Methicillin-resistant Staphylococcus aureus (MRSA) infections in neonatal intensive care unit (NICU) infants are associated with significant morbidity, prolonged hospital stays, and increased healthcare costs.Reference Song, Perencevich, Campos, Short and Singh 1 , Reference Schultz, Tanaka, Goldberg, Benjamin and Smith 2 Colonization with MRSA is an important risk factor for development of infection, as colonized infants are 24-fold more likely to develop infection during their hospitalization than those who are not colonized.Reference Zervou, Zacharioudakis, Ziakas and Mylonakis 3 In more than 90% of cases, infection occurs with the strain that previously colonized the infant,Reference Huang, Chou, Su, Lien and Lin 4 demonstrating the importance of MRSA surveillance and interventions to prevent its spread.
Guidelines for the management of MRSA outbreaks in NICUs recommend use of molecular analysis to assess relatedness of strains found in patients, healthcare workers (HCWs), and the environment.Reference Gerber, Jones and Scott 5 This information is important because it allows discrimination between sporadic cases and those linked to outbreaks, thereby helping identify potential reservoirs and routes of transmission. Historically, several molecular methods have been used for strain typing: pulsed-field gel electrophoresis (PFGE), surface protein A sequence typing (spa typing), and multilocus sequence typing (MLST).Reference Struelens, Hawkey, French, Witte and Tacconelli 6 Recent studies have demonstrated that typing based on whole-genome sequencing (WGS) can be valuable for investigation of MRSA outbreaks in NICUs, providing better resolution than conventional typing techniques.Reference Koser, Holden and Ellington 7 – Reference Earls, Coleman and Brennan 13
In this study, we describe an apparent outbreak of MRSA colonization and/or infection in a NICU over a 3-year period. We compare the results of WGS and PFGE for investigating strain relatedness; we also describe how these data can be used to guide infection prevention and control (IPAC) interventions.
Methods
Setting
The NICU at Saint Mary’s Campus, Mayo Clinic Hospital in Rochester, Minnesota, is a 26-bed level IV neonatal unit caring for ~370 infants per year. The unit is divided into 4 rooms, with 4–6 bed spaces in each room plus 4 additional adjoining single-room bed spaces used for patients requiring isolation precautions. The average daily census is 18–20 patients.
Screening
Prior to April 2016, targeted screening for S. aureus colonization with reflexive testing for MRSA if positive was performed on infants who were hospitalized in the NICU for 2 weeks or longer and had a central line in place. A single swab sampling the anterior nares, axilla, and groin, was obtained. Clinical cultures were collected as indicated. If MRSA colonization or infection was identified by either a screening or clinical culture, infants occupying adjacent bed spaces were also screened (ie, ring screening). From April 2016 to November 2017, universal weekly screening of all infants was performed. HCWs who had had contact with MRSA-positive infants were identified by clinical documentation and were screened once in the summer or fall of 2016 with swabs of their anterior nares.
Culture collection and processing
Infant swabs were plated on sheep blood agar and Columbia nalidixic acid (CNA) with sheep blood agar plates, incubated at 35°C with CO2, and examined for growth at 16–24 hours and, if negative, at 48 hours. Colonies were identified by Gram stain and coagulase testing, or by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Staphylococcus aureus isolates were identified as MRSA by oxacillin susceptibility testing by agar dilution. HCW swabs were plated on BBL CHROMagar MRSA II (Becton Dickinson, Sparks, MD) and incubated at 35°C in ambient air. Results were reported as positive if characteristic mauve colonies were present after 20–26 hours of incubation. Supplemental testing, including Gram stain and/or coagulase testing, was performed if necessary. Results of screening swabs were reported in the medical record for infants, and the IPAC team was notified of positive results for both infants and HCWs. Environmental sampling was performed by collecting swabs of high-touch surfaces. They were processed as described above for HCW samples. All isolates studied were archived at −80°C in 0.9% sterile saline (Baxter, Deerfield, IL). Only the first MRSA isolate from each infant and HCW was studied. Agar dilution was used for susceptibility testing to all antimicrobial agents studied.
