The Congenital Heart Surgery Nomenclature and Database project defines pulmonary atresia with ventricular septal defect as a group of congenital cardiac malformations in which there is a lack of luminal continuity between both ventricles and the pulmonary arterial tree, with an interventricular communication.Reference Tchervenkov and Roy1 In its severe form, there is an absence of either part or all of the intrapericardial pulmonary arteries. The pulmonary circulation in pulmonary atresia with a ventricular septal defect is heterogeneous and variable, and independent of the intracardiac anatomy. The authors of the paper proposed three subtypes of pulmonary atresia with ventricular septal defect, according to the anatomy of the pulmonary circulation and source of pulmonary blood flow. Type A has intrapericardial pulmonary arteries with pulmonary blood flow from an arterial duct. Type B has both intrapericardial pulmonary arteries and major aortopulmonary collaterals. Type C has no intrapericardial pulmonary arteries, and the pulmonary circulation is supplied by major aortopulmonary collaterals only.Reference Tchervenkov and Roy1 Based on the site of origin and connection to the pulmonary circulation, systemic-to-pulmonary collateral arteries is categorised into three types, including direct aortopulmonary collaterals, indirect aortopulmonary collaterals, and true bronchial arteries. Only the first two are considered to be the major aortopulmonary collaterals.Reference Rabinovitch, Herrera-deLeon, Castaneda and Reid2
The above categorisations proposed by the Congenital Heart Surgery Nomenclature and Database project have been overtaken by those provided by the International Nomenclature Committee, which included most of those involved in preparing the documents that made up the supplement of 2000. The recommendations made by the International Nomenclature Committee, furthermore, have been adopted by the World Health Organization for the 11th iteration of the International Classification of Disease. This classification defines tetralogy of Fallot with pulmonary atresia (synonym: pulmonary atresia with ventricular septal defect, Fallot type) as a congenital cardiovascular malformation that is a variant of tetralogy of Fallot, in which there is no direct communication between the right ventricle and the pulmonary arterial tree (IPCCC code 01.01.26), and IPCCC code 01.01.57, when there are collateral blood vessels between the systemic and pulmonary arteries.Reference Franklin, Béland and Colan3
Common arterial trunk is a congenital malformation in which a single great artery arises from the heart, overrides the interventricular septum, and supplies the systemic, pulmonary, and coronary circulations.Reference Franklin, Béland and Colan3–Reference Anderson, Penny, Anderson, Baker, Penny, Redington, Rigby and Wernovsky6 With common arterial trunk, there are no remnants of a separate pulmonary valve or ventricular-to-pulmonary artery continuity, distinguishing it from many forms of pulmonary atresia with ventricular septal defect.Reference Mavroudis, Backer, Mavroudis and Backer7
The pulmonary arterial supply in patients with tetralogy of Fallot with pulmonary atresia can be via the arterial duct or from collateral arteries arising directly or indirectly from the aorta (systemic-to-pulmonary artery collaterals) or rarely both.Reference Rossi, Hislop, Anderson, Martins and Cook8 The rarest sources of pulmonary blood flow are aortopulmonary window and fistulous communication with the coronary artery.Reference Rossi, Hislop, Anderson, Martins and Cook8–Reference Anderson, Anderson, Spicer, Hlavacek, Cook and Backer12 The previously described fifth arch as a source of pulmonary blood flow has no scientific evidence, as the latest literature suggests that there is no embryonic fifth arch artery.Reference Baker, Anderson, Anderson, Baker, Penny, Redington, Rigby and Wernovsky11,Reference Graham, Poopalasundaram, Shone and Kiecker13
Herein, we describe an outflow tract malformation, tetralogy of Fallot with pulmonary atresia and aortopulmonary window, which was misdiagnosed as common arterial trunk, despite definitive morphological differences. We do not suggest that this is a new morphological entity, and probably other teams have encountered such anatomy, diagnosed and managed as common arterial trunk.
Tetralogy of Fallot with pulmonary atresia is a subset of the overall group of patients who can be described as having pulmonary atresia with ventricular septal defect. This subset of tetralogy of Fallot with pulmonary atresia, however, is important because it is only this subset when associated with aortopulmonary window as the source of unifocal pulmonary arterial supply, produces the confusion with common arterial trunk. It would be unlikely that patients having congenitally corrected transposition, or regular transposition, or isomerism, in association with pulmonary atresia and a ventricular septal defect would be misdiagnosed as having common arterial trunk.
