Introduction
Binge eating refers to episodes in which uncontrolled eating is experienced during consumption of an objectively large amount of food. DSM-5 includes two eating disorders (EDs) defined by recurrent binge eating: bulimia nervosa (BN) and binge eating disorder (BED; APA, 2013). BN also involves inappropriate compensatory behaviors intended to prevent weight gain. The lifetime prevalence of BN and BED are approximately 0.9–1.0% and 1.9–2.8%, respectively (Hudson et al. Reference Hudson, Hiripi, Pope and Kessler2007; Swanson et al. Reference Swanson, Crow, Le Grange, Swendsen and Merikangas2011). Both involve major impairment (APA, 2013), with long-term treatment remissions in the range of 30–50% for BN and 50–70% for BED (Kass et al. Reference Kass, Kolko and Wilfley2013). Significant diagnostic crossover between BN and BED (Allen et al. Reference Allen, Byrne, La Puma, McLean and Davis2008; Stice et al. Reference Stice, Marti and Rohde2013) suggests shared etiologies. Indeed, BN and BED have similar risk factors (Hilbert et al. Reference Hilbert, Pike, Goldschmidt, Wilfley, Fairburn, Dohm, Walsh and Striegel Weissman2014) and onset patterns, with peak incidence in late adolescence (Stice et al. Reference Stice, Marti, Shaw and Jaconis2009; Swanson et al. Reference Swanson, Crow, Le Grange, Swendsen and Merikangas2011; Hilbert et al. Reference Hilbert, Pike, Goldschmidt, Wilfley, Fairburn, Dohm, Walsh and Striegel Weissman2014). Jointly examining risk factors for these ‘binge-type’ EDs may inform theory.
Loss-of-control (LOC) eating (APA, 2013), the most salient feature of binge eating, occurs when individuals perceive an inability to control their eating, regardless of the amount consumed (Wolfe et al. Reference Wolfe, Baker, Smith and Kelly-Weeder2009; Tanofsky-Kraff et al. Reference Tanofsky-Kraff, Shomaker, Olsen, Roza, Wolkoff, Columbo, Raciti, Zocca, Wilfley, Yanovski and Yanovski2011a , Reference Tanofsky-Kraff, Yanovski, Yanovski, Striegel-Moore, Wonderlich, Walsh and Mitchell b ). LOC eating is a childhood precursor to BED (Tanofsky-Kraff et al. Reference Tanofsky-Kraff, Shomaker, Olsen, Roza, Wolkoff, Columbo, Raciti, Zocca, Wilfley, Yanovski and Yanovski2011a , Reference Tanofsky-Kraff, Yanovski, Yanovski, Striegel-Moore, Wonderlich, Walsh and Mitchell b ; Hilbert et al. Reference Hilbert, Hartmann, Czaja and Schoebi2013) and BN (Brewerton et al. Reference Brewerton, Rance, Dansky, O'Neil and Kilpatrick2014). High rates of diagnostic progression (Stice et al. Reference Stice, Marti and Rohde2013) suggest that binge- and LOC eating patterns may be early manifestations of binge-type EDs.
Elucidating developmental risk models for binge-type EDs requires exploration of biological and behavioral constructs that interact to promote BN, BED, and their precursors. We apply the Research Domain Criteria (RDoC) framework (Insel et al. Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn, Sanislow and Wang2010) to binge-type EDs (Tanofsky-Kraff et al. Reference Tanofsky-Kraff, Engel, Yanovski, Pine and Nelson2013), using the RDoC Negative Valence Systems (NVS) domain. Animal models and adult findings are described where pediatric data are absent. A working model is offered and areas for future research are highlighted. Importantly, and in accordance with the RDoC approach (Sanislow et al. Reference Sanislow, Pine, Quinn, Kozak, Garvey, Heinssen, Wang and Cuthbert2010), we refer to LOC eating and binge-type EDs throughout to capture syndromes with similar presentations, as opposed to DSM diagnoses.
RDoC
Heterogeneity in binge-type EDs suggests poor concordance between nosology and pathophysiology (Cuthbert & Insel, Reference Cuthbert and Insel2013), a motivation for the RDoC framework (Cuthbert & Insel, Reference Cuthbert and Insel2013). RDoC focuses on mechanisms that isolate primary behavior components in five fundamental ‘domains’: NVS, positive-valence systems, cognitive processes, social processes, and arousal/modulation (Sanislow et al. Reference Sanislow, Pine, Quinn, Kozak, Garvey, Heinssen, Wang and Cuthbert2010). Domains are divided into basic neuro-behavioral constructs, dysfunction of which are linked to numerous psychopathologies (Sanislow et al. Reference Sanislow, Pine, Quinn, Kozak, Garvey, Heinssen, Wang and Cuthbert2010). Assessment of construct functioning can occur across multiple levels, with assessments targeting constructs varying on a continuum, with mental illnesses representing extremes of these dimensions (Cuthbert, Reference Cuthbert2014). Thus, we focus on LOC eating patterns rather than diagnostic categories. Development is also crucial in RDoC to map trajectories that differentially lead to variations along these continua (Cuthbert, Reference Cuthbert2014).
