Introduction
Transgenic technology as a powerful tool allows researchers to generate genetically modified animals for biomedical, veterinary and agricultural applications (Lavitrano et al., Reference Lavitrano, Busnelli, Cerrito, Giovannoni, Manzini and Vargiolu2005). Several methods such as direct DNA microinjection (Love et al., Reference Love, Gribbin, Mather and Sang1994), viral vectors (Lillico et al., Reference Lillico, Sherman, McGrew, Robertson, Smith, Haslam, Barnard, Radcliffe, Mitrophanous, Elliot and Sang2007), and nuclear transfer (Van de Lavoir et al., Reference Van de Lavoir, Diamond, Leighton, Mather-Love, Heyer, Bradshaw, Kerchner, Hooi, Gessaro, Swanberg, Delany and Etches2006) have been developed to produce transgenic animals. The development of new strategies for gene transfer using sperms would be useful in this regard.
Sperm is a natural carrier and can transfer genetic information into the oocyte. Thus, sperm-mediated gene transfer (SMGT) would be a convenient method to generate transgenic organisms. SMGT is simple and does not require advanced tools and expertise compared with other methods such as nuclear transfer and microinjection (Alderson et al., Reference Alderson, Wilson, Laible, Pfeffer and L’Huillier2006). Brackett et al. (Reference Brackett, Baranska, Sawicki and Koprowski1971) for the first time reported that rabbit sperm have the ability to take up and carry foreign DNA. It has been shown that sperm isolated from the epididymis when incubated with a plasmid can transfer foreign DNA to oocytes (Brackett et al., Reference Brackett, Baranska, Sawicki and Koprowski1971). Subsequently, SMGT in chickens (Nakanishi and Iritani, Reference Nakanishi and Iritani1993), buffalo (Hassanane et al., Reference Hassanane, El Makawy, Helalia, Abdoon, Khalil, Ghanem, Tohamy, Sun and Shen2017), pigs (Lavitrano et al., Reference Lavitrano, Bacci, Forni, Lazzereschi, Di Stefano, Fioretti, Giancotti, Marfé, Pucci, Renzi, Wang, Stoppacciaro, Stassi, Sargiacomo, Sinbaldi, Turchi, Giovannoni, Della Cassa, Seren and Rossi2002) and zebrafish (Khoo, Reference Khoo2000) have been reported.
In SMGT, the external gene must pass through a barrier such as a sperm membrane that binds to the DNA and forms a barrier to entry. DNA after escaping from the sperm membrane rarely gets into the genome. Intracytoplasmic nucleases in sperm cells can cause random insertion of DNA in the suspect regions in the genome. So far, two internal factors in sperm cells have been identified that prevent the entry of external DNA molecules. These are
1. Inhibitory Factor 1 (IF-1): This is abundant in the seminal fluid of mammals and inhibits binding of external DNA to sperm, preventing mammalian sperms from taking up exogenous DNA molecules under normal physiological conditions (Zani et al., Reference Zani, Lavitrano, French, Lulli, Maione, Sperandio and Spadafora1995).
2. Nuclease enzymes in sperm cells are activated when sperm come in contact with external DNA (Parrington et al., Reference Parrington, Coward and Gadea2011).
It is believed that binding and integration of exogenous DNA in the sperm genome is not random and is mediated by some specific binding proteins on the surface of the sperm. Recent studies have suggested that CD4 and major histocompatibility complex class II proteins play important roles in this binding (Lavitrano et al., Reference Lavitrano, Maione, Forte, Francolini, Sperandio, Testi and Spadafora1997). A series of chromatin sites has been identified in sperm genome, these structures act as entry points for an external molecule of DNA into the sperm genome (Smith and Spadafora, Reference Smith and Spadafora2005).
As sperm cells act as carriers for introducing foreign DNA, researchers have tried to develop and increase the efficiency of this technique. Shen and his colleagues developed a highly efficient and simple method to obtain transgenic offspring called dimethyl sulfoxide (DMSO)-SMGT. In this method, sperm cells are transfected with external DNA/DMSO under heat shock. This technique increases the permeability of sperm cells to DNA. DMSO can increase DNA uptake in mouse sperm (Shen et al., Reference Shen, Li, Pan, Min, Dong and Deng2006) and short-term storage of sperm in electrolyte-free medium (EFM) at 4°C is better than conventional media such as human tubular fluid (HTF) (Riel et al., Reference Riel, Yamauchi, Huang, Grove and Ward2011). To our knowledge, there are no previous reports on the effect of DMSO and EFM on DNA uptake by mouse sperm to generate plasmid-positive blastocysts. Therefore, the present study aimed to investigate the effect of the addition of DMSO to EFM on sperm motility, viability and DNA uptake.