Contact precautions and decolonization for MRSA-positive infants and HCWs
Infants identified as colonized or infected with MRSA were cared for using contact precautions in single bed space rooms or were cohorted in a single room with multiple bed spaces. Colonized or infected infants were treated with nasal mupirocin twice daily for 5 days and remained in contact precautions for the remaining duration of their hospitalization; they did not undergo further screening. Colonized HCWs were treated with nasal mupirocin twice daily and chlorhexidine baths once daily for 5 days and were rescreened after decolonization to demonstrate MRSA eradication.
Pulsed-field gel electrophoresis
Pulsed-field gel electrophoresis (PFGE) was performed by normalizing a broth culture in brain-heart infusion broth to a turbidity of 0.58–0.63 using a turbidometer (Dade Behring, Deerfield, IL). The normalized suspension was concentrated and resuspended in 500 μL of EET buffer (100 mM EDTA, 10 mM EGTA, 10 mM TRIS, pH 8.0). A 500 μL volume of agarose was added to each sample to prepare a plug. Sample plugs were subjected to cell lysis, restriction endonuclease digestion (using SmaI), and electrophoresis. Determination of clonal groups was performed as previously described.Reference McDougal, Steward, Killgore, Chaitram, McAllister and Tenover 14 Further details are available in the supplemental content.
Whole-genome sequencing
Archived isolates of MRSA were recultured on trypic soy agar with 5% sheep blood and incubated at 35°C in room air. Isolates were passaged once prior to WGS. DNA was extracted from cultured isolates using the Zymo Research Quick-DNA Fungal/Bacterial Miniprep kit (Zymo Research, Irvine, CA) with a modified final elution volume of 200 µL. DNA was quantified with a Quantus fluorometer (Promega, Madison, WI) using the QunatiFluor ONE dsDNA System (Promega). DNA was normalized to 0.5 µM and paired-end (PE), dual-indexed DNA libraries were prepared with a Nextera XT PE library preparation kit (Illumina, San Diego, CA). Sequencing was performed on an Illumina MiSeq with V2 2×250-bp chemistry and a maximum of 12 sample libraries multiplexed per flow cell. Raw sequencing reads were processed for adapter and index cleaning using the MiSeq reporter software in real time.
Genome assembly and core-genome multilocus sequence typing
Following delivery of the processed data, read files were imported into SeqSphere+, version 4.0 software (Ridom, Munster, DE). The vendor derived de novo assembly, and the genome assembly and core-genome multilocus sequence typing (cgMLST) pipeline option was exercised in the software suite following default settings and an optional cgMLST target threshold of 90%. Minimum spanning trees were generated from the typing data table for visualization. Thresholds of ≤8 (related), 9–29 (possibly related), and ≥30 (unrelated) allelic differences were applied.Reference Cunningham, Chia and Jeraldo 15 Groupings obtained using WGS typing were qualitatively compared to PFGE groupings.
Results
Apparent outbreak
In the years prior to 2013, 0–7 cases (median, 3) of infants with MRSA colonization or infection per year were identified by targeted screening and clinical cultures. An increased number of cases was recognized when 9 MRSA colonized or infected infants were identified in November of 2014 (Fig. 1A). Following published guidelines for preventing MRSA transmission and infection,Reference Gerber, Jones and Scott 5 , Reference Calfee, Salgado and Milstone 16 basic practices to prevent transmission of MRSA were reinforced. This included improving adherence to hand hygiene and standard precautions, use of single bed space isolation rooms or cohorting of MRSA-positive infants into adjacent bed spaces when the number of MRSA patients exceeded the number of isolation rooms, improving adherence to contact precautions for MRSA-positive infants, enhancing environmental cleaning, and staff and patient and family education. Cohorting of nursing assignments for colonized or infected infants was done when possible. Mothers of MRSA-positive infants were encouraged to use their own breast pumps in a separate area rather than utilizing the lactation room. No further cases were identified during the following 5 months.
