INTRODUCTION
Parasitic organisms are characterized by a ‘dual’ environment. On the one hand, this environment is represented by their hosts that provide parasites with both food resources and a place for living, mating and reproducing. On the other hand, it is represented by abiotic factors surrounding the hosts. This is especially true for ectoparasitic arthropods that are strongly affected by the off-host environment (see Marshall, Reference Marshall1981 for review). However, duration of a contact with a host varies among taxa of ectoparasitic arthropods. Based on this duration, Lehane (Reference Lehane2005) proposed to distinguish temporary, periodic and permanent ectoparasites. Temporary ectoparasites are largely free-living and visit a host for enough time to take a blood meal (e.g. mosquitos). Periodic ectoparasites spend considerably longer time on a host than is required merely to obtain a blood meal but nevertheless spend a significant amount of time off-host (e.g. most fleas and gamasid mites). Permanent ectoparasites spend their entire life on a host which thus represents their ultimate habitat (lice).
Obviously, the relative effects of factors associated with the abiotic environment and factors associated with the hosts per se on distribution of ectoparasites are expected to differ among the three categories. Temporary ectoparasites are affected mainly by the abiotic environment, permanent ectoparasites mainly by the host-related factors and periodic parasites equally by both. These expectations were largely supported by many studies of temporary (e.g. Jore et al. Reference Jore, Vanwambeke, Viljugrein, Isaksen, Kristoffersen, Woldehiwet, Johansen, Brun, Brun-Hansen, Westermann, Larsen, Ytrehus and Hofshagen2014) and periodic (e.g. Linardi and Krasnov, Reference Linardi and Krasnov2013) ectoparasites. In contrast, studies of the environment- and host-related effects on distribution of permanent ectoparasites such as sucking lice (Insecta: Anoplura) are rare (but see Balashov et al. Reference Balashov, Bochkov, Vashchenok, Grigor'eva and Tret'akov2002). In fact, the majority of studies on sucking lice (apart from studies of lice parasitic on humans) are either taxonomical (e.g. Durden and Eckerlin, Reference Durden and Eckerlin2001; Durden and Timm, Reference Durden and Timm2001; Musser et al. Reference Musser, Durden, Holden and Light2010) or present description(s) of the assemblages of lice recorded on one or more host species from a defined geographic region (e.g. Haitlinger, Reference Haitlinger1983; Durden et al. Reference Durden, Kollars, Patton and Gerhardt1997; Smith et al. Reference Smith, Light and Durden2008; Oguge et al. Reference Oguge, Durden, Keirans, Balami and Schwan2009). Ecological studies of sucking lice are scarce and unevenly distributed among host taxa. For example, and taking aside studies of lice of high medical and veterinary importance (those parasitic on humans and domestic animals), the ecology of lice parasitic on aquatic mammals (pinnipeds and sea otters; see review in Leonardi and Palma, Reference Leonardi and Palma2013) is known better than the ecology of lice parasitic on small mammals.
Earlier studies that considered the ecology of sucking lice on small mammals were purely descriptive and presented a narrative rather than formal analyses of the factors affecting abundance and/or distribution of lice (e.g. Sosnina et al. Reference Sosnina, Nazarova and Sadekova1981). A few recent studies that analysed these factors usually focused on a single host species and either single louse species (e.g. Mize et al. Reference Mize, Tsao and Maurer2011; Archer et al. Reference Archer, Bennett, Ueckermann and Lutermann2014) or the pooled data on several louse species (Fernandes et al. Reference Fernandes, Cruz and Linhares2012) and did not reveal whether the effects they found represented a general trend. Here, we studied the relative effects of the environment- (habitat and season) and host-related (sex and body mass) factors on the occurrence of louse infestation in six rodent–louse associations from Eastern Slovakia. We asked how these factors influenced the occurrence of lice on an individual host and whether different rodent–louse associations demonstrated consistent trends in these effects.
