Introduction
Purple witchweed [Striga hermonthica (Delile) Benth.], a root hemiparasitic weed, is one of the most devastating constraints to corn (Zea mays L.) production in Africa. Striga causes yield losses of up to 100%, translating to more than US$7 billion yearly, and this affects livelihoods of more than 300 million people worldwide (Ejeta Reference Ejeta2007). Among the five major Striga species (S. hermonthica, Asiatic witchweed [Striga asiatica (L.) Kuntze], cowpea witchweed [Striga gesnerioides (Willd.) Vatke], Striga aspera (Willd.) Benth., and Striga forbesii Benth.), S. hermonthica and S. asiatica are the most economically devastating.
Striga has a highly coordinated life cycle. Its association with the host starts with perception of strigolactones—hormones exuded by host roots that serve as germination stimulants. After germination, Striga immediately develops a specialized feeding structure—the haustorium—in response to haustorium-inducing factors from the host. The haustorium penetrates the host root tissues until it connects to the host xylem and siphons nutrients, leading to host growth retardation, stunting, and chlorosis. Striga then emerges from the soil and flowers to produce up to 20,000 viable seeds (Teka Reference Teka2014).
Striga management approaches include: intercropping hosts with trap crops that induce suicidal germination of Striga seeds, application of soil amendments such as fertilizer and manure, hand pulling of emerged Striga, and application of herbicides (Atera et al. Reference Atera, Ishii, Onyango, Itoh and Azuma2013; Ejeta Reference Ejeta2007; Teka Reference Teka2014). These strategies are only moderately effective, because Striga continues to expand its natural range and cause more crop losses.
The most effective and sustainable control strategy is an integrated approach that uses innate host-derived resistance. Therefore, identification of new sources of Striga resistance has been prioritized in Striga-resistance breeding programs. Sources of resistance to Striga have been identified in maize (Amusan et al. Reference Amusan, Rich, Menkir, Housley and Ejeta2008), rice (Oryza sativa L.) (Gurney et al. Reference Gurney, Slate, Press and Scholes2006), sorghum [Sorghum bicolor (L.) Moench.] (Haussmann et al. Reference Haussmann, Hess, Omanya, Folkertsma, Reddy, Kayentao, Welz and Geiger2004; Mbuvi et al. Reference Mbuvi, Masiga, Kuria, Masanga, Wamalwa, Mohamed, Odeny, Hamza, Timko and Runo2017; Mohamed et al. Reference Mohamed, Ellicott, Housley and Ejeta2003), and cowpea [Vigna unguiculata (L.) Walp.] (Menkir Reference Menkir2006). Such host-based Striga-resistance mechanisms act either before (preattachment resistance) or after physical contact with the host (postattachment resistance). Preattachment resistance occurs when a host produces low amounts of strigolactones or when Striga receptors that perceive germination stimulants are insensitive to the strigolactone produced by the host. This is because strigolactone must bind to hypersensitive to light receptors in Striga for germination to occur. Binding causes degradation of an F-box protein, which in turn activates gene regulatory processes that lead to Striga germination (Lumba et al. Reference Lumba, Holbrook-Smith and McCourt2017). There is a great diversity of strigolactones produced by different hosts, each with different receptor-binding efficiencies based on their chemical structures (Yoneyama et al. Reference Yoneyama, Awad, Xie, Yoneyama and Takeuchi2010). For example, the Striga-resistant sorghum variety ‘SRN39’ was found to produce low amounts of orobanchol, resulting in low Striga germination (Gobena et al. Reference Gobena, Shimels, Rich, Ruyter-Spira, Bouwmeester, Kanuganti, Mengiste and Ejeta2017). Preattachment resistance can also be due to less production of haustorial initiation factors, therefore causing failure in effective development of haustorium (Rich et al. Reference Rich, Grenier and Ejeta2004).