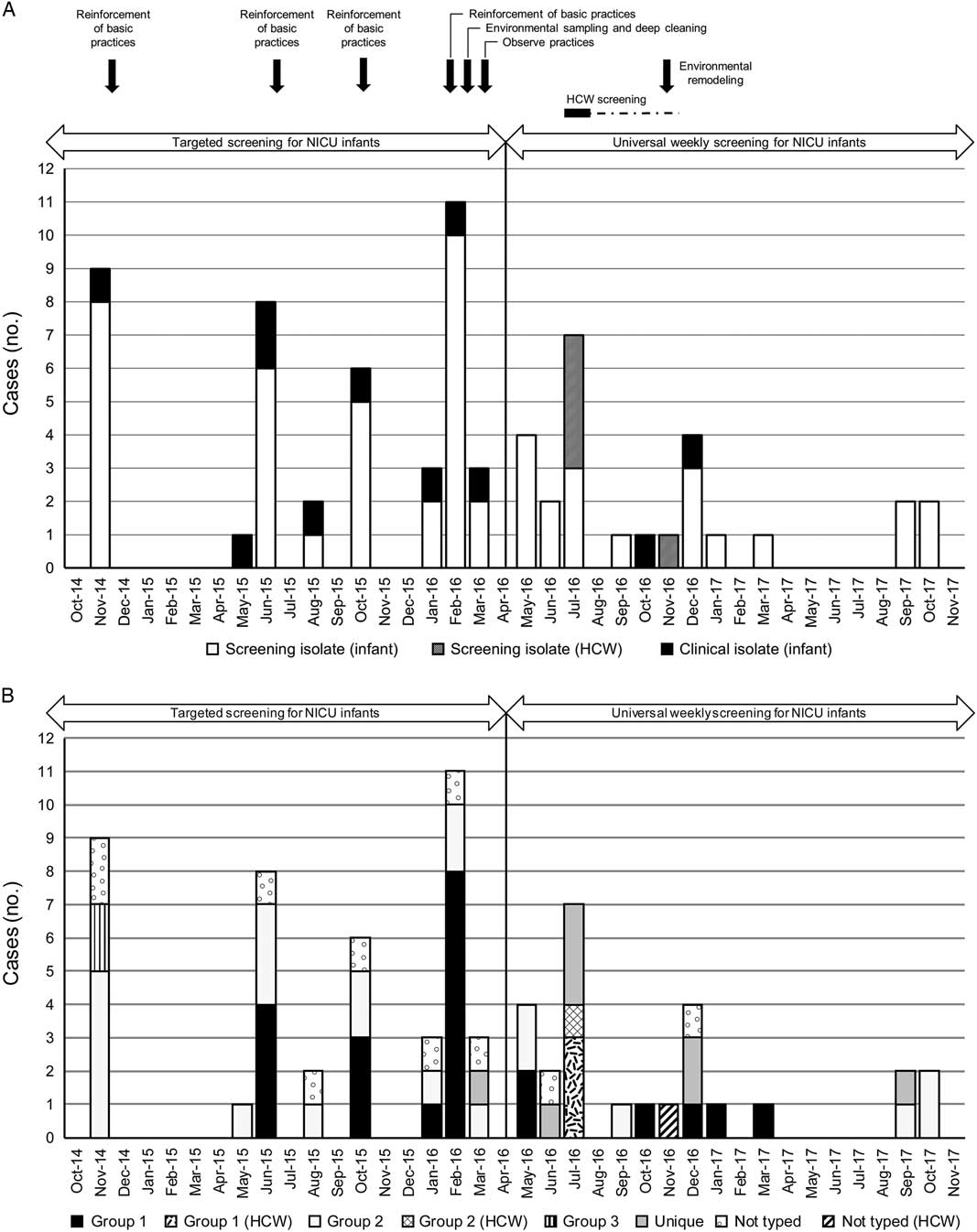
Fig. 1 (A) Timeline of MRSA cases in NICU infants and HCWs from 2014 to 2017. Targeted screening for MRSA in NICU infants was conducted from 2014 to March 2016; weekly universal weekly screening began in April 2016. Cases of MRSA in NICU infants identified by screening cultures (white) or clinical cultures (black) are displayed by month of initial detection. Only the first isolate obtained from each infant was included. Clinical isolates were obtained from respiratory secretions (n=4), blood (n=3), eye drainage (n=3), and a surgical wound (n=1). One-time screening of HCWs was conducted from July to November 2016. Cases of MRSA colonization in HCWs identified by screening cultures (grey-striped) are displayed by month of initial detection. The timing of interventions to prevent and control the spread of MRSA is shown above the graph. (B) Timeline of MRSA cases in NICU infants and HCWs from 2014 to 2017 by WGS group. MRSA cases in NICU infants and HCWs shown in Figure 1A are redisplayed by WGS group. Cases labeled as unique include eight distinct isolates, unrelated to the three WGS groups and to each other. Those labelled “not typed” represent MRSA isolates from infants and HCWs that were unavailable for typing by WGS.
Between May and October 2015, 5 MRSA-positive cases were identified by clinical cultures and 12 more were identified by screening cultures (Figure 1A). Basic prevention practices were reinforced. When 17 more cases were identified between January and March of 2016, environmental sampling was performed to rule out ongoing transmission from a common environmental source. High-touch surfaces, such as drawers, counter tops, phones, patient vital-sign monitors, multiple areas in infant incubators, as well as other objects in patient rooms, utility and storage rooms and the lactation room, were sampled. None of the environmental samples grew MRSA, but adenosine triphosphate testing demonstrated an elevated bioburden on some surfaces. In response to these results, 4 patient rooms were closed in succession for deep cleaning, and environmental services staff received additional training on proper cleaning and disinfection procedures. Procedures for reprocessing ophthalmologic equipment were improved. Compliance with contact precautions and hand hygiene was assessed through direct observations, and feedback was provided to staff by the IPAC team.