Case series
Over the past 3 years, three patients, with a referral diagnosis of common arterial trunk with aortic dominance (two with minimal and one with significant separation of intrapericardial pathways), were found to have tetralogy of Fallot with pulmonary atresia and aortopulmonary window. Echocardiography, cardiac CT scan, operative findings, and post-operative course details were reviewed. All three patients were presented with respiratory distress, feeding difficulty, and failure to thrive. All were tachypneic with chest retractions, systolic murmurs, cardiomegaly, and pulmonary plethora.
The diagnosis of tetralogy of Fallot with pulmonary atresia and aortopulmonary window was made echocardiographically (Fig 1), and confirmed with a CT scan in two patients (Figs 2 and 3). In one, the diagnosis was made intraoperatively.

Figure 1. Transthoracic echocardiography of tetralogy of Fallot with pulmonary atresia and aortopulmonary window (1 – ascending aorta, 2 – pulmonary trunk, 3 – interventricular communication, 4 – aortopulmonary window, arrow – aortic valve, broken arrow – atretic pulmonary valve, arrowhead – inferior aortopulmonary septum).
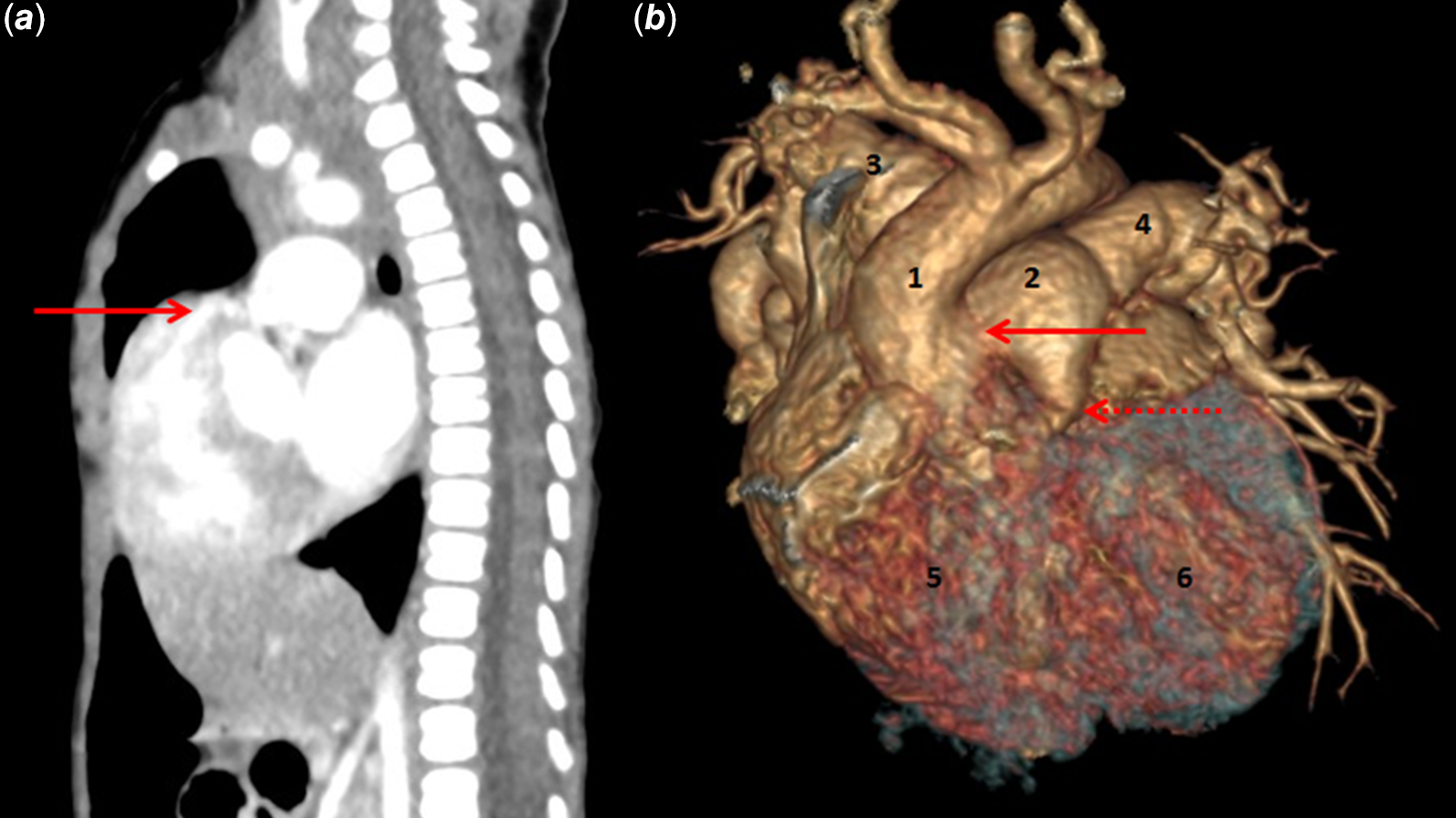
Figure 2. CT scan of tetralogy of Fallot with pulmonary atresia and aortopulmonary window. ( a ) Sagittal section demonstrating sub-pulmonary infundibulum with blindly ending sub-pulmonary outflow tract (arrow). This sub-pulmonary infundibulum is conspicuously absent in common arterial trunk. ( b ) Three-dimensional reconstruction of tetralogy of Fallot with pulmonary atresia and aortopulmonary window demonstrating separate aortic and pulmonary roots (with atretic pulmonary valve). 1 – ascending aorta, 2 – pulmonary trunk, 3 – right pulmonary artery, 4 – left pulmonary artery, 5 – right ventricle, 6 – left ventricle, arrow pointing aortopulmonary window, broken arrow pointing pulmonary root.