Materials and methods
All chemicals used in this study were purchased from Sigma Aldrich Chemical Company (St. Louis, MO, USA), unless otherwise stated. This study and all murine procedures used were approved by the research and ethics committee of Shahid Beheshti University of Medical Sciences, Iran.
Experimental animals
Six- to 8-week-old B6D2F1 male and female mice were obtained from the Pasteur Institute (Tehran, Iran). The mice were fed ad libitum with a standard diet and maintained in a temperature and light controlled room.
Preparation of exogenous DNA
The pEGFP–N1 vector containing the enhanced green fluorescent protein (EGFP) reporter gene was used. These plasmids were propagated in a DH5α competent bacterial strain and purified using a DNA extraction kit (MN, Germany) according to the manufacturer’s instructions.
Preparation of sperm and uptake of exogenous DNA with DMSO
Mouse sperm were collected from the caudal epididymis of 8- to 10-week-old BDF1 male mice. The sperm suspensions were placed in 100 μl of HTF containing 4 mg/ml bovine serum albumin (BSA) and were then incubated at 37°C with 5% CO2 for 60 min for capacitation. Subsequently, the suspension was divided into three groups (1 × 107 cells/ml) (Shen et al., Reference Shen, Li, Pan, Min, Dong and Deng2006). The first group was considered as the control. The second and third groups were centrifuged at 500 g for 10 min. The supernatant was discarded. Sperm suspension in the second group was centrifuged at 500 g for 10 min with EFM (0.33 M glucose, 4 mg/ml BSA) twice. Then 100 μl EFM containing the 3% DMSO and circle pEGFP–N1 DNA (20 µg/ml) was added to sperm pellet. The third group was prepared in a way similar to the second group, although HTF was added instead of EFM. The second and third groups were cooled to 4°C for 10–15 min.
Sperm motility and viability analysis in HTF and EFM
Sperm motility was analyzed manually according to the protocol recommended by WHO. The sperm motility of each sample was graded as follows: progressive, non-progressive, and immotile (Cooper and Yeung, Reference Cooper and Yeung2006). Sperm viability was estimated using eosin Y stain (Björndahl et al., Reference Björndahl, Söderlund and Kvist2003). Sperm motility and viability in the control group, the EFM and HTF groups were assessed after cooling them down to 4°C.
In vitro fertilization
Six- to 8-week-old B6D2F1 mice were superovulated with 10 IU of pregnant mare serum gonadotropin (PMSG) followed by 10 IU of human gonadotropin (HCG) 48 h later. Metaphase II oocytes were collected from the oviduct ampulla 14–16 h after injection of HCG. The oocyte–cumulus complexes were released into flushing holding medium (FHM). Subsequently, they were placed in drops composed of 50 μl HTF and covered with mineral oil. The sperm suspension from the control, EFM and HTF groups was added to the oocyte medium in separate drops and placed in an incubator. Next 5–6 h after IVF the oocytes cultures were transferred into KSOM supplemented with non-essential and essential amino acids, 4 mg/ml BSA and stored at 37°C with 5% CO2. The development of embryos up to the blastocyst stage (96 h post fertilization) was assessed in each group (Hosseini et al., Reference Hosseini, Dehghani-Mohammadabadi, Ghafarri Novin, Haji Molla Hoseini, Arefian, Mohammadi Yeganeh and Salehi2017).
Gene expression analysis of GFP-positive blastocyst
The GFP-positive blastocyst was selected under a fluorescence microscope and genomic DNA extracted using the standard method. The GFP gene was detected by polymerase chain reaction (PCR) amplification with forward primer: sequence AGAAGAACGGCATCAAGG; and reverse primer: sequence GCTCAGGTAGTGGTTGTC; and 2% agarose electrophoresis gel. This process resulted in a 136-bp product.
Differential staining of inner cell mass (ICM) and trophectoderm (TE) of the blastocyst
Blastocysts from all three groups (control, HTF, and EFM) were zona freed using Tyrode acid solution. They were then incubated in rabbit anti-mouse serum in FHM (1:2) solution at 37°C for 30 min. Afterward, they were briefly washed in FHM and incubated in 30% guinea pig complement in FHM supplemented with 10 μg/ml propidium iodide (PI) and 10 μg/ml Hoechst H33342 stain for 30 min. The blastocysts were fixed in ethanol and mounted in a microdrop of glycerol onto glass slides. In order to count nuclei, the prepared samples were immediately observed and photographed under a fluorescence microscope (Salehi et al., Reference Salehi, Kato and Tsunoda2014).