Universal weekly screening of all infants in the unit was implemented in April 2016 and continued throughout the remainder of the study (Fig. 1A). Because of additional cases, screening of HCWs began in July 2016 and was completed in November 2016. In total, 96 HCWs were selected for screening based on documentation of contact with MRSA colonized or infected infants in their electronic medical records (EMRs). These included physicians, advanced practice providers, nurses, physician assistants, sonography technologists, respiratory therapists, occupational therapists, child life specialists, and patient care assistants. Overall, 85 HCWs underwent screening, mostly in July 2016; the others were either unavailable for testing or were lost to follow-up. Five HCWs (6%) tested positive for MRSA (Fig. 1A) and underwent decolonization; all had subsequent negative testing in the weeks following decolonization. Screening was not repeated.
Additional infant cases were identified from August to December of 2016 but at a lower frequency than immediately prior to that time (Figure 1A). Environmental remodeling occurred in November 2016, including replacing the flooring, countertops and sinks, as well as minor updates to lighting and the ceiling. In 2017, 6 colonized infants were identified through surveillance screening.
Clinical information
Of the 64 MRSA-positive infants identified between November 2014 and November 2017, 53 (83%) were initially identified by screening swabs. The other 11 infants (17%) were identified by cultures obtained due to clinical illness: surgical wound infection (n=1), bloodstream infection (BSI) (n=3), conjunctivitis (n=3), and suspected ventilator-associated pneumonia or tracheitis (n=4) (Fig. 1A). Of the 53 infants identified by screening, 15 (28%) developed confirmed or suspected MRSA infections later in their hospitalization; the remainder (72%) had no clinical MRSA infection.
