The composition of bovine milk fat is essential for the milk value regarding its nutritive value and processability. Milk fat is one of the most complex natural sources of fatty acids (FA) from C2 to C28, including even- and odd-numbered, saturated, monounsaturated, polyunsaturated cis and trans, linear and branched and various keto- and hydroxy-FA (Sommerfeld, Reference Sommerfeld1983; Ledoux et al. Reference Ledoux, Chardigny, Darbois, Soustre, Sébédio and Laloux2005). Some of these FA, like short-chain saturated FA (SCSFA) and positional and geometrical isomers of octadecadienoic acid with conjugated double bonds called CLA, have beneficial biological, physiological and nutritional properties (Watkins et al. Reference Watkins, Carter, Mak, Tsau, Yamamoto and German1999; Pariza et al. Reference Pariza, Park and Cook2001).
Major CLA isomer, c-9, t-11 C18:2, was recently shown as potentially beneficial for human health having, e.g., potent cancer-fighting properties (Pariza et al. Reference Pariza, Park and Cook2001). The richest dietary sources of CLA are ruminant products such as bovine meat, milk and milk products. Although CLA is known to be generated in the rumen, there is good evidence that much of it present in bovine milk is actually synthesised within the mammary gland from t-11 18:1. This is mediated by the stearoyl-CoA desaturase (Δ9-desaturase), an enzyme capable of adding a cis-9 double bond to t-11 C18:1, to render c-9, t-11 C18:2 (CLA). The results obtained by Corl et al. (Reference Corl, Baumgard, Dwyer, Griinari, Phillips and Bauman2001) indicated that rumen is a source of CLA in milk fat, rumen production of t-11 C18:1, a substrate for the endogenous synthesis of CLA, being predominant.
The composition of bovine milk fat is influenced by many factors both internal (cattle breed, age, stage of lactation, etc.) and external (feeding systems, seasonal changes, milking frequency and milking system; cf. Jensen, Reference Jensen2002; Kalač & Samková, Reference Kalač and Samková2010; Morales-Almaráz et al. Reference Morales-Almaráz, Soldado, González, Martinez-Fernández, Dominguez-Vara, De La Roza-Degaldo and Vicente2010). Another important factor is geographical, which determines the plant variety underlying the feeding of ruminants. The effects of seasonal and geographical factors on FA composition of the bovine milk fat were reported by many authors (Faulkner et al. Reference Faulkner, Brechany, Mabon and Pollock1986; Lock & Garnsworthy, Reference Lock and Garnsworthy2003; Alonso et al. Reference Alonso, Brana and Bada2004; Thorsdottir et al. Reference Thorsdottir, Hill and Ramel2004; Frelich et al. Reference Frelich, Slachta, Hanus, Spicka and Samkova2009; Rutkowska & Adamska, Reference Rutkowska and Adamska2011).
A specific FA composition of milk from mountainous regions, e.g. in Switzerland, France, Italy and in Czech Republic was recently reported (Leiber et al. Reference Leiber, Scheeder, Wettstein and Kreuzer2004; Collomb et al. Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008; Frelich et al. Reference Frelich, Slachta, Hanus, Spicka and Samkova2009). Mountainous regions in Poland also seem very promising for producing milk due to a high quality of forage devoid of concentrates and with low content of corn silage commonly used in lowland farms. Moreover, mountainous regions represent an important grassland zone and are, therefore, well suited for milk production. However, Polish mountainous regions substantially differ from the Alpine ones—over 80% of farms in the former are small-scale and the arable land is of low fertility. The economic status of those farms is low and, for that reason, the dairy cow husbandry is based on extensive breeds resistant to poor climatic conditions. Animal nutrition is based exclusively on locally produced feeds as the human relations in those areas are very strong. The aim of this study was thus to evaluate the fatty acid profile of milk fat in mountain farms in Poland in relation to lipid intake in feeds and their FA composition.