Figure 3. Post-operative (patch closure of aortopulmonary window and central shunt between ascending aorta and main pulmonary artery) CT scan of tetralogy of Fallot with pulmonary atresia and aortopulmonary window. ( a ) Three-dimensional reconstruction demonstrating separate aortic and pulmonary roots. 1 – ascending aorta, 2 – pulmonary trunk, arrow – central shunt, broken arrow – pulmonary trunk arising from the ventricle. The aortopulmonary communication is distal, extending into the right pulmonary artery and is not clear here (see below). The right coronary artery can be seen arising from the anterior sinus. ( b ) Same as Figure 3a. ( c ) Transverse section demonstrating the patch separating the distal main pulmonary artery and right pulmonary artery from aorta (arrowhead). Pulmonary trunk arising from the ventricle and right coronary artery can be seen clearly. 1 – ascending aorta, 2 – pulmonary trunk, 3 – right pulmonary artery, 4 – left pulmonary artery, 5 – descending thoracic aorta.
One neonate underwent a patch closure of aortopulmonary window, and a central shunt with a polytetrafluoroethylene graft. This child underwent ventricular septal defect closure, transannular patch, and shunt takedown 2 years later. The remaining two infants underwent single-stage repair (separation of pulmonary trunk, closure of ventricular septal defect, and valved polytetrafluoroethylene right ventricle to pulmonary artery conduit).Reference Kasturi and Prabhu14 All three patients survived surgery and are well currently.
Morphology
External anatomy
There was cardiomegaly with biventricular hypertrophy in all three patients. The pulmonary trunk was tense and dilated, arising from the right ventricle without luminal continuity (Fig 4). The source of pulmonary blood flow was an aortopulmonary window, which provided a unifocal pulmonary arterial supply (none of the three patients had a persistent arterial duct). The pulmonary trunk could be looped proximally and distally to the aortopulmonary communication. The coronary arteries were compared to those in a normal heart (right coronary artery and left main coronary artery dividing into left anterior descending artery and left circumflex artery) with origin having a counterclockwise rotation (Figs 2–4).

Figure 4. Intraoperative photographs of tetralogy of Fallot with pulmonary atresia and aortopulmonary window. ( a ) External appearance: 1 – aorta, 2 – pulmonary trunk, 3 – right ventricle. ( b ) After the division of great arteries: 1 – aortic cannula, 2 – superior caval vein cannula, 3 – inferior caval vein cannula, 4 – ascending aorta, 5 – pulmonary trunk, 6 – vessel loop around right pulmonary artery, 7 – vessel loop around left pulmonary artery, 8 – aortic root with bicuspid aortic valve, 9 – pulmonary root with atretic pulmonary valve, arrow indicates inferior aortopulmonary septum, broken arrow indicates ostium of left main coronary artery. Normal origin of right coronary artery can be seen.
Intracardiac anatomy
All three patients had a remnant of a separate pulmonary valve (Fig 4), but no communication with either ventricle. One patient had a bicuspid aortic valve (anterior and posterior aortic leaflets) (Fig 4). The remaining two patients had tricuspid aortic valves.
The aortopulmonary communication was proximal in two patients (Figs 2 and 4) and distal in one (extending into the right pulmonary artery) (Fig 3). The inferior aortopulmonary septum was present in all. During the embryological development of a normal human embryo, the aortopulmonary septum is a transient structure and does not persist in the postnatal heart.Reference Anderson, Chaudhry and Mohun15 This aortopulmonary septum gets transformed into the separate walls of the aorta and pulmonary trunk even in the setting of aortopulmonary window.