In contrast, postattachment Striga-resistance mechanisms act after Striga has attached and attempted to penetrate the host. These mechanisms result in physiological or biochemical barriers that prevent the Striga haustorium from connecting to the host’s xylem. Host plants can also produce secondary metabolites that block parasite ingression or induce a hypersensitive immune response at the host–parasite interface (van Dam and Bouwmeester Reference van Dam and Bouwmeester2016). In some instances, Striga produces enzymes that degrade host tissues and barriers before making a connection to the host’s xylem (Maiti et al. Reference Maiti, Ramaiah, Bisen and Chidley1984; Rogers and Nelson Reference Rogers and Nelson1962).
Sorghum coevolved with Striga in the African savanna and therefore harbor some resistance to the weed. In contrast, maize is alien to Africa and generally more susceptible to the weed. Until now, Striga resistance in maize has mainly come from its wild grass relatives like diploperennial teosinte [Zea diploperennis (Itis, Doebley & Guzman) (Amusan et al. Reference Amusan, Rich, Menkir, Housley and Ejeta2008; Lane et al. Reference Lane, Child, Moore, Arnold and Bailey1997) and eastern gamagrass [Tripsacum dactyloides (L.) L.] (Gutierrez-Marcos et al. Reference Gutierrez-Marcos, Pennington, Costa and Dickinson2003). Introgression from such sources has led to development of a Striga-resistant maize inbred line ‘ZD05’ suitable for integration in breeding programs in western Africa (Amusan et al. Reference Amusan, Rich, Menkir, Housley and Ejeta2008).
In eastern Africa, the open-pollinated KSTP 94 has been used as a Striga-resistant maize variety since 1995, especially in western Kenya, a Striga-prone region. KSTP 94 exhibits remarkable resistance to Striga under field conditions; a characteristic that has made it a subject of intense research in the region. Such research has found the resistance of KSTP 94 to be due to production of low amounts of the strigolactone sorgomol (Yoneyama et al. 2015). Sorgomol is a strigolactone that does not efficiently induce Striga germination, and the resistance of KSTP 94 was therefore concluded to be due to preattachment resistance.
To further characterize host-based resistance in KSTP 94, we sought to determine whether it also exhibits postattachment Striga resistance. To achieve this, we used a soil-free laboratory assay based on rhizotrons (Mbuvi et al. Reference Mbuvi, Masiga, Kuria, Masanga, Wamalwa, Mohamed, Odeny, Hamza, Timko and Runo2017) to compare the resistance response of KSTP 94 with a susceptible inbred maize line (CML 144).
Materials and Methods
Postattachment Resistance Assays for Striga
Preconditioning of Striga hermonthica Seeds
Striga hermonthica seeds (obtained from maize-growing fields in Kibos, western Kenya in 2015) were used for postattachment resistance assays. Seeds were preconditioned as described in Gurney et al. (Reference Gurney, Grimanelli, Kanampiu, Hoisington, Scholes and Press2003) before germination. First, Striga seeds (25 mg) were surface sterilized using 10% (v/v) NaOCl for 10 min with gentle agitation, rinsed three times with sterilized distilled water, and then spread on a glass fiber filter paper (Whatman GFA) placed on sterile petri dishes. Approximately 5000 Striga hermonthica seeds were then incubated for 11 d at 29 C. Finally, seeds were germinated by treatment with 3 ml of 0.1 ppm GR24 (Chirax, Amsterdam) and incubated overnight at 29 C. Germination efficiency of the Striga seedlings was determined using a Leica MZ7F microscope (Leica, Germany), and only plates showing >70% germination were used to infect maize roots.