PFGE of MRSA isolates
In total, 58 MRSA isolates, 54 from infants and 4 from HCWs, were analyzed using molecular typing. Ten isolates from infants and 1 isolate from a HCW were unavailable for typing. PFGE identified 2 large clusters of related isolates (PFGE groups 1 and 2), with 25 isolates in each cluster: For HCWs, 3 HCW isolates were in PFGE group 1 and 1 HCW isolate was in PFGE group 2. In addition, 7 infant isolates were unrelated to each other and to PFGE groups 1 or 2. A single infant isolate failed PFGE analysis.
WGS of MRSA isolates
Among the 58 MRSA isolates, cgMLST also identified 2 major clusters (WGS group 1 and WGS group 2) and 1 minor cluster (WGS group 3) (Fig. 2). WGS group 1 was most closely related to the USA300 strain, and WGS groups 2 and 3 were most closely related to the USA100 strain (Fig. 2). Another 8 isolates were unique (Fig. 2). Infant cases included in WGS groups 1 and 2 occurred in parallel and were distributed over long periods of time (Fig. 1B).
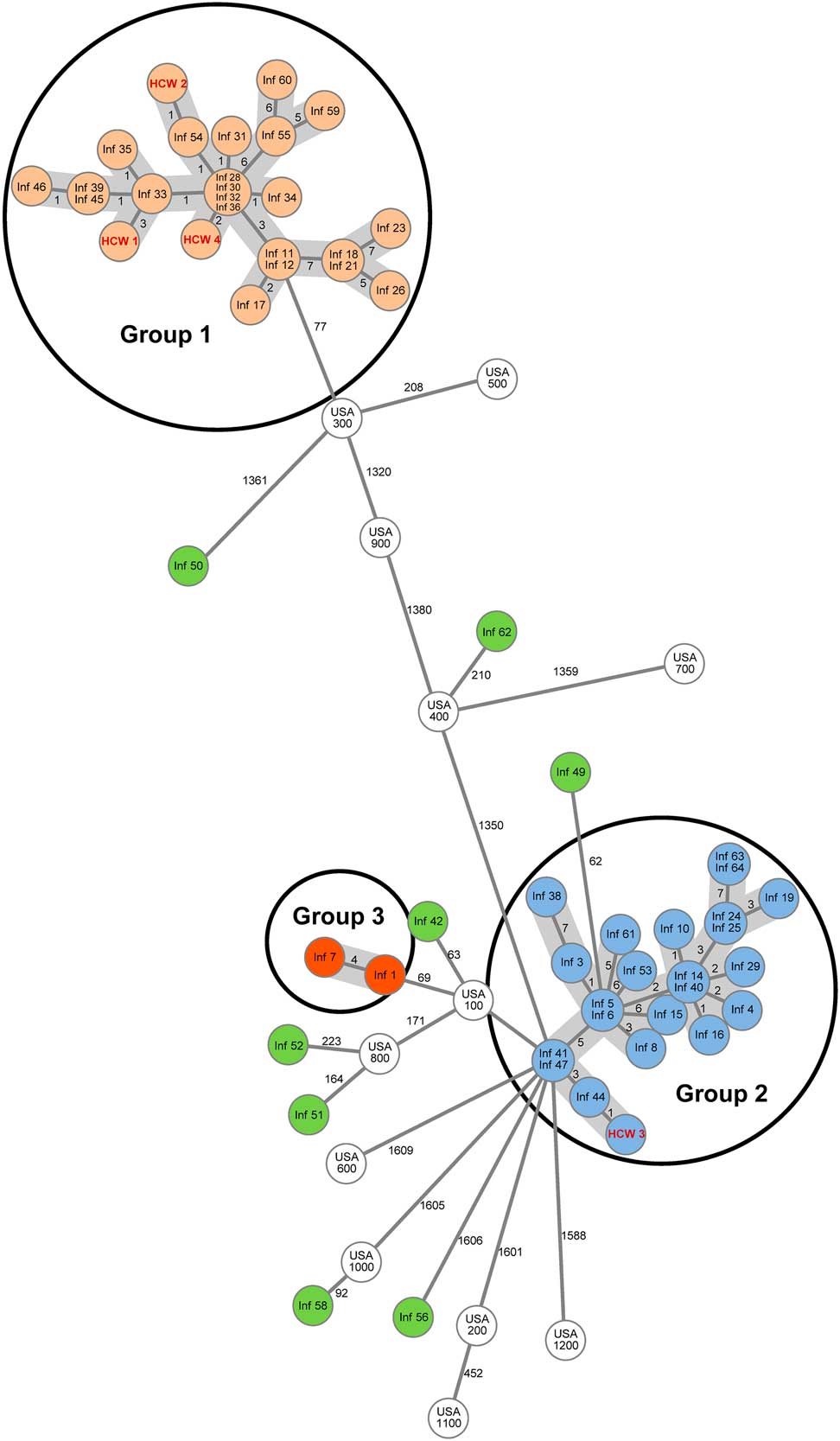
Fig. 2 Collective cgMLST minimum spanning tree. Infant (labeled “Inf”) and healthcare worker (labeled “HCW”) isolates are numbered consecutively based on date of isolation of MRSA. Numbers next to lines show the number of allelic differences between isolates as determined by cgMLST. The line lengths have been shortened for better graphic display and are not proportional to the number of allelic differences. The angles between the lines are randomly determined. Background grey shading links isolates in the same group. PFGE group 1 is the same as WGS group 1, PFGE group 2 includes WGS group 2 and 3. USA isolates USA100, USA200, USA300, USA400, USA500, USA600, USA700, USA800, USA1000, and USA1100 were courtesy of Henry F. Chambers; USA isolates USA900 (ATCC BAA-1749) and USA1200 (ATCC BAA-1765) were obtained from the American Type Culture Collection.Reference Cunningham, Chia and Jeraldo 15 WGS groups 2 and 3 isolates are most closely related to USA100, WGS group 1 isolates are most closely related to USA300.
Comparison of PFGE and WGS T of MRSA isolates