Dysfunctions within each RDoC domain likely contribute to binge-type ED symptomatology (Tanofsky-Kraff et al. Reference Tanofsky-Kraff, Engel, Yanovski, Pine and Nelson2013), but herein we restrict our focus to the NVS domain, mapping the ways in which NVS parameters relate specifically to LOC eating. Negative affect is prominent in existing theories, which propose that LOC eating represents a maladaptive attempt to escape or alleviate negative emotions in response to psychosocial stressors (Hawkins & Clement, Reference Hawkins, Clement, Hawkins II, Fremouw and Clement1984; Heatherton & Baumeister, Reference Heatherton and Baumeister1991; Fairburn et al. Reference Fairburn, Cooper and Shafran2003; Rieger et al. Reference Rieger, Van Buren, Bishop, Tanofsky-Kraff, Welch and Wilfley2010). Yet, to our knowledge, there is no biologically driven conceptual model relating NVS construct functioning to LOC eating symptoms. Data on co-morbidity between binge-type EDs and depressive or anxiety disorders (Swanson et al. Reference Swanson, Crow, Le Grange, Swendsen and Merikangas2011; Aspen et al. Reference Aspen, Weisman, Vannucci, Nafiz, Gredysa, Kass, Trockel, Jacobi, Wilfley and Taylor2014) underscore the relevance of NVS to LOC eating.
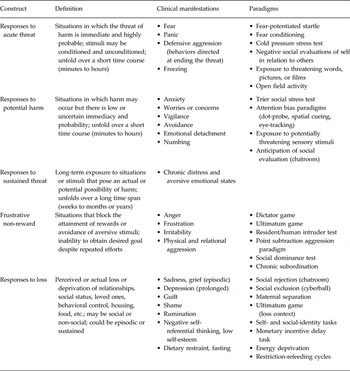
The NVS domain encompasses defensive or avoidance motivational systems, and largely functions to re-establish internal biological stability in response to threats and losses (Sanislow et al. Reference Sanislow, Pine, Quinn, Kozak, Garvey, Heinssen, Wang and Cuthbert2010). NVS functioning is defined by reactions to noxious stimuli and situations, including responses to acute threat, potential harm, sustained threat, frustrative non-reward, and loss (See Table 1 for construct descriptions; RDoC Working Group, 2011). LOC eating may be one phenotypic outcome of numerous dysfunctional NVS responses. We discuss each NVS construct in relation to risk for LOC eating and binge-type EDs.
Table 1. Research Domain Criteria negative valence systems constructs
Genetics
Genetic factors influencing negative emotionality temperaments confer vulnerability for LOC eating (Racine et al. Reference Racine, Keel, Burt, Sisk, Neale, Boker and Klump2013; Koren et al. Reference Koren, Munn-Chernoff, Duncan, Bucholz, Madden, Heath and Agrawal2014), suggesting a potential link to molecular genetic mechanisms that impact NVS functioning. Numerous possible mechanisms may include those affecting the signaling of serotonin, glucocorticoids, catecholamines, neuropeptide-Y, and brain-derived neurotrophic factor (BDNF; McEwen, Reference McEwen2007). Most molecular genetic studies of binge-type EDs emphasize candidate genes. The genetic architecture of BN is unclear based on null findings from one, albeit significantly underpowered, genome-wide association study (Boraska et al. Reference Boraska, Davis, Cherkas, Helder, Harris, Krug, Pei-Chi Liao, Treasure, Ntalla and Karhunen2012). NVS genetic findings in relation to LOC eating are not well replicated, so inferences based upon existing studies should be considered tentative and taken with caution.
Candidate gene association studies
Candidate gene association studies examined many NVS-related gene variants in relation to LOC eating (see Supplementary Table S1). Polymorphisms affecting serotonergic and BDNF signaling generally are not directly linked to the presence of binge-type EDs, but are associated with greater negative affect and binge eating severity in those reporting the behavior (Trace et al. Reference Trace, Baker, Peñas-Lledó and Bulik2013). As such, these genetic predispositions may confer heightened emotional response to NVS stressors that exacerbate LOC eating tendencies, but may not necessarily determine their initial onset. Adults with binge eating, BN, or BED are more likely to carry low-function variants of glucocorticoid receptor (GR) polymorphisms (Cellini et al. Reference Cellini, Castellini, Ricca, Bagnoli, Tedde, Rotella, Faravelli, Sorbi and Nacmias2010; Steiger et al. Reference Steiger, Bruce, Gauvin, Groleau, Joober, Israel, Richardson and Kin2011, Reference Steiger, Gauvin, Joober, Israel, Badawi, Groleau, Bruce, Kin, Sycz and Ouelette2012). Thus, GR gene variants that promote inhibitory feedback insensitivity of the hypothalamic-pituitary-adrenal (HPA) axis may predispose individuals to LOC eating via secondary neurophysiological effects promoting non-homeostatic (e.g. eating due to negative states) feeding.
Gene–environment interplay
Limited data suggest that genetic and environmental factors interact to influence binge-type EDs. Among carriers of the 5-HTTLPR s allele, repeated childhood exposure to maltreatment escalates risk for greater binge eating severity in adolescent girls (Akkermann et al. Reference Akkermann, Kaasik, Kiive, Nordquist, Oreland and Harro2012). Similarly, carriers of at least one C allele of the BclI GR polymorphism with exposure to child maltreatment are at greatest risk for BN (Steiger et al. Reference Steiger, Bruce, Gauvin, Groleau, Joober, Israel, Richardson and Kin2011). Consistent with a diathesis-stress model, acute threat exposure may promote the expression of latent genetic risk-factors for LOC eating via expressed NVS dysfunction in serotonergic and glucorticoid signaling (Trace et al. Reference Trace, Baker, Peñas-Lledó and Bulik2013). There is increasing recognition, however, that genetic and environmental factors perpetually impact each other to modulate risk for LOC eating (Campbell et al. Reference Campbell, Mill, Uher and Schmidt2011; Strober et al. Reference Strober, Peris and Steiger2014). Epigenetic responses to sustained childhood threats are likely mechanisms for the development of binge-type EDs. Indeed, preliminary data implicate altered DNA methylation in specific GR and BDNF promoter regions in BN (Steiger et al. Reference Steiger, Labonté, Groleau, Turecki and Israel2013; Thaler et al. Reference Thaler, Gauvin, Joober, Groleau, de Guzman, Ambalavanan, Israel, Wilson and Steiger2014), which may program early vulnerability to stressors that manifest as persistent anxiety, depression, and LOC eating later in development.