We predicted that the probability of an individual rodent being infested by lice will be higher in lowland than in mountain habitats, during warm rather than cold seasons, in males rather than females and will either increase or decrease with increasing body mass. Higher probability of louse infestation in lowlands as compared with mountains and during warm as compared to cold seasons was expected because of the positive effect of higher temperature on fecundity and rate of development in lice (see Marshall, Reference Marshall1981 for review). Slovakian lowlands are, in general, warmer than mountains (Mazur and Jakal, Reference Mazur and Jakal1982). In addition, earlier observations reported higher abundance and prevalence of lice on rodents during warm seasons (e.g. Sosnina et al. Reference Sosnina, Nazarova and Sadekova1981). Male-bias in louse infestation was reported for a variety of louse–host associations (Krasnov and Matthee, Reference Krasnov and Matthee2010; Matthee et al. Reference Matthee, McGeoch and Krasnov2010; Fernandes et al. Reference Fernandes, Cruz and Linhares2012) and has been considered as a manifestation of a commonly observed phenomenon of male-biased parasitism in small mammals (see Krasnov et al. Reference Krasnov, Bordes, Khokhlova and Morand2012 for review). The effect of body mass was expected due to the correlation between body mass and age of a rodent. Earlier studies of age-dependence of non-louse ectoparasites on rodents demonstrated contrasting patterns of the effect of host age on parasite abundance and/or prevalence (either predominantly positive or predominantly negative), being dependent on natural history parameters of a host species, such as patterns of post-natal growth and dispersal, spatial distribution and the structure of shelters (Krasnov et al. Reference Krasnov, Stanko and Morand2006a ; see also Pacala and Dobson, Reference Pacala and Dobson1988).
In this study, we used data from a broad ectoparasitological survey that was aimed to investigate abundance and distribution of various taxa of blood-feeding arthropods parasitic on small mammals. Data on fleas, ticks and gamasid mites from this survey can be found elsewhere (e.g. Stanko, Reference Stanko1994; Stanko et al. Reference Stanko, Miklisová, Goüy de Bellocq and Morand2002, Reference Stanko, Krasnov, Miklisová and Morand2007; Krasnov et al. Reference Krasnov, Stanko and Morand2006b , Reference Krasnov, Stanko and Morand2007, Reference Krasnov, Stanko and Morand2010).
MATERIALS AND METHODS
Data collection
Small mammals were sampled and lice were collected during 15 years across Eastern Slovakia. Mammals were captured using snap-traps following the same protocol at each of 102 trapping sessions (see details in Stanko, Reference Stanko1988, Reference Stanko1994). In brief, traps were distributed in lines of 50 traps with 10 m distance between the consecutive traps. Trapping sessions (on average, 700 traps per session, ranging from a total of 100–2000 traps/nights) lasted one to six nights. In each session, traps were opened in the late evening, checked early in the morning and operated, on average, for 7 h. The number of trapped mammals ranged from eight to 395 per trapping session. Each trapped animal was identified, sexed, weighed and examined for ectoparasites. The animal's fur was combed thoroughly, using a toothbrush, over a plastic pan and ectoparasites (lice, fleas, ticks and mites) were carefully collected. Trapping grids were distributed across two main habitat types, namely the lowlands (70 trapping sessions) and the mountains (32 trapping sessions). Lowland habitats were situated at elevations between 100 and 200 m above sea level. They included lowland river valleys with floodplain forests (dominated by Fraxinus angustifolia, Quercus robur, Carpinus betulus, Salix alba, Salix fragilis and Populus alba), woodland belts (represented by 3–8 rows of a poplar, Populus canadensis, and various shrubs such as Prunus sp., Rosa sp., Sambuccus nigra, with herbal floor composed mainly of Urtica dioica), and agricultural fields (mainly wheat and maize as well as stubble and shrubbery dominated by Prunus spinosus, Rosa canina and Crataegus sp. with sporadic occurrence of poplar and willow trees). Mountain habitats were situated at elevations from 300 to 1100 m above sea level. They included submontane and montane brook valleys (dominated by Alnus glutinosae, Alnus incanae, Fagus sylvaticus and C. betulus), submontane (oak-hornbeam) and montane (beech and beech-maple) forests, shrubbery patches on pastures (P. spinosus, Corylus avellana and R. canina) as well as gardens and orchards in public green spaces within cities at elevation of 650–750 m above sea level. Mean July and January air temperatures in the lowlands are 20 and −4 °C, respectively; while in the mountains they are 15·5 and −6 °C, respectively (Mazur and Jakal, Reference Mazur and Jakal1982). Mean annual amount of rainfall is 550–560 mm in the lowlands and 800–1000 mm in the mountains (Mazur and Jakal, Reference Mazur and Jakal1982).