Infection of Maize Roots with Striga Seedlings
Maize inbred line CML 144, obtained from the International Maize and Wheat Improvement Center (Nairobi, Kenya), and open-pollinated variety KSTP 94 from the Kenya Agricultural Livestock Research Organization (Kakamega, Kenya) were screened for postattachment Striga resistance. Seeds were first germinated in 10 cm by 10 cm by 7 cm pots filled with vermiculite. At 5 d postplanting, maize seedlings were transferred to rhizotrons (25 cm by 25 cm by 5 cm Perspex® chambers) (Mbuvi et al. Reference Mbuvi, Masiga, Kuria, Masanga, Wamalwa, Mohamed, Odeny, Hamza, Timko and Runo2017) prepared as follows: chambers were lined with 25 cm by 5 cm by 5 cm foam strips at the bottom to absorb excess water and packed with vermiculite, then a 50-micron-thick mesh was placed on top. A germinated maize seedling was placed on the mesh, the chamber closed, and wrapped with aluminum foil. Plants were then maintained in the glasshouse under a 12-h light/12-h dark photoperiod with 60% humidity and day and night temperatures of 28 and 24 C, respectively. During growth on rhizotrons, plants were drip irrigated with 25 ml of 40% Long Ashton nutrient solution (Hudson Reference Hudson1967). Maize seedlings with well-developed roots (10 d on rhizotrons) were then infected with 25 mg pregerminated S. hermonthica seeds (per plant) by aligning the Striga seeds along the maize roots with a soft paintbrush. Five plants per genotype were screened in a randomized complete block design in three replicates (single experimental run).
Analysis of Postattachment Striga Resistance in Maize
Measures of Striga Resistance
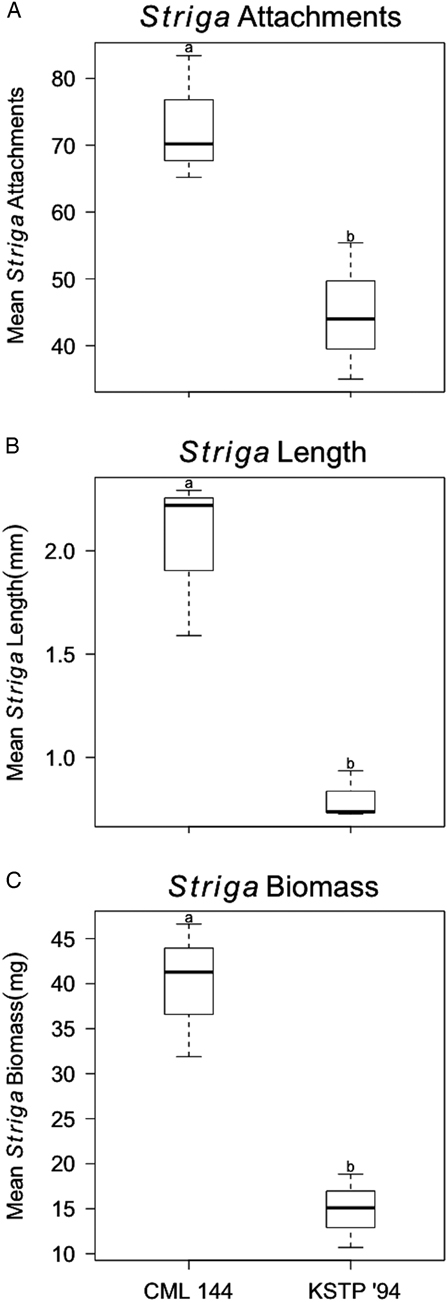
Infected maize roots were screened for Striga resistance at 9 and 21 d after infection (DAI). At 9 DAI, Striga seedlings attached on maize roots were observed and documented using a stereomicroscope (Leica MZ4 fitted with a DFC320FX camera (Leica, Germany). At 21 DAI, all Striga attached to maize roots were harvested, placed on 90-mm petri plates, and photographed. Image analysis using ImageJ v. 1.45 (http://rsb.info.nih.gov/ij) was then carried out to determine the length and the number of Striga parasitizing each host plant. To determine the total Striga biomass attached on maize roots, harvested Striga seedlings were oven-dried for 7 d at 45o C and weighed. ANOVA was carried out to compare the means for biomass, length, and number of infecting Striga using statistical analysis software (SAS v. 9.1, SAS Institute, Cary, NC, USA) and presented as box plots prepared in R software. Significant differences between the means were according to Tukey’s honest significant difference test at a 95% confidence interval.