Neural circuits
LOC eating and binge-type EDs may reflect structure and functioning in circuits encompassing the amygdala, insula, and prefrontal cortex (PFC), that mediate NVS responses (Phillips et al. Reference Phillips, Ladouceur and Drevets2008; Dillon et al. Reference Dillon, Rosso, Pechtel, Killgore, Rauch and Pizzagalli2014). Aberrant circuitry responses upon exposure to NVS stressors may override homeostatic feeding circuitry and result in non-homeostatic LOC eating behaviors generating increased motivation to eat and/or degraded executive functioning (Dallman, Reference Dallman2010). While structural MRI studies are useful (see Supplementary Table S2), the current review emphasizes neural circuit functioning tied to specific NVS constructs. Since diverse methodologies make directly comparing findings across studies challenging, findings are hypothesis-generating.
Acute- and potential-threat paradigms
Corticolimbic hyperactivation during acutely threatening social evaluations of one's body may contribute to binge-type EDs. When comparing their own body with other women's thin bodies, women with BN have greater response in the insula and cerebellum than healthy controls (Van den Eynde et al. Reference Van den Eynde, Giampietro, Simmons, Uher, Andrew, Harvey, Campbell and Schmidt2013). Enhanced responsivity could indicate greater NVS engagement during social-evaluative processing, suggesting distorted emotional experiences of acute threat may promote LOC eating. No group differences in corticolimbic activation emerge when women with and without BN evaluate their own bodies relative to line drawings of other women, perhaps because drawings (v. images) are less threatening (Uher et al. Reference Uher, Murphy, Friederich, Dalgleish, Brammer, Giampietro, Phillips, Andrew, Ng, Williams, Campbell and Treasure2005). Yet, in women with BN only, greater amygdala and medial PFC response is associated with higher fear and disgust ratings of women's bodies (Uher et al. Reference Uher, Murphy, Friederich, Dalgleish, Brammer, Giampietro, Phillips, Andrew, Ng, Williams, Campbell and Treasure2005), supporting a role of these structures in aversive body-related emotions that may trigger LOC eating.
Neural mechanisms underlying the known association between social-appearance anxiety, a potential NVS construct, and BN symptoms (Koskina et al. Reference Koskina, Van den Eynde, Meisel, Campbell and Schmidt2011; Levinson et al. Reference Levinson, Rodebaugh, White, Menatti, Weeks, Iacovino and Warren2013) are examined when women imagine that someone is comparing their own bodies to other women (Spangler & Allen, Reference Spangler and Allen2012). Women with BN demonstrate greater activity in the rostral-ventral anterior cingulate cortex than healthy controls upon exposure to obese (v. thin) bodies (Spangler & Allen, Reference Spangler and Allen2012). Women with BN also have greater ventromedial PFC response than controls when selecting between potentially ego-threatening (v. neutral) body image words (Miyake et al. Reference Miyake, Okamoto, Onoda, Kurosaki, Shirao, Okamoto and Yamawaki2010a , Reference Miyake, Okamoto, Onoda, Shirao, Okamoto, Otagaki and Yamawaki b ). Findings suggest that binge-type EDs may be linked to dysregulated NVS engagement during potentially threatening social evaluations of ‘fatness’ and/or disturbed emotion processing with regard to cognitive aspects such as emotion identification, appraisal, and recall (Phillips et al. Reference Phillips, Ladouceur and Drevets2008; Dillon et al. Reference Dillon, Rosso, Pechtel, Killgore, Rauch and Pizzagalli2014).
Importantly, negative self-evaluations in various contexts are tied to perturbed engagement in the amygdala, medial PFC, and dorsolateral PFC among women with BN (Miyake et al. Reference Miyake, Okamoto, Onoda, Kurosaki, Shirao, Okamoto and Yamawaki2010a , Reference Miyake, Okamoto, Onoda, Shirao, Okamoto, Otagaki and Yamawaki b ; Pringle et al. Reference Pringle, Ashworth, Harmer, Norbury and Cooper2011). Comparable perturbations occur in other clinical scenarios relevant to the NVS domain (Rottenberg et al. Reference Rottenberg, Gross and Gotlib2005; Schlund et al. Reference Schlund, Hudgins, Magee and Dymond2013; Dillon et al. Reference Dillon, Rosso, Pechtel, Killgore, Rauch and Pizzagalli2014). LOC eating, therefore, may occur as a dysfunctional means to avoid negative emotions or compensate for emotion blunting. In contrast to patients, increased response in these corticolimbic structures is observed among healthy controls (Miyake et al. Reference Miyake, Okamoto, Onoda, Kurosaki, Shirao, Okamoto and Yamawaki2010a , Reference Miyake, Okamoto, Onoda, Shirao, Okamoto, Otagaki and Yamawaki b ; Pringle et al. Reference Pringle, Ashworth, Harmer, Norbury and Cooper2011), potentially indicating that normative anxiety levels are not well-tolerated and adaptive regulatory mechanisms are deficient in those with binge-type EDs. It is possible that such poor regulatory capacity upon exposure to threats may contribute to impulsive-decision making or a reduced ability to control the type or quantity of food consumed by individuals with LOC eating.