A total of 9490 individuals belonging to 23 species of small mammals (rodents and insectivores) were trapped, of which 2348 individuals were infested with lice belonging to five species (see Results section).
Data analysis
We analysed factors affecting the occurrence of lice separately for each host and each louse species. For these analyses, we selected only those host–louse associations in which (a) at least 50 host individuals were captured and (b) mean louse abundance and prevalence attained at least 0·10 lice per individual or 10%, respectively. This resulted in six associations used in the analyses (see Results section).
We chose to analyse the occurrence rather than the number of lice on an individual host because the majority of animals were not infested, thus resulting in a large number of zeroes in the dataset. Consequently, our response variable was dichotomous and took a value of either 1 or 0 if an individual was either infested with at least one louse or not, respectively. We analysed the response variable using generalized linear mixed-effects models (GLMM) with the binomial error and logit-link function. Categorical independent variables were habitat (lowland vs mountain), sex, and season (warm vs cold). We considered 16 April till 15 October as the warm season and 16 October to 15 April as the cold season. In addition, we included in the models body mass of an individual mammal (a proxy for age) as a continuous variable. This variable was log-transformed prior to analysis. Because more than one sample was taken per year, we included a year of sampling as a random variable in our models. To fit GLMMs, we used the function glmer from the package ‘lme4’ (Bates et al. Reference Bates, Maechler, Bolker and Walker2014a , Reference Bates, Maechler, Bolker and Walker b ) implemented in R 3·0 environment (R Core Team, 2013). First, we ran the models with all fixed effects, the interactions between season and either sex or habitat, and a random effect (a year of sampling). We included (a) the interaction between sex and season because host sex-related pattern of infestation by ectoparasites could depend on season (Krasnov et al. Reference Krasnov, Morand, Hawlena, Khokhlova and Shenbrot2005a , Reference Krasnov, Bordes, Khokhlova and Morand2012; Kiffner et al. Reference Kiffner, Stanko, Morand, Khokhlova, Shenbrot, Laudisoit, Leirs, Hawlena and Krasnov2013) and (b) the interaction between habitat and season because seasonal variation in climate can be pronounced differently in lowland and mountain habitats, resulting in differential seasonal effects on ectoparasites. Then, we selected the best model based on Akaike Information Criterion (AIC) using the function dredge from package ‘MuMIn’ (Barton, Reference Barton2014) implemented in R, and ran the best model again. This repeatedly fits models with different number of predictors extracted from the global model (Barton, Reference Barton2014). We calculated both marginal (that is for the model containing fixed effects only) and conditional (for the model containing both fixed effects and a random effect) coefficients of determination (R 2) following Nakagawa and Schielzeth (Reference Nakagawa and Schielzeth2013). Finally, to evaluate the overall fit of the best model, we compared it and the model with an intercept and a random effect only using the likelihood ratio test.
Confidence intervals for values of prevalence for the six host–louse associations in dependence of habitat, sex and season were calculated as the adjusted Wald-Sterne's intervals (Reiczigel, Reference Reiczigel2003) using Quantitative Parasitology 3·0 (Rózsa et al. Reference Rózsa, Reiczigel and Majoros2000). Differences in rodent densities between habitat types were analysed using Student's t-test.
RESULTS
Data on mean abundance and prevalence of five louse species recorded on 23 species of small mammals are presented in Table 1. Based on the cut-off values (see Methods section), in the following analyses we focused on six host–louse associations, namely Apodemus agrarius–Hoplopleura affinis, Apodemus agrarius–Polyplax serrata, Apodemus flavicollis–P. serrata, Apodemus uralensis–P. serrata, Microtus arvalis–Hoplopleura acanthopus and Myodes glareolus–Hoplopleura edentula.
Table 1. Mean abundance (A) and prevalence (P, %) of five louse species recorded on 23 small mammalian species in Eastern Slovakia
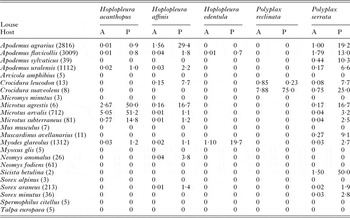
In parenthesis – number of captured and examined individuals.