Materials and Methods
Sampling and sample treatment
The study was carried on 5 cow herds reared in low-input mountain farms located at altitudes of 670–780 m above sea level (Beskid Mountains, Poland), 9 cows per herd, on average. The cows were of Black-White breed, their average milk yield amounting to 4465 kg per standard lactation (305 d). The farms were typical of the Polish mountainous region in terms of area and in the number of reared animals. The nutrition of cows was typical of that region and depended on soil and climatic conditions and on the economical status of farms. From 1st May to 15th October the cows were pastured with supplementation of fodder maize in colder periods. In the evenings, when back to the stalls, the cows were administered coarse grains (triticale and oat). During the indoor period (from mid-October to the end of April), the cows were fed corn silage and hay prepared from grass cut in June and July. The details on cow feeding are presented in Table 1. Samples of every forage were pooled across the 5 studied farms.
Table 1. Composition of the feed ration (kg fresh weight per cow and day) administered to cows (n = 45)
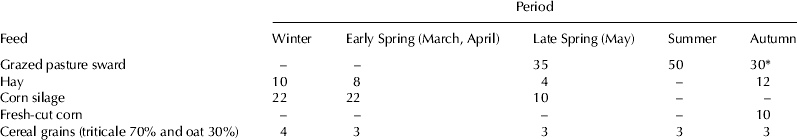
* From 1st September to 15th October
Cows were milked twice daily at 06:00 and 18:00 using bucket machine. The pooled evening and morning milk were stored at 4 °C in temperature-controlled bulk tanks and on the following morning milk samples were taken and transported in cooled box to the laboratory. Milk fat was immediately extracted. A total of 83 milk samples were collected from November 2009 to November 2010 and analysed by the season. One milk sample was taken from each herd every month in the indoor feeding period, i.e. 17 samples in the winter (November–February) and 10 samples in early spring (March, April). In the grazing period, two samples were taken from each herd every month, i.e. 10 samples in late spring (May), 30 samples in the summer (June–August), and 16 samples in the autumn (September, October). Less samples were taken in the autumn due to approaching calving of some cows and in the winter—due to suspected cold-related disorders.
Extraction of lipids from milk and from forage samples
Milk lipids were extracted and determined using the Röse–Gottlieb method (AOAC No 905.02). Well ground samples of forage (3 g) were homogenised in chloroform-methanol 2:1 v/v and filtered (Folch et al. Reference Folch, Lees and Sloane-Stanley1957). The filter was washed several times with small amounts of that mixture, the total filtrate was supplemented with 20% water and shaken for 5 min in a separating funnel with deaeration, then covered with aluminium foil and left for 2 h to separate layers. The chloroform layer was dried with sodium sulphate, filtered into a 150-ml round-bottomed flask, chloroform was evaporated on a pressure evaporator and the residue was dried till constant weight up to 3 decimal places. Dry mass of samples (5 g) was determined in duplicates in a preheated and cooled weighing bottle, dried for 2 h at 105 °C, then cooled for 30 min and weighed. Drying and cooling was repeated until constant weight (within 1 mg).
Fatty acid analysis of lipids in milk and in forage samples
Methyl esters of FA (FAME) were prepared by transmethylation of fat samples using a mixture of concentrated H2SO4 (95%) and methanol (AOCS Official Method Ce 2-66); FAMEs were analysed by gas chromatography (GC) using an Agilent 6890N (USA) chromatograph equipped with flame ionisation detector (FID), a split/splitless injector, operated with a split ratio of 1:50, and capillary column with stationary phase of high polarity (100 m × 0·25 mm I.D., film thickness 0·1 μm; Rtx 2330 Restek). Oven temperature was initially 120 °C for 40 min then ramped to 155 °C at 1·5°/min and held for 50 min, then ramped again at 2°/min to 210 °C and held for 35 min. Injector and detector temperatures were maintained at 250 °C, the carrier gas (helium) flow rate being 0·9 ml/min.