Loss paradigms
Aberrant NVS responses to social defeat are linked to pediatric LOC eating (Jarcho et al. Reference Jarcho, Tanofsky-Kraff, Nelson, Engel, Vannucci, Field, Romer, Hannallah, Brady and Demidowich2015). In overweight girls with LOC eating, reduced engagement is observed in the ventromedial and dorsolateral PFC in response to peer rejection. By contrast, girls without LOC eating increase engagement in the ventromedial PFC, while their dorsolateral PFC response does not differ between rejection and acceptance feedback. In addition, greater activity in the fusiform face area in response to rejecting feedback from desirable peers is associated with more subsequent energy intake at a test meal among girls with LOC eating only. These data suggest that NVS-mediated alterations in perceptual processing, potentially in combination with reduced corticolimbic engagement of regulatory mechanisms, during social-evaluative losses may promote LOC eating patterns.
NVS hypoactivation during the anticipation, but not receipt, of non-social losses may contribute to LOC eating (Balodis et al. Reference Balodis, Kober, Worhunsky, White, Stevens, Pearlson, Sinha, Grilo and Potenza2013). When anticipating monetary loss (v. no outcome), BED is associated with reduced engagement of numerous corticolimbic structures. Failure of individuals with BED to engage corticolimbic circuitry sufficiently in response to potential threat suggests a lack of adaptive anticipatory anxiety (Nitschke et al. Reference Nitschke, Sarinopoulos, Mackiewicz, Schaefer and Davidson2006), which could motivate impulsive, emotion-driven behaviors (Knutson & Greer, Reference Knutson and Greer2008), such as LOC eating. Striatal hypoactivation during the anticipatory phase may also reflect inefficient learning from loss outcomes, placing individuals with BED at higher risk of continuing to enter defeating situations.
Negative self-referential processing is considered a loss construct because it involves a LOC over limiting reflections on negative personal thoughts and emotions (i.e. rumination). When self-evaluating their body shape, women with BN have decreased neural responses in the default mode network structures, whereas healthy controls exhibit increased responses (Uher et al. Reference Uher, Murphy, Friederich, Dalgleish, Brammer, Giampietro, Phillips, Andrew, Ng, Williams, Campbell and Treasure2005; Miyake et al. Reference Miyake, Okamoto, Onoda, Kurosaki, Shirao, Okamoto and Yamawaki2010a , Reference Miyake, Okamoto, Onoda, Shirao, Okamoto, Otagaki and Yamawaki b ; Vocks et al. Reference Vocks, Busch, Grönemeyer, Schulte, Herpertz and Suchan2010; Van den Eynde et al. Reference Van den Eynde, Giampietro, Simmons, Uher, Andrew, Harvey, Campbell and Schmidt2013). This could reflect aberrant attention shifts (Whitfield-Gabrieli & Ford, Reference Whitfield-Gabrieli and Ford2012). LOC eating may, therefore, be precipitated as an attempt to reestablish an emotional and neurophysiological balance. Findings may also reflect negative ruminative and self-evaluative tendencies (Whitfield-Gabrieli & Ford, Reference Whitfield-Gabrieli and Ford2012) that could lead to LOC eating as a means to escape self-awareness.
Physiology
NVS neuroendocrine systems include the sympathetic-adrenomedullary (SAM) axis and HPA axis. Both the SAM and HPA axes are crucial to the regulation of NVS stressor responses and energy intake. As such, NVS physiological dysfunction may at least partly underlie the known relationship among stress, negative affect, and LOC eating patterns. Again, there is a paucity of pediatric data and so developmental implications are extremely tentative.
SAM axis
The SAM axis is activated, triggering the release of epinephrine and some norepinephrine from the adrenal medulla, when stressors are perceived as imminently threatening or demanding, yet controllable, in non-clinical samples (Adam & Epel, Reference Adam and Epel2007). Most adult studies of binge-type EDs demonstrate blunted SAM axis activity at rest (Pirke et al. Reference Pirke, Platte, Laessle, Seidl and Fichter1992; Koo-Loeb et al. Reference Koo-Loeb, Costello, Light and Girdler2000) and during acute psychosocial stress (Pirke et al. Reference Pirke, Platte, Laessle, Seidl and Fichter1992; Koo-Loeb et al. Reference Koo-Loeb, Pedersen and Girdler1998; Messerli-Bürgy et al. Reference Messerli-Bürgy, Engesser, Lemmenmeier, Steptoe and Laederach-Hofmann2010; Monteleone et al. Reference Monteleone, Scognamiglio, Canestrelli, Serino, Monteleone and Maj2011; Het et al. Reference Het, Vocks, Wolf, Hammelstein, Herpertz and Wolf2015). SAM axis hypoactivity reflects a reduced physiological capacity to respond adaptively to acute threats and predictable NVS stressors, as well as a biased perception that seemingly innocuous stressors are uncontrollable (Charmandari et al. Reference Charmandari, Tsigos and Chrousos2005). Exaggerated/maladaptive emotional responses to such stressors may drive individuals toward LOC eating as an alternative means of coping. Yet, relationships between SAM axis hypoactivity and LOC eating persist when adjusting for mood variables (Pirke, Reference Pirke1996; Koo-Loeb et al. Reference Koo-Loeb, Pedersen and Girdler1998), suggesting an independent role in binge-type EDs.