GLMMs indicated significant effects of at least one environment-related factor and at least one host-related factor on the occurrence of lice on a host individual in five of six host–louse associations (except for A. uralensis–P. serrata association; Table 2). Effects of the categorical factors were manifested in the patterns of prevalence of louse infestation within host–louse associations between habitats, sexes and seasons (Table 3). The significant effect of habitat was found in two associations (A. agrarius–P. serrata and M. glareolus–H. edentula). The sign of the estimate suggests that the occurrence of lice was more frequent in individual rodents occupying lowland than mountain habitats (Tables 2 and 3). The effect of season on the occurrence of lice was significant in five associations with the estimate being positive (higher occurrence during warm seasons) in four associations and negative (higher occurrence during cold seasons) in one association (M. arvalis and H. acanthopus) (Tables 2 and 3). Sex of an individual affected significantly the occurrence of lice in three associations (A. agrarius and A. flavicollis with P. serrata and M. arvalis with H. acanthopus) being higher in males (Tables 2 and 3). Finally, the effect of host body mass on the occurrence of lice was found in all five associations, being negative in A. agrarius and A. flavicollis and positive in M. glareolus and M. arvalis (Table 2, see illustrative examples with A. agrarius and M. arvalis in Fig. 1).
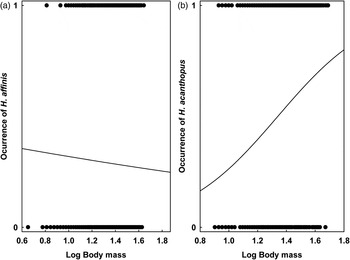
Fig. 1. The effect of body mass of an individual (a) A. agrarius and (b) M. arvalis on the occurrence of lice.
Table 2. Summary of GLMM (binomial error and logit-link function) of the effects of habitat, host sex, season and host body mass on the occurrence of lice on an individual host for six host–louse associations
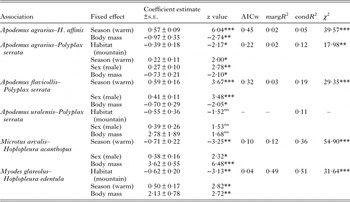
Year of sampling was introduced as a random effect in each model. Reference levels for the fixed effects were lowland (for habitat), female (for sex) and cold (for season). AICw – Akaike Information Criterion weight of the best model from a set of models derived from a full model (all fixed effects, the interactions between sex and season and between habitat and season, and a year of sampling as a random effect; see main text for explanations). margR2 – marginal coefficient of determination, condR2 – conditional coefficient of determination. Significance levels: * – <0·05, ** – <0·01, *** – <0·001ns – non-significant. χ 2 – likelihood ratio χ 2 when comparing the best model with the model of intercept and a random effect only.
Table 3. Prevalence (%) (in parentheses 95% confidence intervals) of infestation of five rodent species by four lice species in dependence of habitat, host sex and season

DISCUSSION
Although the explanatory power of models of the occurrence of lice on an individual host as affected by the environment- and host-associated factors was not especially high, results of our study indicate that these factors affect the probability of an individual host to be infested. We found this indication in the majority (five of six) of studied host–louse associations. It is unclear why no significant effect of any factor was found for the occurrence of P. serrata on A. uralensis. Extremely low numbers and prevalence of this louse on this host is the most likely explanation of this lack of significant effects (Table 1). In addition, A. uralensis is the least promiscuous among Apodemus species, so that each animal usually contacts with a very limited number of individuals of the opposite sex (Bryja et al. Reference Bryja, Patzenhauerová, Albrecht, Mošanský, Stanko and Stopka2008) which may impede the transmission of lice.
Furthermore, we found consistent trends in the effect of a factor on the louse occurrence for some factors (habitat and sex) and contrasting patterns for other factors (season and body mass). The mechanism behind these effects is interplay between louse and host ecology. This is because the occurrence of lice on a host is determined by a variety of factors including the effects of physiology, immunology and ant-parasitic behaviour of an individual host on louse reproduction and survival as well as the effects of the host population density and its social/spatial behaviour on the processes of louse transmission.