Statistical analysis
All statistical analysis was performed using the statistical program SPSS version 22 (SPSS, Chicago, IL, USA). The means of cleavage and development rates up to the blastocyst stage were compared by non-parametric analysis test (Kruskal–Wallis test). Analysis of variance (ANOVA) and post hoc Tukey test were used to compare the average number of cells in blastocysts obtained under each culture condition within the studied group. Data are expressed as means±standard deviation (SD). A statistically significant difference was considered at P-values lower than 0.05.
Results
Sperm motility and viability in EFM and HTF groups
The sperm motility rate consists of progressive, non-progressive, and immotile per cent in the control, EFM, and HTF groups. HTF and EFM groups were analyzed 15 min after storage at 4ºC with DNA/DMSO. Progressively motile sperm percentage in the control, HTF, and EFM groups was 37.5±5.4, 9.25±1.25, and 19.25±2.5, respectively. There was a significant difference in progressive motility among control, HTF, and EFM groups. The percentage of non-progressive motile sperm was also 11.75±3.09, 18.25±1.25 and 13.5±1.29 respectively. There was a significant difference between control and HTF groups. Conversely, we observed a non-significant difference between the control and EFM groups. It is apparent from Table 1 that the percentage of immotile sperm significantly increased in HTF and EFM compared with the control group, but no significant difference was found between HTF and EFM groups. The viability of sperms in each group was also investigated. According to Table 1, sperm viability declined dramatically in the HTF group (42.19±6.22) compared with the EFM and control groups (75.21±1.92 and 74.83±1.78 respectively).
Table 1 Sperm viability and mobility rates for control, HTF and EFM groups

a,b,c Within the same column, values followed by different letters were significantly different (P < 0.05). EFM, electrolyte-free medium; HTF, human tubular fluid, SD, standard deviation.
Mouse embryo development and fertilization rate after IVF
Fertilization rates for the control, HTF and EFM groups were determined and they were 85.54±3.37%, 38.22±8.7%, and 72.31±5.5% respectively. There was a significant difference between HTF, EFM, and the control groups (P < 0.05) but no significant difference was observed between the control and EFM groups (Table 2). In each group, mouse embryo development was evaluated and the results were summarized in Table 2. Our finding indicated that the percentage of blastocyst formation in EFM group (59.84±5) was significantly higher than HTF group (29.39±11.25), while there were significant differences among the control, HTF and EFM groups (P < 0.05).
Table 2 Mouse embryo development and fertilization rates in control, EFM, and HTF groups

a,b,c Within the same column, values followed by different letters were significantly different (P < 0.05). EFM, electrolyte-free medium; GFP, green fluorescent protein; HTF, human tubular fluid, SD, standard deviation.
GFP detection in the mouse embryo
The expression of GFP in plasmid-positive blastocysts was examined under fluorescence microscope (Fig 1). Forty-six per cent of blastocysts in the HTF group were EGFP positive. GFP expression was detected in 62% of the blastocysts derived from the EFM group (Table 2). Our findings demonstrated that the GFP-positive rate at the blastocyst stage was significantly higher in the EFM group, compared with the HTF group (P < 0.05). GFP detection by PCR is pictured in Fig. 1.