Peak areas were corrected by the response factors for FAME responses of FID, and area% of FAME was appropriately converted to weight% of FA. A butter reference standard (CRM 164; Commission of the European Communities, Community Bureau of Reference, Brussels, Belgium) was used to determine the recovery rates and correction factors for individual FAs in milk samples. In addition, the Supelco 37 No. 47885-U standard (Sigma Aldrich) was applied for fatty acid identification and recoveries of FAME. The retention times of unsaturated FAME: linoleic—c-9 c-12 C18:2, α-linolenic—c-9 c-12 c-15 C18:3, γ-linolenic C18:3 n-6) were confirmed by those recorded for natural plant oils (rapeseed, sunflower, evening primrose), for which those acids are characteristic. The contents of individual FAs were expressed as g/100g FA. Because of the analytical techniques used, the term ‘fatty acid’ or its acronym ‘FA’ means actually methyl ester of the respective acid (FAME). Thus, the absolute or relative contents of FAs are those of FAME throughout the text. The activity of Δ9-desaturase was assessed from the product:substrate ratio according to Lock & Garnsworthy (Reference Lock and Garnsworthy2003).
Data analysis
The between-season differences in individual FA in milk samples were assessed using one-way ANOVA and Tukey's HSD post-hoc test for uneven sample sizes. Statistica 9PL software (StatSoft Inc., 2010) was used, the level of P ⩽ 0·05 being considered significant.
Results
Fatty acid composition of forages and fat intake by cows
Highest lipid content was found in fresh grass (4·2–4·7%; Table 2). Other forages contained much less fat (1·5–2·4%) and for that reason highest fat intake by cows was noted in the grazing season (Fig. 1), mainly during summer, when the animals were grazing 10–12 h daily and the grass intake amounted to about 50 kg (Table 1). Grass from the grazed pasture was rich in polyunsaturated FA (PUFA)—64·1 and 51·8 g/100 g FA in the summer and autumn seasons, respectively; PUFA content in other forages was lower and ranged from 22·57 (hay) to 32·74 g/100 g FA (grains; Table 2). Grass from various seasons differed with respect to individual PUFA: May grass (late spring) contained more α-linolenic acid than the summer one – 50·54 and 40·54 g/100 g FA, respectively. Regarding linoleic acid, its content in autumn grass was drastically lower compared with that from summer or late spring (1·36 vs. 12·95 g/100 g FA, respectively).
Table 2. Mean content (g·100 g−1FA) of major fatty acids in forages

* Collected from 31st May to 3rd June
ND – not detected

Fig. 1. Daily intakes (g/d) of fat from various forage sources
Seasonal differences were also found in saturated FA (SFA; mainly C16:0 and C18:0): green forages (grass and corn) contained less SFA (16·11–22·27 g/100 g FA) than grains, corn silage or hay (24·52–38·83 g/100 g FA). Moreover, cereal grains and fresh corn were rich in oleic acid (c-9 C18:1; Table 2).
Fatty acid composition of milk
Application of the high-resolution GC enabled measurement of the levels of 47 FA in milk fat. The composition of milk fat in relation to season is shown in Tables 3 and 4.
Table 3. Fatty acid composition of bovine milk fat (g·100 g−1FA) from a mountainous region in Poland

Values with different superscripts significantly (P ⩽ 0·05) differ from each
Table 4. Fatty acid composition of odd- and branched-chain (OBCFA) of bovine milk fat (g/100 g FA) from a mountainous region in Poland means±sd

nd = not detected
Values with different superscripts significantly (P ⩽ 0·05) differ from each other
Short-chain saturated FA (SCSFA) were represented by C4:0 – C12:0 compounds; in general, their content was highest in winter (14·10 g/100 g FA) and lowest in summer (11·32 g/100 g FA). Samples from the winter period were significantly (P ⩽ 0·05) higher in the contents of butyric (C4:0), caproic (C6:0) caprylic (C8:0) and capric (C10:0) acids compared with the summer ones.