Habitat-dependence
In both host–louse associations for which the effect of habitat was significant, higher frequency of infestation occurred in the lowlands. Earlier studies that reported habitat differences in louse infestation often explained this pattern by difference in the host density that, in turn, could affect the transmission of lice among host individuals (e.g. Fernandes et al. Reference Fernandes, Cruz and Linhares2012 for lice on Oligoryzomys nigripes in Brazil). However, this explanation does not look feasible in our study because in both hosts for which the significant habitat effect on louse infestation was found, densities (calculated as the number of animals captured per 100 trap/nights) in lowland and mountain habitats where similar (Student's t-test; t = −0·28 for A. agrarius and t = 0·12 for M. glareolus; d.f. = 100, P > 0·80 for both), while they differed in those hosts which did not demonstrate habitat-dependence of infestation (t = −7·28 for A. flavicollis and t = 2·55 for M. arvalis; d.f. = 100, P < 0·05 for both). It is likely therefore that habitat dependence of the occurrence of lice was due to abiotic variables that undoubtedly differ between the two habitat types.
Lowland habitats in Slovakia are warmer than the mountains (Mazur and Jakal, Reference Mazur and Jakal1982). Consequently, the occurrence of lice could be higher in the lowlands because higher temperature (although not too high) favours survival of pre-imaginal lice (e.g. Colwell, Reference Colwell2014) as well as their rate of development (e.g. Leeson, Reference Leeson1941) and the rate of oviposition by adults (e.g. Schrader et al. Reference Schrader, Schmolz, Könning, Dahl, Robinson and Bajomi2008). However, Slovakian lowlands are also drier than the mountains (Mazur and Jakal, Reference Mazur and Jakal1982). The results of studies of the effects of habitat-associated moisture on lice parasitic on mammals are contradictory and differ among louse species, host species and geographic regions. For example, infestation of small mammals by sucking lice was found to be higher in drier than wetter habitats in North America (Mize et al. Reference Mize, Tsao and Maurer2011), but the opposite was true in Africa (Oguge et al. Reference Oguge, Durden, Keirans, Balami and Schwan2009; see also Moyer et al. Reference Moyer, Drown and Clayton2002 and Calvete et al. Reference Calvete, Estrada, Lucientes and Estrada2003 for chewing lice parasitic on birds in North America and Southern Europe, respectively). This contradiction could arise due to, for example, among-louse species variation in the response to humidity.
The reason why the effect of habitat was found in only two host–louse associations is still unclear and warrants further investigation. On the one hand, the fact that this pattern was found in P. serrata but not in H. affinis, both parasitic on the same host (A. agrarius) suggests that some between-louse differences in sensitivity to abiotic factors play a role. On the other hand, habitat-dependence of the louse occurrence was found in P. serrata parasitic on A. agrarius but not on A. flavicollis. This suggests also a role of some host-associated mechanisms. However, P. serrata exploiting A. agrarius and those exploiting A. flavicollis might belong to different lineages (see Štefka and Hypša, Reference Štefka and Hypša2008) and thus the former explanation seems more likely.
Seasonal changes
Three of four hosts (four of five host–louse associations) demonstrated higher occurrences of louse infestation during the warm season. Seasonal differences in abundance and prevalence of sucking lice on small mammals have been reported for a variety of geographic regions (Sosnina et al. Reference Sosnina, Nazarova and Sadekova1981; Wilson et al. Reference Wilson, Telford and Forrester1991; Archer et al. Reference Archer, Bennett, Ueckermann and Lutermann2014). Similarly to our results, the highest abundance and prevalence of lice in the temperate areas have been found in the warmer months (Sosnina et al. Reference Sosnina, Nazarova and Sadekova1981; Haitlinger, Reference Haitlinger1983; Krištofík and Lysy, Reference Krištofík and Lysy1992). This was explained not only by the favourable air temperature but also by an increase in density and mobility of hosts (Sosnina et al. Reference Sosnina, Nazarova and Sadekova1981). The latter presumably facilitates transmission of lice among host individuals, thus not only increasing louse prevalence but also mating chances of lice. This, in turn, may lead to higher reproductive rates and, eventually, to higher abundances. The only host species in our study in which more individuals were infested by lice during cold than warm seasons was the common vole, M. arvalis. In contrast to other studied rodents, this species is colonial. In winter, non-hibernating small mammals of the temperate zone, such as M. arvalis, spend much time in the subnivean space (i.e. between the snow cover and the ground) (e.g. Bashenina, Reference Bashenina1962; Fuller, Reference Fuller1967; Pruitt, Reference Pruitt and Merritt1984; Korslund and Steen, Reference Korslund and Steen2006) and their activity level decreases at this time (e.g. Eccard and Herde, Reference Eccard and Herde2013). Thus, in M. arvalis, time spent in contact with other members of the colony might substantially increase in the cold season, which results in a greater rate of the louse transmission between individuals and, eventually, in a higher occurrence of infestation. In addition, the summer nests of M. arvalis are usually occupied by a single female with its offspring, whereas the winter nests are occupied by many unrelated individuals (Chełkowska, Reference Chełkowska1978). This is another, but not necessarily an alternative mechanism of the higher number of individual voles infested by lice during cold as compared to warm seasons.