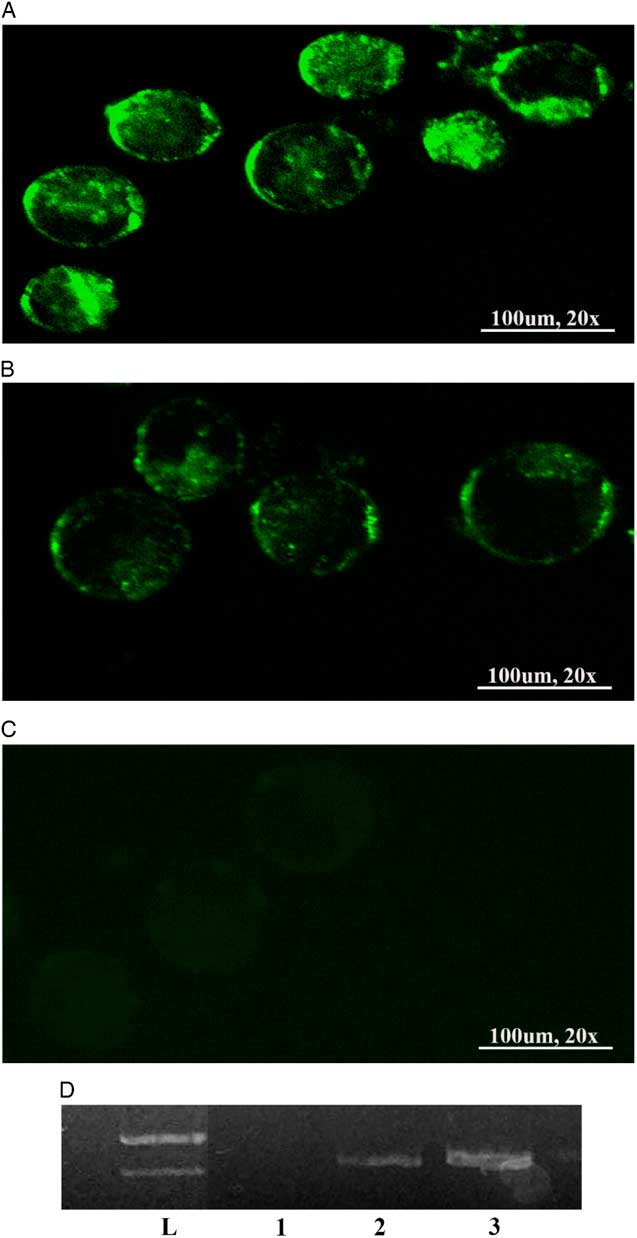
Figure 1 The plasmid-positive blastocysts by in vitro fertilization (IVF) expressed enhanced green fluorescent protein (EGFP) in vitro. Embryos at blastocyst stages. (A–C) represent the green embryos under the fluorescence microscope in electrolyte-free medium (EFM), human tubular fluid (HTF) and control groups respectively. (D) The GFP band was extracted from blastocysts. Lanes 1, 2, 3, and L represent the negative control and the GFP band extracted from the blastocysts from the HTF and EFM groups, and the ladder DNA respectively.
The number of total, ICM, and TE cells in blastocysts of each group
The cell numbers of ICM and TE of blastocysts from each group were examined using differential staining (Fig. 2). In each group, the blastocysts were double stained in order to determine the cell number of ICM and TE (Fig. 2). The result showed that there was a significant decrease in TE and total cells from the blastocyst-derived HTF group in comparison with EFM and control groups (P < 0.05). There was no significant difference between the EFM and control groups (P>0.05).
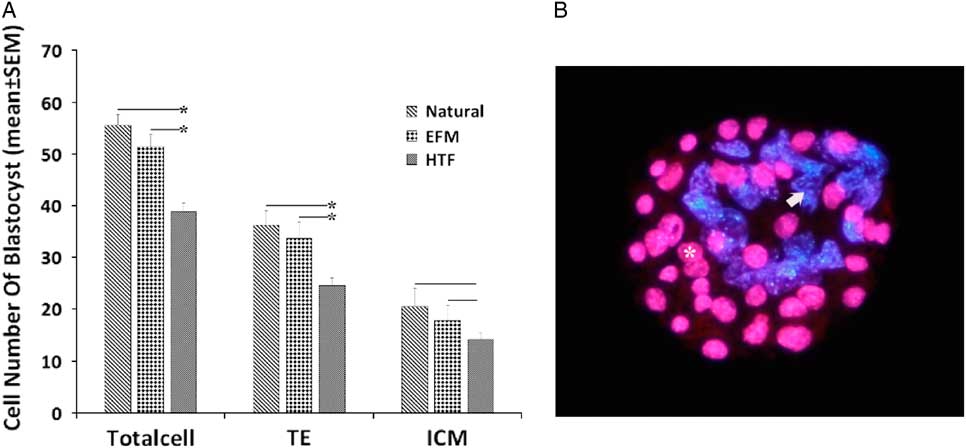
Figure 2 (A) Effect of electrolyte-free medium (EFM) and human tubular fluid (HTF) medium on the cell numbers for blastocysts (mean±standard deviation (SD)). (B) Fluorescence micrograph of a differentially labelled mouse blastocyst showing trophectoderm (TE) nuclei (asterisk, pink) and inner cell mass (ICM) nuclei (arrow, blue).
