Since angiogenesis is a prerequisite for cholesteatoma growth and maintenance, it is conceivable that chronic otitis media with thick mucous secretions in the middle-ear spaces and mastoid air cells may contribute to hypoxic conditions and microvessel occlusion, eventually leading to apoptosis and deterioration of cholesteatoma tissue components.Reference Chole, Sudhoff and Cummings8, Reference Adunka, Gstoettner, Knecht and Kiener40, Reference Sudhoff, Dazert, Gonzales, Borkowski, Park and Baird52 Established cholesteatomas are marked by inflammatory granulation and fetid exudates with flakes of keratin.Reference Becker, Naumann and Pfaltz24
Pre-cholesteatoma and cholesteatoma conditions
Description
For the purposes of this review, a clear distinction is made between pre-cholesteatoma and cholesteatoma conditions. The presence of mucocutaneous junctions, marked by cell migration without inflammation, is considered a pre-cholesteatoma condition. Tympanic membrane retraction pockets and invaginations (such as found in atelectasis and adhesive otitis media) are also conditions that may lead to cholesteatoma. Cholesteatomas, triggered by chronic inflammation and marked by epidermal hyperplasia or diseased mucosa, present as cysts or tumour-like (i.e. mass-like) growths. It is argued that, although otitis externa can trigger epidermal hyperplasia (with epidermal projections into the tympanic cavity or epidermal ingrowths into the tympanic membrane), persistent inflammation in the tympanic membrane perimatrix and chronic middle-ear inflammation are factors in cholesteatoma growth, expansion and invasion, whilst chronic otitis media is the culprit where diseased mucosa can contribute to cholesteatoma development. It is argued that recurrent bouts of chronic infection contribute to cholesteatoma persistence and recurrence.Reference Chole, Sudhoff and Cummings8, Reference Sudhoff and Tos15, Reference Mishiro, Sakagami, Kitahara, Kondoh and Okumura21, Reference Mustafa, Heta, Kastrai and Dreshaj22, Reference Smith and Danner53, Reference Vikram, Khaja, Udayashankar, Venkatesha and Manjunath54
Pathological mechanisms
The pathological mechanisms encountered in pre-cholesteatoma and cholesteatoma conditions can be summarised as follows.
(1) Migration of keratinising squamous epithelium into the middle ear after tympanic membrane trauma or perforation, to form mucocutaneous junctions (via the phenomenon of contact guidance), in the absence of inflammation. This is a pre-cholesteatoma condition. (Evidence based on experimental research.)Reference Oktay, Cureoglu, Schachern, Paparella, Kariya and Fukushima13
(2) Hyperproliferation of keratinising squamous epithelium to form epidermal projections which may close membrane perforations (subject to inflammation), or protrusions into the tympanic cavity which progress toward cholesteatoma formation (subject to chronic inflammation). (Evidence based on enhanced perimatrix–matrix interactions and an established link between abnormal wound healing and cholesteatoma pathogenesis.)Reference Oktay, Cureoglu, Schachern, Paparella, Kariya and Fukushima13, Reference Huisman, de Heer, Dijke and Grote16
(3) Hyperplastic growth of keratinising squamous epithelial cells (as papillary ingrowths or cones) from focal areas of the epidermal basal layer within retraction pockets, triggering cholesteatoma formation, growth and expansion (subject to otitis externa and media). (Evidence based on clinical studies.)Reference Sudhoff and Tos15
(4) Hyperplastic growth of keratinising squamous epithelial cells (as papillary ingrowths or cones) from focal areas within an intact tympanic membrane, triggering the formation and growth of intratympanic membrane cholesteatoma (subject to otitis externa and persistent perimatrix inflammation). (Evidence based on clinical observation.)Reference Ekambar, Reddy, Goodyear, Ghosh and Lesser55
(5) Hyperplastic growth of scattered keratinising squamous epithelial cells in the middle ear (after injury or surgery), triggering cholesteatoma formation, growth and expansion (subject to chronic otitis media), with or without secondary tympanic membrane perforation. (A speculation.)
(6) Hyperplastic growth of single-layer cuboidal mucosal epithelium into squamous mucosal epithelium, with excessive mucus production, contributing to pre-cholesteatoma conditions with retraction pockets (subject to chronic otitis media) and cholesteatoma conditions (subject to otitis externa and media). (A postulation.)
(7) Metaplastic transformation of nests (also known as colonies) of middle-ear mucosal epithelial cells into lightly keratinising squamous epithelial cells, triggering cholesteatoma formation, growth and expansion (subject to chronic otitis media), with or without tympanic membrane involvement. (Based on indirect experimental evidence and limited clinical research.) Reference Olszewska, Wagner, Bernal-Sprekelson, Ebmeyer, Dazert and Hildmann1, Reference Chole, Sudhoff and Cummings8
(8) Metaplasia of mucosal cells (i.e. enlarged glands embedded in cholesteatoma perimatrix), with enhanced perimatrix–matrix interactions, leading to cholesteatoma growth and expansion (with or without chronic otitis media). (Based on clinical observation and laboratory research.)Reference Chole, Sudhoff and Cummings8, Reference Nagai, Suganuma, Ide, Shimoda and Kato51
According to Sudhoff and Toss,Reference Sudhoff and Tos15 it is difficult to find any clinical support for the migration and metaplasia theories of cholesteatoma formation. However, research using cell markers has confirmed that migration plays a role during normal or pre-cholesteatoma conditions.Reference Park, Min, Min, Jun, Seo and Kim48 Other investigations have shown that metaplasia of mucosal epithelium into lightly keratinising squamous epithelium like masses can only be feasible in the absence of congenital remnants, skull trauma or previous middle-ear surgery.Reference Isaacson56
Cholesteatoma pathogenesis: stepwise explanations
Although different views on the migration, hyperplasia and metaplasia theories of acquired cholesteatoma formation have been expounded in the literature, it is conceivable that a combination of these theories may contribute to the pathogenesis of acquired cholesteatoma. For the purposes of this review, the pathogenesis of acquired cholesteatoma is approached from the simplest level, according to (1) tympanic membrane trauma (i.e. perforations, displacements and retractions) and (2) inflammation (either prolonged or chronic). The first category represents pre-cholesteatoma conditions, whilst the second category is a prerequisite for cholesteatoma formation, growth and expansion (with or without tympanic membrane involvement). Stepwise explanations of acquired cholesteatoma pathogenesis, according to trauma, prolonged inflammation and chronic inflammation, are given in Tables I to III, and illustrated in Figure 1.
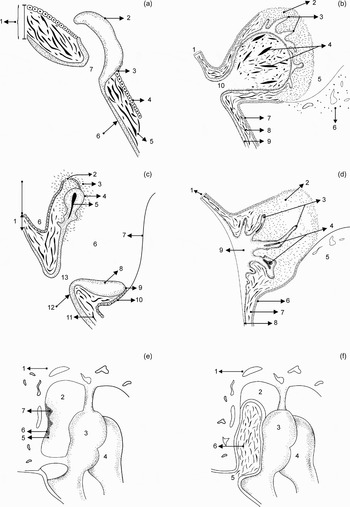
Fig. 1 Schematic representation of cholesteatoma pathogenesis. (a) Epidermal projection: 1 = tympanic membrane; 2 = papillary projection; 3 = mucocutaneous junction; 4 = mucosal layer; 5 = thinsp;connective tissue; 6 = epidermal layer; 7 = perforation. (b) Protruding mass: 1 = tympanic membrane; 2 = eruption of mucosal layer; 3 = chronic infection; 4 = papillary growth; 5 = keratin in sac; 6 = tympanic cavity; 7 = malleus; 8 = papillary projection; 9 = mucocutaneous junction; 10 = mucosal layer. (c) Retraction pocket: 1 = tympanic membrane; 2 = chronic inflammation; 3 = papillary growths; 4 = keratin in retraction pocket; 5 = tympanic cavity; 6 = bone; 7 = mucosal layer; 8 = connective tissue; 9 = keratinising squamous epithelium; 10 = invagination; 11 = connective tissue; 12 = epidermal layer; 13 = perforation. (d) Epidermal hyperplasia: 1 = tympanic membrane; 2 = chronic inflammation; 3 = papillary growths; 4 = keratin in cysts; 5 = bone; 6 = mucosal layer; 7 = connective tissue; 8 = epidermal layer; 9 = epidermal hyperplasia. (e) Mucosal metaplasia: 1 = bone; 2 = epitympanic recess; 3 = malleus; 4 = incus; 5 = chronic inflammation; 6 = mucosal epithelium; 7 = mucosal nests. (f) Cholesteatoma with secondary membrane perforation: 1 = bone; 2 = epitympanum; 3 = malleus; 4 = incus; 5 = secondary perforation; 6 = attic cholesteatoma.
Table I Trauma (pre-cholesteatoma conditions)