In healthy populations, threat-induced SAM axis activation is accompanied by simultaneous reductions in parasympathetic tone (Charmandari et al. Reference Charmandari, Tsigos and Chrousos2005). Following stressor cessation, shifts back toward parasympathetic control are important for adaptive recovery (Charmandari et al. Reference Charmandari, Tsigos and Chrousos2005). BN is distinguished from BED and controls by parasympathetic dominance at rest and during acute stressors (Messerli-Bürgy et al. Reference Messerli-Bürgy, Engesser, Lemmenmeier, Steptoe and Laederach-Hofmann2010; Hilbert et al. Reference Hilbert, Vögele, Tuschen-Caffier and Hartmann2011; Het et al. Reference Het, Vocks, Wolf, Hammelstein, Herpertz and Wolf2015). The more persistent, severe dietary restriction reported by individuals with BN may partly account for this difference, as chronic deprivation contributes to vagal insensitivity (Vögele et al. Reference Vögele, Hilbert and Tuschen-Caffier2009). Following psychosocial stressors, individuals with binge-type EDs exhibit persistent sympathetic activation coupled with little-to-no parasympathetic increase compared with controls (Messerli-Bürgy et al. Reference Messerli-Bürgy, Engesser, Lemmenmeier, Steptoe and Laederach-Hofmann2010; Hilbert et al. Reference Hilbert, Vögele, Tuschen-Caffier and Hartmann2011). This poor physiological NVS capacity to recover from psychosocial stressors may lead individuals toward LOC eating to regulate prolonged negative affective states and physiological disruption.
HPA axis
The HPA axis is preferentially activated in response to stressors perceived as uncontrollable, potentially threatening, defeating, or difficult to manage (Adam & Epel, Reference Adam and Epel2007). In non-clinical samples, exposure to such stressors stimulates glucocorticoid release, primarily cortisol, from the adrenal cortex that triggers metabolic effects to support adaptive responses and provides negative feedback signals to terminate HPA-axis activity (Charmandari et al. Reference Charmandari, Tsigos and Chrousos2005). Sustained elevated cortisol, particularly in palatable food environments, promotes non-homeostatic consumption, thereby linking the NVS to LOC eating (Adam & Epel, Reference Adam and Epel2007).
Individuals with BN exhibit HPA axis hyper-reactivity and inhibitory feedback insensitivity (Copeland et al. Reference Copeland, Herzog, Carr, Klibansk, MacLaughlin and Martin1988; Kaye et al. Reference Kaye, Gwirtsman and George1989; Neudeck et al. Reference Neudeck, Jacoby and Florin2001; Bruce et al. Reference Bruce, Steiger, Israël, Groleau, Ng Ying Kin, Ouellette, Sycz and Badawi2012). An extreme weight loss history amplifies this dysregulation, which may in turn exacerbate LOC eating (Lo Sauro et al. Reference Lo Sauro, Ravaldi, Cabras, Faravelli and Ricca2008). By contrast, obese adults with BED generally display normal HPA axis function at rest, though several studies suggest hyper- or hypo-reactivity (Yanovski et al. Reference Yanovski, Yanovski, Gwirtsman, Bernat, Gold and Chrousos1993; Monteleone et al. Reference Monteleone, Luisi, De Filippis, Colurcio, Monteleone, Genazzani and Maj2003; Gluck et al. Reference Gluck, Geliebter and Lorence2004a , Reference Gluck, Geliebter, Hung and Yahav b ; Rosenberg et al. Reference Rosenberg, Bloch, Ben Avi, Rouach, Schreiber, Stern and Greenman2013). Equivocal findings may stem from co-morbid obesity, which itself is linked to HPA axis dysregulation (Nieuwenhuizen & Rutters, Reference Nieuwenhuizen and Rutters2008) and altered cortisol metabolism (Morton & Seckl, Reference Morton and Seckl2008).
During stressors, individuals with binge-type EDs may exhibit steeper cortisol increases than controls, which has been associated with increased desire to binge (Koo-Loeb et al. Reference Koo-Loeb, Pedersen and Girdler1998, Reference Koo-Loeb, Costello, Light and Girdler2000; Gluck et al. Reference Gluck, Geliebter and Lorence2004a , Reference Gluck, Geliebter, Hung and Yahav b ). Yet, other studies find blunted cortisol response (Pirke et al. Reference Pirke, Platte, Laessle, Seidl and Fichter1992; Ginty et al. Reference Ginty, Phillips, Higgs, Heaney and Carroll2012; Rosenberg et al. Reference Rosenberg, Bloch, Ben Avi, Rouach, Schreiber, Stern and Greenman2013) or no group differences (Tuschen-Caffier & Vogele, Reference Tuschen-Caffier and Vogele1999; Monteleone et al. Reference Monteleone, Scognamiglio, Canestrelli, Serino, Monteleone and Maj2011; Schulz et al. Reference Schulz, Laessle and Hellhammer2011; Gluck et al. Reference Gluck, Yahav, Hashim and Geliebter2014). Blunted cortisol response likely reflects a compensatory downregulation of the HPA axis due to repeated activation from sustained stressors, persistent depression, and/or LOC eating (Rosenberg et al. Reference Rosenberg, Bloch, Ben Avi, Rouach, Schreiber, Stern and Greenman2013). Such downregulation results in prolonged HPA axis responses and impaired recovery from stressors, conferring higher sensitivity to stressors that may lead to exacerbated LOC eating (Rosenberg et al. Reference Rosenberg, Bloch, Ben Avi, Rouach, Schreiber, Stern and Greenman2013).
In light of heterogeneous findings, the nature of HPA axis dysfunction may reflect multicausality, as binge-type EDs may arise from impaired functioning in disparate NVS constructs. Greater HPA axis dysfunction, regardless of the nature or cause, is associated with greater binge eating severity, anxiety, depression, and rumination among individuals with binge-type EDs (Díaz-Marsá et al. Reference Díaz-Marsá, Carrasco, Basurte, Sáiz, López-Ibor and Hollander2008; Lo Sauro et al. Reference Lo Sauro, Ravaldi, Cabras, Faravelli and Ricca2008; Bruce et al. Reference Bruce, Steiger, Israël, Groleau, Ng Ying Kin, Ouellette, Sycz and Badawi2012; Rosenberg et al. Reference Rosenberg, Bloch, Ben Avi, Rouach, Schreiber, Stern and Greenman2013; Monteleone et al. Reference Monteleone, Scognamiglio, Monteleone, Perillo and Maj2014). Thus, aberrant HPA axis responses to potential harm and losses may be linked to exacerbated negative affective states that trigger recurrent LOC eating.