Histological Analysis of Striga Resistance in Maize
To determine the extent of parasite development within the host root, microscopic screening of the connection point between Striga and maize roots was carried out according to Gurney et al. (Reference Gurney, Grimanelli, Kanampiu, Hoisington, Scholes and Press2003). Tissues at the point of host–parasite infection were collected from rhizotrons at 9 DAI and fixed using Carnoy’s fixative (4:1 ethanol:acetic acid). For each variety (CML 144 or KSTP 94), 3 attachments from 5 rhizotrons were collected, making a total of 15 samples per variety. Samples were dehydrated with 100% absolute ethanol for 30 min, followed by pre-infiltration in ethanol-Technovit® (Haraeus Kulzer GmbH) solution for 2 h and a further pre-infiltration step in 100% Technovit® solution for 1 h. These tissues were then left in fresh 100% Technovit® for 3 d. For embedding, samples were placed in Eppendorf lid molds containing 1 part Technovit® and 15 parts hardener and left to set. Embedded tissues were then mounted on wooden blocks using the Technovit® 3040 kit following the manufacturer’s instructions (Haraeus Kulzer GmbH). Small (5-micron-thick) sections were cut using a Leica RM 2145 microtome (Leica, Germany) and transferred to glass slides. The sections were stained using 0.1% toluidine blue O dye in 100 Mm phosphate buffer for 2 min, washed in distilled water, and dried at 65 C for 30 min. The microscope slides were then covered with slips using DePex (BDH, Poole, UK), observed, and photographed using a Leica DM500 microscope mounted with a Leica ICC50 camera (Leica, Germany).
Results and Discussion
An effective measure of host resistance to Striga is achieved by determining the number and size of the parasite seedlings infecting the host and their biomass. A resistance response is characterized by fewer, smaller, and less biomass relative to a susceptible host (Figure 1A and B). We found that CML 144, the susceptible maize inbred line, had significantly more Striga attachments (Figure 2A), longer Striga seedlings (Figure 2B), and a higher Striga biomass (Figure 2C). CML 144 had an average of 72.93±9.40 attachments per plant, while KSTP 94 had significantly fewer attachments (44.80±10.22). Similarly, longer Striga seedlings were observed on CML 144 (2.03±0.39 mm) compared with those on KSTP 94 (0.80±0.12 mm). Finally, the biomass of Striga seedlings harvested from CML 144 was 39.93±7.46 mg, which was significantly higher than that of seedlings from KSTP 94, which averaged 14.88±4.07 mg per CML 144/KSTP 94 plant.

Figure 1 Striga hermonthica seedlings growing on the roots of maize lines screened on rhizotrons 21 d after infection with a S. hermonthica ecotype from Kibos. (A) Susceptible maize inbred line CML 144, characterized by numerous S. hermonthica attachments. (B) Resistant open-pollinated maize KSTP 94, characterized by fewer and smaller attachments. White arrows indicate parasite attachment points to the host. Scale bar: 0.2 mm.

Figure 2 Postattachment resistance evaluation of maize inbred line CML 144 and KSTP 94 following infection with S. hermonthica seeds. (A) Mean number of Striga seedlings attached to host roots; (B) mean length of Striga seedlings; and (C) mean Striga dry biomass of parasite seedlings attached to the roots of each host. Data were collected 21 d after infection and are a mean of three replicates. Letters above each bar indicate significant differences (P≤0.05).