The levels of major long-chain saturated FA (LCSFA) in milk fat (C14:0, C16:0 and C18:0) varied significantly throughout the year. The contents of palmitic (C16:0) and myristic (C14:0) acids were higher in winter: (37·92 and 11·80 g/100 g FA) than in summer (25·00 and 9·80 g/100 g FA, respectively). An opposite trend was observed in case of stearic acid (C18:0) whose highest content was found in summer (11·33 g/100 g FA), and lowest in winter and early spring (7·64 g/100 g FA).
Oleic acid (c-9 C18:1) levels were higher in the grazing season (20·50 and 19·64 g/100 g FA in the summer and spring, respectively) than in the indoor one (14·52 g/100 g FA). Both total trans C18:1 acids and vaccenic acid (t-11 C18:1) were subject to high seasonal variations, highest concentrations being noted in summer and lowest in the winter and early spring months. The content of α-linolenic acid was twice as high in summer (0·77 g/100 g FA) than in the winter period (0·38 g/100 g FA). Greatest variability throughout the year was noted in the CLA content which was highest in summer and lowest in winter and early spring (1·20 and 0·33 g/100 g FA on average).
The contents of the main odd- and branched-chain FAs (OBCFA) isomers (tetradecanoic—iso C14:0, pentadecanoic—C15:0, iso C15:0 and anteiso C15:0, hexadecanoic—iso C16:0 and heptadecanoic—C17:0, iso C17:0 and anteiso C17:0) are presented in Table 4. Total OBCFA content differed significantly (P ⩽ 0·05) between the grazing and indoor seasons: 6·97 and 5·92 g/100 g FA in late spring and in winter, respectively.
Assessment of Δ 9-desaturase activity
The activity of Δ9-desaturase was assessed by comparing the product:substrate ratios of certain FA shown in Table 5. The ratios were season-dependent.
Table 5. Product:substrate ratios reflecting Δ9-desaturase activity in the mammary gland
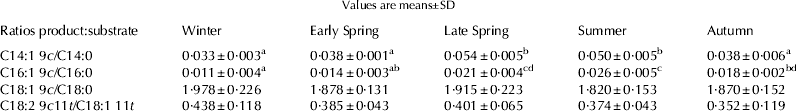
Values with different superscripts significantly (P ⩽ 0·05) differ from each other
Discussion
The study was conducted under natural circumstances of farming and herd management in a Polish mountainous region which remains natural with respect to environmental conditions like climate, soil, vegetation period, etc. The nutrition of studied herds was based solely on forages planted locally, i.e. pasture supplemented with small amounts of grains in the grazing season, and hay and corn silage in the winter season. Those season-related feeding regimes were, obviously, reflected in the FA profiles of milk and forages.
Fat intake was highest in summer (249 g/d) due to a high content of fat in grass and lowest in early spring (55·7 g/d). It should be emphasised that grazed grass was in late spring markedly rich in PUFA, α-linolenic acid being its principal component. However, hay produced from grass in that period contained only one quarter of the α-linolenic acid, the losses being due to wilting and drying. Namely, a mechanical damage to plants combined with air access bring about extensive oxidation of PUFA, especially α-linolenic acid, as reported by Kalač & Samková (Reference Kalač and Samková2010). In the winter season, cereal grains, hay and corn silage were the sources of PUFA but the content of α-linolenic acid was low in them (17·63 g/100 g FA in corn silage).