Behavioral paradigms
Performance in behavioral paradigms capturing NVS constructs suggests that LOC eating patterns are linked to cognitive disturbances in response to threats and social losses. Adolescents with LOC eating have greater inhibitory control degradations during peer exclusion than youth with ADHD and healthy controls (Hartmann et al. Reference Hartmann, Rief and Hilbert2013), indicating that youth with LOC eating experience greater cognitive control difficulties in response to social losses. Adolescents and adults with binge eating or BN exhibit strong interference effects on Emotional Stroop paradigms utilizing stimuli indicating threats to one's weight/shape, social status, self-worth, or autonomy (McManus et al. Reference McManus, Waller and Chadwick1996; Waller et al. Reference Waller, Watkins, Shuck and McManus1996; Kuhnpast et al. Reference Kuhnpast, Gramann and Pollatos2012; Aspen et al. Reference Aspen, Darcy and Lock2013; Cardi et al. Reference Cardi, Di Matteo, Corfield and Treasure2013; Kanakam et al. Reference Kanakam, Krug, Raoult, Collier and Treasure2013). Interference effects may indicate heightened attention orienting toward salient cues, reflecting hypervigilance and anxiety, or poorer cognitive control upon threat exposure, manifesting as intensified negative affect, impulsivity, or rumination. Greater interference effects are further associated with more severe LOC eating (McManus et al. Reference McManus, Waller and Chadwick1996; Lokken et al. Reference Lokken, Marx and Ferraro2006), suggesting that greater executive dysfunction upon exposure to potentially threatening cues may contribute to recurrent LOC eating patterns. Women with LOC eating also exhibit greater cognitive avoidance of ego-threatening stimuli than controls (Meyer et al. Reference Meyer, Serpell, Waller, Murphy, Treasure and Leung2005; Blechert et al. Reference Blechert, Nickert, Caffier and Tuschen-Caffier2009, Reference Blechert, Ansorge and Tuschen-Caffier2010). Overall, these stressor-induced cognitive dysfunctions indicate that LOC eating may represent a maladaptive coping strategy for exacerbated negative affect and negative self-referential processing, and/or an impulsive behavioral response occurring when cognitive resources are degraded.
Emerging data suggest that threat and loss exposure enhances the salience of palatable foods (Adam & Epel, Reference Adam and Epel2007; Dallman, Reference Dallman2010), which may partly explain why individuals with LOC eating turn towards food to cope with NVS stressors. Among adults with LOC eating, those with high social-threat sensitivity are more willing to work to obtain palatable foods (v. non-food alternatives) than adults with low social-threat sensitivity or those without LOC eating (Goldfield et al. Reference Goldfield, Adamo, Rutherford and Legg2008). Among women with BN, higher anxiety, dietary restraint, and weight/shape-related ruminations are associated with greater attention orienting biases toward food stimuli (Brooks et al. Reference Brooks, Owen, Uher, Friederich, Giampietro, Brammer, Williams, Schiöth, Treasure and Campbell2011). These data suggest that NVS perturbations contribute to a pathological motivation to eat among individuals with LOC eating. Women with BN and high negative affect demonstrate greater interference effects for palatable food cues (Rofey et al. Reference Rofey, Corcoran and Tran2004), indicating that NVS disturbances may impair cognitive control over palatable foods in those prone to LOC eating.
Laboratory test-meals
Laboratory test-meal studies directly link psychological indicators of NVS construct dysfunction to LOC eating behaviors, thereby precisely mapping the ways in which the RDoC NVS construct links to a particular behavior relevant to binge-type EDs. Although exposure to sad (v. neutral) mood inductions do not differentially impact overall intake in children with LOC eating relative to those without LOC eating (Hilbert et al. Reference Hilbert, Tuschen-Caffer and Czaja2010; Goldschmidt et al. Reference Goldschmidt, Tanofsky-Kraff and Wilfley2011), overweight girls with LOC eating consume more energy from fat following a sad film (Goldschmidt et al. Reference Goldschmidt, Tanofsky-Kraff and Wilfley2011). Additionally, greater pre-meal negative affect predicts more energy consumed from snack-type foods (Ranzenhofer et al. Reference Ranzenhofer, Hannallah, Field, Shomaker, Stephens, Sbrocco, Kozlosky, Reynolds, Yanovski and Tanofsky-Kraff2013) and an increased likelihood of experiencing LOC during a meal (Goldschmidt et al. Reference Goldschmidt, Tanofsky-Kraff and Wilfley2011). These pediatric findings are consistent with adult studies that also link negative affect to palatable food consumption and the experience of LOC (Telch & Agras, Reference Telch and Agras1996; Levine & Marcus, Reference Levine and Marcus1997; Agras & Telch, Reference Agras and Telch1998; Chua et al. Reference Chua, Touyz and Hill2004). Youth with LOC eating report greater increases in anxiety, confusion, and fatigue, but similar anger, depression, and tension changes, compared to controls (Tanofsky-Kraff et al. Reference Tanofsky-Kraff, McDuffie, Yanovski, Kozlosky, Schvey, Shomaker, Salaita and Yanovski2009). Since confusion and fatigue may align with ‘numbing’ experiences reported by youth during LOC episodes (Tanofsky-Kraff et al. Reference Tanofsky-Kraff, Goossens, Eddy, Ringham, Goldschmidt, Yanovski, Braet, Marcus, Wilfley, Olsen and Yanovski2007), it is possible that youth with LOC eating could dissociate while eating to avoid simultaneous anxiety increases. Other laboratory studies find that youth with LOC eating and adults with binge-type EDs report negative affect reductions after eating (Munsch et al. Reference Munsch, Michael, Biedert, Meyer and Margraf2008; Hartmann et al. Reference Hartmann, Rief and Hilbert2012; Vannucci et al. Reference Vannucci, Tanofsky-Kraff, Crosby, Ranzenhofer, Shomaker, Field, Mooreville, Reina, Kozlosky and Yanovski2012; Ranzenhofer et al. Reference Ranzenhofer, Hannallah, Field, Shomaker, Stephens, Sbrocco, Kozlosky, Reynolds, Yanovski and Tanofsky-Kraff2013). Overall, laboratory findings suggest a regulatory role of LOC eating in alleviating negative affect.