Discussion
Despite the advantages of the SMGT method such as convenience, simplicity and low cost, SMGT efficiency was only about 3–6% to 25%. Ways to increase the efficiency of this method for producing transgenic animals is being researched (Sato, Reference Sato2005). Sperm quality is determined by motility and viability, which are the main factors for achieving high efficiency in the SMGT method (Lavitrano et al., Reference Lavitrano, Busnelli, Cerrito, Giovannoni, Manzini and Vargiolu2005). However, sperm motility and viability usually decrease after treatment with various reagents such as TfxTM-50, lipofectamine, DMSO or electroporation. Therefore there are subsequent reductions in the efficiencies and success rates for generating transgenic embryos following SMGT. Use of a special sperm preparation medium that preserves sperm motility and viability could be an effective strategy to increase the success of SMGT. It has been shown that heat shock with DMSO can improve SMGT efficiency (Shen et al., Reference Shen, Li, Pan, Min, Dong and Deng2006), but sperm motility and viability levels reduce using conventional media such as HTF. The presence of ions in the surrounding medium results in their coupling with the sperm membrane and changes membrane structure during sperm storage at non-physiological temperatures. In addition, sodium–potassium-dependent ATPase pumps, which regulate the concentration of sodium, are very sensitive to hypothermia. When the temperature decreases, the function of this pump may be disrupted and sodium ion uptake from the extracellular environment increases. Sodium ions can change some sperm function (Saito et al. Reference Saito, Kinoshita, Kanno, Iwasaki and Hosaka1996) and an electrolyte-free solution can preserve sperm motility and viability (Riel et al., Reference Riel, Yamauchi, Huang, Grove and Ward2011). Electrolyte-free medium is used for short-term storage of sperm at 4°C. In our study, EFM medium was used to incubate sperm with DNA and increased SMGT efficiency compared with HTF. To preserve sperm motility and viability, EFM with thermal shock at 4°C is a better choice than HTF. Other important factors to increase SMGT efficiency are the choice of detergent or of a suitable method to increase sperm permeability to the external DNA molecules. The SMGT transgenic ratio in rabbits using common reagents like TfxTM-50(62%), lipofectamine (45%), and DMSO (61%) is approximately the same, but in mice a ratio of 42% was reported (Shen et al., Reference Shen, Li, Pan, Min, Dong and Deng2006). This value was consistent with our study findings. In this study, we used DMSO reagent because of the setup and availability of the SMGT protocol in mice. SMGT is carried out in mouse by electroporation, which decreases sperm viability and reduces the transgenic rate to approximately 33% (Furuta et al., Reference Furuta, Ichikawa, Sugimura, Kikuchi, Yoshida, Mukouyama and Tomogane2006). DMSO has been replaced by electroporation, retroviruses, or lipids for the safety reasons associated with retroviruses and the cost of commercial liposomes. DMSO in this method can be used as a medium to transfect sperm and produce positive transgenic mice. At low temperatures, especially at 4°C, transfection with DMSO increased. Our data showed that the use of suitable treatments and media could preserve sperm motility and viability, and improve SMGT efficiency.
In our study, development up to the blastocyst stage and in plasmid-positive blastocysts was better in EFM groups than the HTF group. When incubated in EFM with thermal shock and DMSO the motility and viability of sperm was improved. Subsequently, the rate of IVF and development to the blastocyst stage was higher in the EFM than the HTF groups. These parameters cause an increase in exogenous DNA uptake in the EFM group. Generally, the correct selection of medium and the appropriate reagent, according to the type of sperm treatment, can significantly increase SMGT efficiency. This study showed that the use of DMSO with thermal shock (4°C) in EFM improved SMGT efficiency in mice. Sperm motility and viability rates in EFM compared with a conventional medium such as HTF were improved. For fertilization, embryo development to the blastocyst stage and GFP-positive embryo levels also increased.
In conclusion, in this study, we demonstrated that storage of mouse sperm in EFM with DMSO for 15 min increased in sperm mobility, viability and transfection efficiency. GFP-positive mouse blastocyst rates produced by sperms that were transfected with pEGFP in EFM were increased compared with rates for HTF. Overall, storage of sperm at low temperatures in an electrolyte-free medium such as EFM can be more efficient for DMSO/SMGT and produce a substantial number of GFP-positive mouse embryos.
Financial support
This study was supported by Shahid Beheshti University of Medical Sciences (Tehran, Iran).
Ethical standards
This study and all murine procedures used were approved by the research and ethics committee of Shahid Beheshti University of Medical Sciences.
Conflicts of interest
None.
Acknowledgements
This article has been extracted from a thesis written by Soleiman Kurd. We would like to thank Shokofe Torabi, Maryam Vahdat, and Mahmoud Vahidi for their help.