The effect of PUFA on FA profile of bovine milk is of utmost importance due to the fact that PUFA are substrates of biohydrogenation of the essential fatty acids—vaccenic and a range of conjugated linoleic acids in which the c-9 t-11 C18:2 constitutes 75–90% of total CLA (Bauman et al. Reference Bauman, Corl, Peterson, Sebedio, Christie and Adlof2003). Vaccenic acid is the predominant trans-FA in milk fat (Griinari et al. Reference Griinari, Dwyer, McGuire, Bauman, Palmquist and Nurmela1998). Vaccenic acid can be converted to c-9 t-11 C18:2 – CLA via the Δ9-desaturase. Several authors demonstrated that human beings can convert about 20% of vaccenic acid into CLA, thereby doubling the CLA supply (Palmquist et al. Reference Palmquist, Lock, Shingfield and Bauman2005). In this study, the concentration of vaccenic acid in the summer/autumn milk increased over fourfold compared with the winter/early spring season and the same was true for total trans C18:1 isomers, the increase being nearly fourfold. Those differences could be explained by a higher supply of PUFA during summer (Kepler & Tove, Reference Kepler and Tove1967; Couvreur et al. Reference Couvreur, Hurtaud, Lopez, Delaby and Peyraud2006). Our study confirmed earlier observations (Bargo et al. Reference Bargo, Delahoy, Schroeder, Baumgard and Muller2006; Couvreur et al. Reference Couvreur, Hurtaud, Lopez, Delaby and Peyraud2006; Ferlay et al. Reference Ferlay, Agabriel, Sibra, Journal, Martin and Chilliard2008) that high content of total trans C18:1 in milk fat and of vaccenic acid were due to grazing. Similar variations in total trans C18:1 isomers were found in other studies (Lock & Garnsworthy, Reference Lock and Garnsworthy2003; Collomb et al. Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008). Similar changes were also shown by us for CLA as confirmed by others: Collomb et al. (Reference Collomb, Bütikofer, Sieber, Jeangros and Bosset2002, Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008), Bargo et al. (Reference Bargo, Delahoy, Schroeder, Baumgard and Muller2006), Couvreur et al. (Reference Couvreur, Hurtaud, Lopez, Delaby and Peyraud2006), Frelich et al. (Reference Frelich, Slachta, Hanus, Spicka and Samkova2009).
Due to the differences in PUFA intake, the total PUFA content in milk tended to fluctuate throughout the year; summer and winter milk samples contained 4·23 and 2·20 g/100 g FA, respectively. Also, α-linolenic acid showed seasonal variation; its significantly higher content in summer milk was reported for Switzerland (Collomb et al. Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008) it was attributed to a high content of PUFA in grass and legume silages in contrast to a lower one in concentrates which, in turn, led to development of specific rumen bacteria of intense activity. The feeding regimen based only on fodder prepared from own crops and on grazing (May till mid-October) led to increasing the content of α-linolenic acid in milk fat, and the resulting biohydrogenated FA, CLA and vaccenic acid (Dewhurst et al. Reference Dewhurst, Shingfield, Lee and Scollan2006; Collomb et al. Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008). These effects are amplified by a specific mobilisation of body fat in cows with alpine hypoxia, as well as by a reduced ruminal biohydrogenation due to energy shortage or to secondary plant ingredients like polyphenols and terpenoids that inhibit hydrogenating microorganisms in the rumen (Leiber et al. Reference Leiber, Scheeder, Wettstein and Kreuzer2004, Reference Leiber, Kreuzer, Nigg, Wettstein and Scheeder2005, Collomb et al. Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008).
During the indoor period, the nutrition of cows was based mainly on corn silage and hay supplemented with grains. Those fodders contained much more SFA compared with green products – 38·83 and 32·42 g/100 g FA in hay and corn silage, respectively. In that period, the intake of long-chain SFA was higher than in the summer season which resulted in correspondingly higher content of those SFA in milk – 57·21 and 46·32 g/100 g FA, respectively. Similar results were reported for mountain farms by Ferlay et al. (Reference Ferlay, Agabriel, Sibra, Journal, Martin and Chilliard2008) in France and Frelich et al. (Reference Frelich, Slachta, Hanus, Spicka and Samkova2009) in Czech Republic.