Dietary restraint appears to have little impact on laboratory LOC eating in humans. Neither acute energy deprivation in adults with binge-type EDs (Telch & Agras, Reference Telch and Agras1996; Agras & Telch, Reference Agras and Telch1998; Hetherington et al. Reference Hetherington, Stoner, Andersen and Rolls2000; Moreno-Domínguez et al. Reference Moreno-Domínguez, Rodríguez-Ruiz, Fernández-Santaella, Ortega-Roldán and Cepeda-Benito2012) nor chronic dietary restraint in adults with binge eating (Chua et al. Reference Chua, Touyz and Hill2004) differentially impact energy intake or the occurrence of self-defined binges compared with controls. Dietary restriction also does not interact with negative mood inductions to influence energy intake (Agras & Telch, Reference Agras and Telch1998; Chua et al. Reference Chua, Touyz and Hill2004). These data contrast with findings in animal models (Hagan et al. Reference Hagan, Wauford, Chandler, Jarrett, Rybak and Blackburn2002; Boggiano & Chandler, Reference Boggiano and Chandler2006), potentially resulting from greater experimental control that ensures sufficiently prolonged, severe, and stressful energy deprivation. However, it is also likely that LOC eating in humans emerges from numerous factors not present in rodents.
Although there is no prospective laboratory study in humans, animal models indicate that sustained early social-threats and losses lead to the development of LOC eating. Prolonged maternal separation and low maternal care during the neonatal period leads to increased palatable food intake during the pre-adult period when mice are exposed to novel acute stressors, such as social isolation from peers and fasting-refeeding cycles (Jahng, Reference Jahng2011; Maniam & Morris, Reference Maniam and Morris2012). Chronic subordination and social defeat also induce LOC-like eating (Sanghez et al. Reference Sanghez, Razzoli, Carobbio, Campbell, McCallum, Cero, Ceresini, Cabassi, Govoni and Franceschini2013; Razzoli et al. Reference Razzoli, Sanghez and Bartolomucci2015). Increases in anxiety- and depressive-like behaviors tend to accompany LOC-like eating (Jahng, Reference Jahng2011). Behavioral effects of neonatal stressors were not observed during other developmental periods (Maniam & Morris, Reference Maniam and Morris2012), implicating the pre-adult period as a sensitive time for activating social stressor-induced LOC eating. Based on these preliminary data, it is conceivable that chronic childhood social stressors precipitate NVS dysfunction and manifests as LOC eating in response to novel NVS stressors much later in development.
Psychological self-reports
Ecological momentary assessment (EMA) involves real-time assessment in the natural environment of an individual's emotional state and associated behaviors. Such studies generally indicate that NVS disturbances proximally promote LOC eating. Adult EMA studies consistently find links among negative affect, stress, and binge eating (Haedt-Matt & Keel, Reference Haedt-Matt and Keel2011; Goldschmidt et al. Reference Goldschmidt, Engel, Wonderlich, Crosby, Peterson, Grange, Tanofsky-Kraff, Cao and Mitchell2012a , Reference Goldschmidt, Wall, Loth, Le Grange and Neumark-Sztainer b , Reference Goldschmidt, Crosby, Cao, Engel, Durkin, Beach, Berg, Wonderlich, Crow and Peterson2014a –Reference Goldschmidt, Wonderlich, Crosby, Engel, Lavender, Peterson, Crow, Cao and Mitchell c ; Berg et al. Reference Berg, Peterson, Crosby, Cao, Crow, Engel and Wonderlich2014). Greater daily variability in negative affect, a proxy of stressor sensitivity, also predicts higher binge eating frequency in adults (Engel et al. Reference Engel, Boseck, Crosby, Wonderlich, Mitchell, Smyth, Miltenberger and Steiger2007; Zander & Young, Reference Zander and Young2014). However, pediatric EMA studies do not find such associations (Hilbert et al. Reference Hilbert, Rief, Tuschen-Caffier, de Zwaan and Czaja2009; Ranzenhofer et al. Reference Ranzenhofer, Engel, Crosby, Anderson, Vannucci, Cohen, Cassidy and Tanofsky-Kraff2014), possibly due to methodological or developmental differences. Independent of negative affect, increases in dissociative/numbing experiences are found prior to binge episodes (Engelberg et al. Reference Engelberg, Steiger, Gauvin and Wonderlich2007; McShane & Zirkel. Reference McShane and Zirkel2008).