WGS group 1 included the same 25 isolates (22 infants, 3 HCWs) as PFGE group 1. WGS groups 2 and 3 together included the 25 isolates from PFGE group 2. Isolates in WGS group 3 differed from those in WGS group 2 by >30 alleles and were therefore separated from one another by the higher resolution of WGS cgMLST. The 2 isolates in WGS group 3 were from twin infants. The isolate that was not able to be characterized by PFGE was unrelated to all other isolates according to cgMLST.
Antimicrobial susceptibility
Antimicrobial susceptibilities for MRSA isolates by WGS group are shown in the Supplemental Table. The MRSA isolates in WGS group 1 were mostly susceptible to the tested antimicrobials (aside from oxacillin), whereas isolates in WGS groups 2 and 3 were generally resistant to levofloxacin and clindamycin.
Discussion
The existence of 2 contemporaneous outbreaks described herein would not have been recognized without screening of infants combined with the use of molecular typing. Importantly, 83% of MRSA-positive infants were detected initially by screening swabs. The implementation of universal weekly screening in April 2016 led to more rapid identification of new cases and implementation of additional control measures. With the use of WGS, we discovered that 2 major clusters of related isolates occurred in parallel over a long period of time. Thus, WGS was a valuable tool for this outbreak investigation, offering benefit over antibiogram analysis and PFGE in determining isolate relatedness in more detail. WGS distinguished 2 isolates (WGS group 3) occurring in twin infants as distinct isolates not transmitted over time; PFGE did not distinguish these isolates from WGS group 2. Other researchers using WGS for MRSA outbreak investigation have also shown that WGS can disprove transmission pathways suggested by conventional typing methods and thereby improve the understanding of an outbreak.Reference Azarian, Cook and Johnson 8 , Reference Moore, Cookson and Gordon 17 , Reference Garvey, Pichon, Bradley, Moiemen, Oppenheim and Kearns 18 Notably, the WGS bioinformatics and reporting algorithms we used allow easy and near-real-time longitudinal comparison of isolates. We were able to identify the reappearance of 3 WGS group 2 isolates in the fall of 2017, a year after the previous WGS group 2 isolate occurred in September 2016, in addition to a unique isolate (Fig. 1B).