As compared with other reports (Lock & Garnsworthy, Reference Lock and Garnsworthy2003; Collomb et al. Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008; Frelich et al. Reference Frelich, Slachta, Hanus, Spicka and Samkova2009; Nałęcz-Tarwacka et al. Reference Nałęcz-Tarwacka, Grodzki, Kuczyńska and Zdziarski2009) on various regions and feeding regimens, the content of C4:0 in milk of cows studied by us was much higher but season-related (3·70 and 4·68 g/100 g FA in summer and winter, respectively). Similar season-dependence of the C4:0 content in milk was reported for Spain (Alonso et al. Reference Alonso, Brana and Bada2004), UK (Lock & Garnsworthy, Reference Lock and Garnsworthy2003), France (Ledoux et al. Reference Ledoux, Chardigny, Darbois, Soustre, Sébédio and Laloux2005) and Poland (Rutkowska & Adamska, Reference Rutkowska and Adamska2011) but a reverse trend was observed in a mountainous area in the Czech Republic (Frelich et al. Reference Frelich, Slachta, Hanus, Spicka and Samkova2009). Seasonal changes in the content of other SCFAs (C6:0–C10:0) in milk are worth interest since they are synthesised in part by non-malonyl CoA mechanisms, i.e. not involving acetyl-CoA carboxylase, thus being less likely to be affected by differences in the intakes of PUFA between grazing vs. winter feeding periods (Ferlay et al. Reference Ferlay, Agabriel, Sibra, Journal, Martin and Chilliard2008).
As far as the long-chain SFA are concerned, lower levels of the rather controversial FA (C14:0 and C16:0) in milk were noted by us in the summer than in winter samples. This was particularly evident in case of palmitic acid whose content decreased dramatically from winter to summer (37·92 vs. 25·0 g/100 g FA, respectively). Similar seasonal trends for C16:0 were reported from a mountainous region in France (Ferlay et al. Reference Ferlay, Agabriel, Sibra, Journal, Martin and Chilliard2008), Czech Republic (Frelich et al. Reference Frelich, Slachta, Hanus, Spicka and Samkova2009) and Switzerland (Collomb et al. Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008) but the differences were smaller. Those changes might be beneficial for human health since only the C12:0, C14:0 and C16:0 acids adversely affect plasma cholesterol levels.
The content of stearic acid (C18:0) in ruminant feed is known to be low amounting to 3·50 g/100 g FA on average (Doreau & Chilliard, Reference Doreau and Chilliard1997); thus, an increased concentration of C18:0 acid in milk compared with that in the animal diet is due to extensive biohydrogenation of PUFAs in the rumen (Polan et al. Reference Polan, Mcneill and Tove1964; Lock & Garnsworthy, Reference Lock and Garnsworthy2003). In this study, the concentration of C18:0 in milk fat was significantly increased in summer when the cows stayed all day on pasture. We thus confirmed that fresh grass contained high concentrations of α-linolenic acid convertible in the rumen to vaccenic and stearic acids. The majority of C18:0 is a product of PUFA hydrogenation in rumen (Chilliard et al. Reference Chilliard, Ferlay and Doreau2001; Jenkins et al. Reference Jenkins, Wallace, Moate and Mosley2008). Similar seasonal effects of mountainous pastures on C18:0 content in milk was also reported by other authors (Ferlay et al. Reference Ferlay, Agabriel, Sibra, Journal, Martin and Chilliard2008; Frelich et al. Reference Frelich, Slachta, Hanus, Spicka and Samkova2009), as well as for other areas (Asturia, Spain; Alonso et al. Reference Alonso, Brana and Bada2004). Higher content of C18:0 in butter was also noted in the summer than in winter by Polish and French authors (Ledoux et al. Reference Ledoux, Chardigny, Darbois, Soustre, Sébédio and Laloux2005; Rutkowska & Adamska, Reference Rutkowska and Adamska2011).