Male bias
Male-biased parasitism has long been a popular concept based on numerous observations that mammalian and avian males are usually infested with more parasite individuals and/or species than females (see reviews in Zuk and McKean, Reference Zuk and McKean1996; Krasnov et al. Reference Krasnov, Bordes, Khokhlova and Morand2012). However, recent studies challenged this concept and demonstrated that it is far from being universal rule (Kiffner et al. Reference Kiffner, Stanko, Morand, Khokhlova, Shenbrot, Laudisoit, Leirs, Hawlena and Krasnov2013, Reference Kiffner, Stanko, Morand, Khokhlova, Shenbrot, Laudisoit, Leirs, Hawlena and Krasnov2014). Nevertheless, whenever a significant effect of host sex on the occurrence of lice was found in our study (three of six host–louse associations), it indicated that the probability being infested was higher for a male than a female rodent. Male-biased infestation by sucking lice has been found in a variety of louse and host species in different geographic regions including Polyplax arvicanthis on Rhabdomys pumilio in South Africa (Matthee et al. Reference Matthee, McGeoch and Krasnov2010) and several Hoplopleura lice on O. nigripes in South America (Fernandes et al. Reference Fernandes, Cruz and Linhares2012). However, the lack of any gender bias in louse infestation has been reported as well (Scantlebury et al. Reference Scantlebury, Maher McWilliams, Marks, Dick, Edgar and Lutermann2010; Viljoen et al. Reference Viljoen, Bennett, Ueckermann and Lutermann2011). Explanations of male-biased infestation usually involve either lower immunocompetence in males due to the immunosuppressive effect of testosterone or their higher mobility resulting in higher exposure to parasites or both. Both these mechanisms could be implied for the explanation of the patterns found in our study. In other words, gender-specific behaviour and physiology of the host are the most likely reasons behind gender-biased infestation. For example, male M. arvalis are more often involved in aggressive interactions than females and the testosterone levels in these males are elevated (Gromov and Voznesenskaya, Reference Gromov and Voznesenskaya2010). In contrast, male M. glareolus usually avoid one another (Łopucki, Reference Łopucki2007). These differences explain why the male bias in louse infestation was found in the former but not in the latter species. Although lice spend their entire life on the hosts, they are transmitted between hosts via their contacts. Males of M. arvalis continuously migrate from colony to colony while this is not the case for females (Gauffre et al. Reference Gauffre, Petit, Brodier, Bretagnolle and Cosson2009). This dispersal pattern might be another mechanism underlying male-biased parasitism in this rodent. However, we also found male-biased infestation of the same host species (A. agrarius) by one (P. serrata) but not another (H. affinis) louse species. This suggests that the manifestation of gender-biased parasitism may depend not only on the life history and/or physiological traits of a host species, but also on some, still unknown, traits of a parasite species.
Effect of host body mass: age or body condition?