To further elucidate the underlying resistance mechanism of KSTP 94 after infection, we carried out histological analyses of Striga–host interactions at the attachment point at 9 DAI. Striga parasitism is considered to be successful when the vascular connection between host and parasite is established followed by efficient nutrient flow into the parasite. We found that in KSTP 94, 53% (n = 15) of Striga seedlings penetrated host tissue up to the cortical cells but did not go beyond the endodermis, hence failing to make xylem–xylem connections (Figure 3Ai and ii). However, this was not the case with CML 144, in which the parasite successfully attached and established xylem–xylem connections in all tissues sectioned (Figure 3Bi and ii).

Figure 3 Histological analysis of postattachment resistance mechanisms to S. hermonthica. (Ai) Resistance interaction in KSTP 94, in which the parasite penetrates the host but exits (white arrow). Scale bar: 1 mm. (Aii) A transverse section through the haustorium of the resistant maize line KSTP 94. The parasite penetrates the cortex but is unable to breach the endodermal barrier and grows around the host vascular cylinder. Scale bar: 0.1 mm. (Bi) Susceptible interaction in CML 144 showing host penetration by the parasite for xylem–xylem connection. Scale bar: 1 mm. (Bii) Transverse section of a stained root tissue of CML 144 maize line 9 d after infection showing penetration of the host root cortex and endodermis as well as connections between the host and parasite xylem (Hx-Px). Scale bar: 0.1 mm. H, host, P, parasite, Px, parasite’s xylem. In the susceptible interaction, the parasite penetrates the cortex and endodermis and connects to the xylem vessels of the host, allowing the haustorium to differentiate.
The ability of Striga to penetrate a host and make vascular connections is critical for the survival of this parasite. Our results suggest that Striga was able to successfully achieve parasitism in the susceptible variety CML 144 about 2.6-fold more frequently compared with KSTP 94. The significantly larger Striga size in the susceptible variety resulted in higher parasite biomass on susceptible line CML 144. A comparison of Striga’s ability to penetrate its host and complete its life cycle from our study and previous work on a resistant maize inbred line ZD05 developed from wild maize show striking similarities (Amusan et al. Reference Amusan, Rich, Menkir, Housley and Ejeta2008). The frequency of formation of xylem–xylem connections between S. hermonthica and ZD05 was 12%, resulting in 88% fewer infections. This translated to significantly fewer Striga attachments.
Our study and earlier studies have reported host and Striga incompatibility. For example, Gurney et al. (Reference Gurney, Slate, Press and Scholes2006) described this resistance mechanism between rice variety ‘Nipponbare’ and S. hermonthica. Similarly, the inbred line ZD05 described earlier showed incompatibility with S. hermonthica in Amusan et al. (Reference Amusan, Rich, Menkir, Housley and Ejeta2008). In all these cases, the parasite penetrates the host cortex but is deflected before it gets to the endodermis. The exact mechanism for this parasite’s inability to penetrate the endodermis is unknown, but it seems plausible that molecules that mediate interactions between Striga and hosts play an important role in resistance. Particularly, the resistance can be attributed to biochemical or physiological barriers from the host (Yoshida and Shirasu Reference Yoshida and Shirasu2009) such as a tough sclerenchyma (Amusan et al. Reference Amusan, Rich, Menkir, Housley and Ejeta2008).
Our findings emphasize the need to continuously screen germplasm for pre- and postattachment Striga resistance. We have identified an additional mechanism of resistance that protects against Striga in maize. Postgermination Striga resistance had been previously demonstrated in maize. However, this is the first time it has been shown in an open-pollinated maize variety that is not introgressed with wild germplasm. These results have significant Striga management implications in eastern Africa and demonstrate the greater importance of KSTP 94 than previously thought. This research underscores the need for further integration of KSTP 94 in breeding programs as well as determination of genetic mechanisms underlying this resistance.
Acknowledgments
The authors wish to acknowledge the International Foundation for Science for funding the project and the Plant Transformation Laboratory at Kenyatta University where this work was done. No conflicts of interest have been declared.