The clusters in our study were most closely related to USA100 (WGS groups 2 and 3) and to USA300 (WGS group 1). The USA100 strain is a common healthcare-associated strain that is typically multidrug resistant,Reference McDougal, Steward, Killgore, Chaitram, McAllister and Tenover 14 and the USA300 strain is considered a community-associated strain that is generally susceptible to most antibiotics,Reference McDougal, Steward, Killgore, Chaitram, McAllister and Tenover 14 consistent with our findings. However, surveillance studies have demonstrated that USA300 is now as common as or more common than USA100 as a cause of nosocomial infection.Reference Diekema, Richter and Heilmann 19 , Reference Richter, Heilmann and Dohrn 20 Indeed, USA300 has been the cause of other reported outbreaks within NICUs, Reference Azarian, Cook and Johnson 8 , Reference McAdams, Ellis, Trevino and Rajnik 21 and several authors have described the concomitant presence of USA100 and USA300 in their units,Reference Popoola, Budd and Wittig 22 , Reference Nelson, Bizzarro, Baltimore, Dembry and Gallagher 23 which highlights the increasingly blurred distinction between hospital and community strains. In addition to the 2 major clusters, we found 8 unique isolates, which is commonly observed as well.Reference Azarian, Maraqa and Cook 10 , Reference Popoola, Budd and Wittig 22 , Reference Nelson, Bizzarro, Baltimore, Dembry and Gallagher 23 These findings demonstrate that multiple sources of transmission exist within as well as outside the unit. Our report underscores the complexity of the transmission dynamics and the difficulty in identifying effective prevention and control strategies.
Previous studies indicate that MRSA surveillance with cohorting of colonized infants and the use of contact precautions may reduce the transmission of MRSA to other infants.Reference Nelson, Bizzarro, Baltimore, Dembry and Gallagher 23 , Reference Kaushik, Kest, Zauk, DeBari and Lamacchia 24 This is important because colonized infants have a higher risk of developing infection with MRSA than those who are not colonized,Reference Zervou, Zacharioudakis, Ziakas and Mylonakis 3 , Reference Huang, Chou, Su, Lien and Lin 4 , Reference Kaushik, Kest, Zauk, DeBari and Lamacchia 24 and decolonization is often unsuccessful in this population.Reference Popoola, Budd and Wittig 22 In our study a quarter of colonized infants developed confirmed or suspected infection with MRSA during their hospitalization, a rate similar to other reports.Reference Huang, Chou, Su, Lien and Lin 4 , Reference Popoola, Budd and Wittig 22 In our study, as in others,Reference Popoola, Budd and Wittig 22 , Reference Gregory, Eichenwald and Puopolo 25 infection control practices alone with or without infant decolonization were insufficient to stop transmission. Environmental screening did not identify an environmental source, and deep cleaning of infant rooms was not successful in preventing ongoing transmission. As WGS data suggested horizontal transmission, HCWs were screened. Those who were positive were colonized primarily with WGS group 1 isolates (n=3), with 1 HCW harboring a WGS group 2 isolate. The disappearance of WGS group 1 after March 2017 suggests that screening and treatment of colonized HCWs may have assisted in control efforts. Other investigators have reported success with this strategy.Reference Khoury, Jones, Grim, Dunne and Fraser 26 However, WGS group 2 reappeared in the fall of 2017, which suggests persistent reservoirs in our unit that have not been identified. This reappearance could also represent recolonization of a previously colonized HCW, as described by others studies in which 80% of previously treated HCWs were positive again several months later.Reference Popoola, Budd and Wittig 22 The HCWs in our study were rescreened after decolonization and were negative, but no further screening was performed to assess the long-term success of decolonization.