Body mass of an individual rodent could be indicative of either its age or body condition. It is commonly known that parasite abundance and the pattern of its distribution often vary between younger and older hosts (e.g. Goater and Ward, Reference Goater and Ward1992). Moreover, studies from the number of host–parasite systems demonstrated that shape of the relationships between host age and parasite abundance and/or distribution varies among host–parasite associations because these relationships are generated by different mechanisms (see review in Hudson and Dobson, Reference Hudson, Dobson, Grenfell and Dobson1995). Indeed, substantial variation in the relationships between abundance and distribution of parasites and host age/body size has been shown for small mammals and fleas (Krasnov et al. Reference Krasnov, Stanko and Morand2006a ). Similar results were found for lice in this study. The most likely reason for the variation in host age–louse occurrence patterns is differences in the natural history of host species. We found a decrease in louse occurrence with increasing body mass in both wood mice (Apodemus) and the opposite trend in both voles (Myodes and Microtus). The explanation for this difference is that the relationship between host age and louse infestation is affected by the pattern of the host's postnatal growth. Indeed, wood mice attain a definitive size at about 40 days and grow extremely slow afterwards (e.g. Apodemus semotus; Lin et al. Reference Lin, Nishino and Shiraishi1993). In contrast, period of continuous growth in voles is at least twice (Microtus cabrerae; Fernández-Salvador et al. Reference Fernández-Salvador, García-Perea and Ventura2001) or thrice (Microtus montebelli; Nakatsu, Reference Nakatsu1975) longer. Consequently, the heaviest cohort of wood mice is a mix of individuals of median age and old individuals, whereas the heaviest cohort of voles is represented mainly by old individuals. The immune function in the individuals of median age is strong, whereas it deteriorates in old individuals (see review in Miller, Reference Miller1996). Lower defensibility of old animals makes them better patches for lice. Consequently, the difference in age composition of the heaviest individuals between wood mice and voles could be manifested in the contrasting patterns of body mass–louse infestation relationship.
The effect of the host's body condition on parasites has been studied in a variety of animals (e.g. Oppliger et al. Reference Oppliger, Christe and Richner1996; Brown et al. Reference Brown, Loosli and Schmid-Hempel2000; Krasnov et al. Reference Krasnov, Khokhlova, Arakelyan and Degen2005b ). On the one hand, a host in good condition may provide parasites with resources of a higher quality than a host in poor condition (Dawson and Bortolotti, Reference Dawson and Bortolotti1997). On the other hand, parasites may benefit from exploiting hosts of the poorer body condition because their immune system is weaker (Simon et al. Reference Simon, Thomas, Blondel, Lambrechts and Perret2003). It is not surprising, therefore, that studies of the effect of the host's body condition on parasites produced contradictory results with parasite performance in hosts in the good conditions being either better (e.g. Blanco et al. Reference Blanco, Tella and Potti1997) or worse (e.g. Whiteman and Parker, Reference Whiteman and Parker2004) than in hosts in the poor conditions. Moreover, the opposite patterns of parasite performance in hosts of different nutritional statuses were reported for parasites belonging to the same taxon (fleas; Krasnov et al. Reference Krasnov, Khokhlova, Arakelyan and Degen2005b vs Tschirren et al. Reference Tschirren, Bischoff, Saladin and Richner2007). In our study, we found negative relationships between host body mass and the occurrence of lice in three associations and positive relationships in two associations. These opposite patterns could stem from differences in the co-evolutionary history of different host–parasite systems (Tschirren et al. Reference Tschirren, Bischoff, Saladin and Richner2007). This explanation is indirectly supported by the fact that we found similar patterns in closely-related (among mice or among voles) but different patterns in distantly related hosts (between mice and voles). We also found the same pattern in the same louse (P. serrata) parasitic on either A. agrarius or A. flavicollis. Moreover, the opposite patterns of the relationships between infestation and the host's body condition among host–parasite associations may arise due to either differential immunological responses of the same host to attacks by different parasites or differential responses of the same parasite to the defence efforts of different host or both (e.g. Khokhlova et al. Reference Khokhlova, Spinu, Krasnov and Degen2004).
We conclude that, despite being permanent ectoparasites, the distribution of sucking lice among host individuals was influenced not only by the host-related but also by the environment-related factors. However, the effects of the environment-related factors could be mediated via life history parameters of a host such as reproductive and dispersal patterns, social behaviour and spatial distribution.
ACKNOWLEDGEMENTS
The authors thank two anonymous referees for their helpful comments. Allan Degen read an earlier version of the manuscript, made helpful comments and improved our English prose. The study was carried out under the licenses of the Ministry of Environment of the Slovak Republic No. 297/108/06-3·1 and No. 6743/2008-2·1. The authors also thank L. Mošanský and M. Onderová for field and laboratory assistance.
FINANCIAL SUPPORT
This study was supported by grants from VEGA (1/0390/12 to M.S.) and (partly) the Israel Science Foundation (grant No. 26/12 to B.R.K. and I.S.K.). This is publication number 857 of the Mitrani Department of Desert Ecology.