The interest in OBCFAs is steadily increasing due to their anticarcinogenic effects and for being potential indicators of dairy product intake by human beings (Vlaeminck et al. Reference Vlaeminck, Fievez, Cabrita, Fonesca and Dewhurst2006). Although OBCFAs in milk fat are largely derived from rumen bacteria, we attributed the variability in milk OBCFA levels to the composition of fodders. Fresh grass feeding resulted in a higher total content of OBCFA in milk compared with indoor feeding. Similar effect was also reported by Collomb et al. (Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008), Ferlay et al. (Reference Ferlay, Agabriel, Sibra, Journal, Martin and Chilliard2008) and Frelich et al. (Reference Frelich, Slachta, Hanus, Spicka and Samkova2009) in milk samples originated from mountains in the Switzerland, France and Czech Republic, respectively. Our results confirmed earlier observations of Ferlay et al. (Reference Ferlay, Agabriel, Sibra, Journal, Martin and Chilliard2008) that microbial synthesis of branched-chain FA (BCFA) seems to be enhanced by fibre-rich diets since the relative content of fibre in the forage was closely related to the relative content of anteiso C15:0 in rumen bacteria. The content of anteiso C15:0 varied from 0·63 to 0·85 g/100 g FA and was generally higher compared with other reports (Collomb et al. Reference Collomb, Bisig, Bütikofer, Sieber, Bregy and Etter2008; Ferlay et al. Reference Ferlay, Agabriel, Sibra, Journal, Martin and Chilliard2008; Frelich et al. Reference Frelich, Slachta, Hanus, Spicka and Samkova2009) due probably to a high fibre content in the forage and low quantities of concentrates in the diet. We also noted higher contents of C17:0 and anteiso C17:0 in the period of intensive biohydrogenation of PUFA than reported by others (Vlaeminck et al. Reference Vlaeminck, Fievez, Cabrita, Fonesca and Dewhurst2006).
In addition, direct and indirect effects of diet on the FA composition of milk fat and the role of Δ9-desaturase ought to be considered. Mammary gland cells contain the Δ9-desaturase complex (often referred to as stearoyl-CoA desaturase), responsible for catalysing the oxidation of acyl-CoA esters, that results in the introduction of a cis double bond between carbon atoms 9 and 10 (Shingfield et al. Reference Shingfield, Chilliard, Toivonen, Kairenius, Givens and Bosze2008). The 4 main products of Δ9-desaturase activity in the mammary gland of ruminants (C14:1, C16:1, c-9 C18:1 and CLA) are produced from C14:0, C16:0, C18:0, and t-11 C18:1 acids, respectively.
The product:precursor ratio C14:1 : C14:0 was used as a proxy for the assessment of Δ9-desaturase activity in the mammary gland regarding possible effects of season and dietary changes. That ratio is the most appropriate since the whole amount of the C14:0 in milk fat is produced via de novo synthesis in the mammary gland, hence all of the C14:1 in milk is produced via desaturation of C14:0 in the mammary gland (Corl et al. Reference Corl, Baumgard, Bauman and Griinari2000). We noted significantly higher activities of Δ9-desaturase during the late spring (May) and summer confirmed by higher C14:1 : C14:0 ratios (0·054) than in winter (0·033) and the same was true for the C16:1 : C16:0 ratio. Our observations were supported by the results of Lock & Garnsworthy (Reference Lock and Garnsworthy2003).
A marked seasonal change in the c-9 C18:1 content in milk fat was noted. Highest supply of the c-9 C18:1 acid took place in autumn because of two sources: fresh corn and grains (27·62 and 33·90 g/100 g FA, respectively). The c-9 C18:1 : C18:0 ratio indicated no between-season differences in the Δ9-desaturase activity due to the fact that a steady concentration of c-9 C18:1 acid in milk fat throughout the year would arise only if maximal activity of Δ9-desaturase had been achieved, since 40–50% of c-9 C18:1 acid in milk fat is produced from C18:0 in the mammary gland via Δ9-desaturase (Chilliard et al. Reference Chilliard, Ferlay, Mansbridge and Doreau2000; Lock & Garnsworthy, Reference Lock and Garnsworthy2003).
Summing up, milk fat was nutritionally more valuable in the grazing period, when fat intake was highest, and the FA profile of dietary fat was favourable, than in the winter season. Since the content of fatty acids beneficial for human health in milk was higher in the spring and summer, mainly because of the high quality of locally produced forages, increasing the population of dairy cattle in the mountainous regions, reared under natural pasture conditions, would increase the output of dairy products with health promoting properties and improve the economic status of households in such regions.