With the use of WGS, a database of NICU MRSA isolates was established. This allowed analysis of isolates in real time as new cases were identified, and it will continue to be used for longitudinal comparison of new isolates to tailor replace with IPAC strategies. For example, the recurrence of previous outbreak strains could lead to repeated screening and decolonization of HCWs, whereas the ongoing introduction of unique strains could focus interventions on education and reinforcement of hand hygiene in visitors and family members, or potentially family member screening and decolonization. Although this approach could be accomplished with sophisticated PFGE analysis software and databases, it would be technically more challenging than using WGS. Furthermore, the WGS data are portable and can be used for comparison with other isolates within our institution or can potentially be shared with other institutions without the difficulty of interlaboratory comparison and standardization expected with PFGE data. Researchers in Europe have already applied MRSA WGS technology on a larger scale,Reference Earls, Coleman and Brennan 13 , Reference Coll, Harrison and Toleman 27 – Reference Aanensen, Feil and Holden 29 demonstrating the power of this technology for investigating outbreaks as well as the potential for pathogen surveillance on regional, national, and international levels.
This study has several limitations. Universal weekly screening of infants was not implemented until April 2016; therefore, some cases of MRSA colonization before that time may have gone undetected. The HCWs were selected for screening based on contact with MRSA colonized or infected infants documented in the EMR. This selection strategy was expected to capture most providers, but it may have missed HCWs whose care activities were not documented in the EMR. In addition, a few HCWs were not available for screening, and visitors and family members of infants were not screened. Finally, only 1 isolate from each infant and HCW was sequenced; therefore, colonization with >1 strain of MRSA would not have been detected.
In summary, WGS is a powerful tool for MRSA outbreak investigation that provides more detailed information on isolate relatedness than other conventional typing techniques, such as PFGE. This approach may be helpful in dissecting complex transmission pathways. In addition, WGS data allows for easy and near-real-time longitudinal comparison of isolates by establishing a database of sequenced isolates. Thus, WGS characterization of strains is expected to become the preferred method for molecular typing with the potential for data sharing and application on a wide scale.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.239
Acknowledgments
We thank the physicians, nurses, and other staff in the NICU for their excellent patient care and help with this investigation. We also thank the staff in the clinical microbiology laboratory for their help with isolate acquisition, storage, processing, and analysis. Finally, we thank the Mayo Clinic Center for Individualized Medicine for their assistance with development of the WGS platform.
Financial support
This research was graciously supported by the Mayo Clinic Center for Individualized Medicine.
Conflicts of interest
Dr Patel reports grants from CD Diagnostics, BioFire, Curetis, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, Allergan, and The Medicines Company. Dr Patel is or has been a consultant to Curetis, Qvella, St. Jude, Beckman Coulter, Morgan Stanley, Heraeus Medical GmbH, CORMATRIX, Specific Technologies, Diaxonit, Selux Dx, GenMark Diagnostics, LBT Innovations Ltd, PathoQuest and Genentech; monies are paid to Mayo Clinic. In addition, Dr. Patel has a patent on Bordetella pertussis/parapertussis PCR, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an antibiofilm substance. Dr Patel receives travel reimbursement from ASM and IDSA and an editor’s stipend from ASM and IDSA, as well as honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course. Dr Sampathkumar served as a consultant as a member of an advisory board on an experimental Zika vaccine. All other authors declare no conflicts of interest.