Table II Prolonged inflammation (cholesteatoma conditions)

Table III Chronic inflammation (cholesteatoma conditions)

Conclusions
Despite previous controversies, there is now progress toward a better understanding of the pathogenesis of acquired cholesteatoma. The onset of the disease is clearly diverse, and different growth patterns are encountered. Trauma and/or disease of the tympanic membrane is generally involved, and the various growth patterns are marked by: (1) epidermal migration, mucocutaneous junction formation, and formation of tumours or masses with haphazard growth and dispersed keratin deposits; or (2) epidermal hyperplasia, papillary cone formation and fusion with keratin deposits in lacunae or cysts, and expansion into a typical cholesteatoma (i.e. a keratin sac surrounded by matrix and perimatrix), depending on the stage of development during clinical observation. The metaplasia theory cannot be over-ruled; however, substantial support for this theory is still lacking. It is evident that diseased mucosa in the tympanic cavity (with chronic otitis media with effusion) can contribute to pre-cholesteatoma conditions (i.e. tympanic membrane retraction pockets or invaginations) which may progress to cholesteatoma when subject to local and chronic inflammation. It is also plausible that embedded mucosal glands and mucous secretions within the cholesteatoma perimatrix may enhance chronic inflammation, by which means cholesteatoma growth could occur in the absence of chronic otitis media.
The various cholesteatoma triggers are clinically relevant when contemplating surgical correction, while the role of inflammation in cholesteatoma formation, growth and expansion is important when considering preventative therapy. While advanced antibiotic therapy can reduce the chronic inflammation associated with cholesteatoma development, complete surgical removal of the cholesteatoma and any diseased mucosa is required to prevent disease recurrence.