Interventricular communication was in the form of perimembranous ventricular septal defect in two patients with postero-inferior margin probably related to the conduction tissue. The third patient had a muscular border all around (the postero-inferior limb of the septomarginal trabeculation fused with the ventriculo-infundibular fold). A sub-pulmonary infundibulum (blindly ending sub-pulmonary outflow tract) was present in all. The ventricular septal defect was separated from the blindly ending infundibulum by a malaligned muscular outlet septum. Extensive malalignment of the outlet septum (fibrous or muscular) is the essence of Fallot-type pulmonary atresia with ventricular septal defect, and was present in all three of our cases. On the basis of this phenotypic anatomy, we have grouped our patients together.
Intrapulmonary anatomy
Rossi RN et al have mentioned that in patients with pulmonary atresia with ventricular septal defect, rare sources of pulmonary arterial supply normally do not usually bring problems since the segmental arterial supply to the lungs is normally distributed.Reference Rossi, Hislop, Anderson, Martins and Cook8 Our three patients had unifocal and symmetric pulmonary arterial supply with increased pulmonary blood flow status. CT scan in two patients demonstrated normal segmental distribution of pulmonary arteries without hypoplasia of intraparenchymal arteries.
Discussion
Patients with tetralogy of Fallot with pulmonary atresia and aortopulmonary window clinically and echocardiographically resemble those with common arterial trunk. Presentation can be late, as the patients are not overtly cyanotic. Surgical management is also similar to common arterial trunk and early surgical intervention (staged or total correction) is needed to avoid the establishment of irreversible pulmonary artery hypertension or Eisenmengerization.
In cases of tetralogy of Fallot with pulmonary atresia and aortopulmonary window, depending on the type of aortopulmonary window, there can be confusion with common arterial trunk with aortic dominance.Reference Mori, Ando, Takao, Ishikawa and Imai16–Reference Jacobs, Quintessenza, Gaynor, Burke and Mavroudis18 If the aortopulmonary communication is proximal or intermediate, it can be confused with common arterial trunk with aortic dominance and minimal separation of the intrapericardial pathways (two cases in our series). And if the aortopulmonary communication is distal or total, it can be confused with common arterial trunk with aortic dominance and significant separation of the intrapericardial pathways (one case in our series).
Patients with tetralogy of Fallot with pulmonary atresia and aortopulmonary window have a sub-pulmonary infundibulum, a remnant of a separate pulmonary valve, and a pulmonary trunk arising from the ventricle (without luminal continuity). There is a true ventricular septal defect. These are four features that are conspicuous by their absence in common arterial trunk (in patients with common arterial trunk, the interventricular communication is bordered superiorly by the truncal valve, not ventricular septum).
Aortopulmonary window as a unifocal source of pulmonary arterial supply in tetralogy of Fallot with pulmonary atresia is considered to be a rare entity.Reference Rossi, Hislop, Anderson, Martins and Cook8,Reference Liao, Edwards, Julsrud, Puga, Danielson and Feldt9,Reference Baker, Anderson, Anderson, Baker, Penny, Redington, Rigby and Wernovsky11,Reference Anderson, Anderson, Spicer, Hlavacek, Cook and Backer12 We suggest that this entity, rather than being rare, is misdiagnosed and managed as a common arterial trunk.
The morphological differences between the tetralogy of Fallot with pulmonary atresia and aortopulmonary window and common arterial trunk are shown in Table 1.
Table 1. Morphological differences between common arterial trunk and tetralogy of Fallot with pulmonary atresia and aortopulmonary window.

* Data from more TOF-PA-APW patients is needed to categorise the ventricular septal defect type, morphology of ventricular outlet valve, anatomy of the arterial duct, and coronary artery pattern.
Conclusion
We have described an outflow tract malformation, tetralogy of Fallot with pulmonary atresia and aortopulmonary window, which can be misdiagnosed as common arterial trunk, but which has definitive morphological differences. Data from more such patients is needed to categorise the type of ventricular septal defect, morphology of ventricular outlet valve, anatomy of arterial duct, and coronary artery pattern.
Acknowledgements
None.
Financial support
None.
Conflicts of interest
None.
Ethical standards
The ethics committee of the Narayana Institute of Cardiac Sciences approved the study (NHH/AEC-CL-2021 636) and waived need for the individual consent.