Specific NVS constructs are salient LOC eating triggers. Greater body-related cognitions precede LOC episodes in children (Hilbert et al. Reference Hilbert, Rief, Tuschen-Caffier, de Zwaan and Czaja2009), but do not predict binge-episodes in adults with binge-type EDs (Hilbert & Tuschen-Caffier, Reference Hilbert and Tuschen-Caffier2007). This developmental difference highlights the potential importance of weight/shape-related ruminations in contributing to pediatric LOC eating, perhaps prior to self-reported negative affect predominating as an LOC eating trigger in adulthood or full-syndrome binge-type EDs. Interpersonal stressors, primarily involving conflicts or defeat, predict the onset of LOC episodes in adolescents (Ranzenhofer et al. Reference Ranzenhofer, Engel, Crosby, Anderson, Vannucci, Cohen, Cassidy and Tanofsky-Kraff2014) and adults (Steiger et al. Reference Steiger, Gauvin, Jabalpurwala, Séguin and Stotland1999; Freeman & Gil, Reference Freeman and Gil2004; Goldschmidt et al. Reference Goldschmidt, Crosby, Cao, Engel, Durkin, Beach, Berg, Wonderlich, Crow and Peterson2014a –Reference Goldschmidt, Wonderlich, Crosby, Engel, Lavender, Peterson, Crow, Cao and Mitchell c ). Moreover, increases in negative affect and self-referential criticisms mediate this relationship in adults (Steiger et al. Reference Steiger, Gauvin, Jabalpurwala, Séguin and Stotland1999; Goldschmidt et al. Reference Goldschmidt, Crosby, Cao, Engel, Durkin, Beach, Berg, Wonderlich, Crow and Peterson2014a –Reference Goldschmidt, Wonderlich, Crosby, Engel, Lavender, Peterson, Crow, Cao and Mitchell c ), indicating that aberrant responses to NVS social-threats and losses contribute to LOC eating. NVS dysfunction in acute threat, frustrative non-reward, and loss constructs are linked to LOC eating, as state ratings of fear, anger/hostility, sadness, and guilt increase prior to, and decrease following, LOC episodes in women with BN (Smyth et al. Reference Smyth, Wonderlich, Heron, Sliwinski, Crosby, Mitchell and Engel2007; Berg et al. Reference Berg, Crosby, Cao, Peterson, Engel, Mitchell and Wonderlich2013). Guilt remains a robust predictor of LOC eating even after adjusting for other facets of negative affect (Berg et al. Reference Berg, Crosby, Cao, Peterson, Engel, Mitchell and Wonderlich2013), implicating an important role for loss response disturbances. The role of dietary restraint in predicting binge eating is unclear due to mixed findings, likely resulting from divergent assessment methods and complex interactions with restraint chronicity, stress, and coping strategies (Engelberg et al. Reference Engelberg, Gauvin and Steiger2005; Zunker et al. Reference Zunker, Peterson, Crosby, Cao, Engel, Mitchell and Wonderlich2011; Holmes et al. Reference Holmes, Fuller-Tyszkiewicz, Skouteris and Broadbent2014). Together, these findings suggest that aberrant NVS responses to social threats and loss, with potentially the exception of dietary restraint, may be particularly salient to momentary causes of LOC eating.
Importance of development
The role of NVS in LOC eating risk may developmentally vary. Childhood anxiety disorders, especially social anxiety disorder, may predict later-life binge-type EDs (Bulik et al. Reference Bulik, Wade and Kendler2001; Kaye et al. Reference Kaye, Bulik, Thornton, Barbarich and Masters2004), but findings from prospective data are inconsistent (Tanofsky-Kraff et al. Reference Tanofsky-Kraff, Shomaker, Olsen, Roza, Wolkoff, Columbo, Raciti, Zocca, Wilfley, Yanovski and Yanovski2011a , Reference Tanofsky-Kraff, Yanovski, Yanovski, Striegel-Moore, Wonderlich, Walsh and Mitchell b ; Hilbert et al. Reference Hilbert, Hartmann, Czaja and Schoebi2013; Hilbert & Brauhardt, Reference Hilbert and Brauhardt2015). Body dissatisfaction, elevated depressive symptoms, low self-esteem, and ruminative tendencies during early adolescence more consistently predict the onset and persistence of LOC eating in late adolescence and young adulthood (Stice et al. Reference Stice, Presnell and Spangler2002; Nolen-Hoeksema et al. Reference Nolen-Hoeksema, Stice, Wade and Bohon2007; Goldschmidt et al. Reference Goldschmidt, Crosby, Cao, Engel, Durkin, Beach, Berg, Wonderlich, Crow and Peterson2014a –Reference Goldschmidt, Wonderlich, Crosby, Engel, Lavender, Peterson, Crow, Cao and Mitchell c ). While adolescent dieting and extreme weight control behaviors also predict LOC eating patterns (Haines & Neumark-Sztainer, Reference Haines and Neumark-Sztainer2006; Keel & Forney, Reference Keel and Forney2013), negative affect may be more salient when both constructs are considered together (Goldschmidt et al. Reference Goldschmidt, Engel, Wonderlich, Crosby, Peterson, Grange, Tanofsky-Kraff, Cao and Mitchell2012a , Reference Goldschmidt, Wall, Loth, Le Grange and Neumark-Sztainer b ). Overall, these data (see Supplementary Table S3) suggest that the NVS alone is insufficient to characterize LOC eating, and identify adolescence as a potential sensitive period for the increasing influence of NVS disturbances on LOC eating.
Severe and chronic stressors may disrupt typical developmental processes, causing increased risk for the early onset of LOC eating and emergence of binge-type EDs in adolescence and young adulthood. Reports of childhood abuse and other traumatic experiences predict risk for binge-type EDs in adulthood (Striegel-Moore et al. Reference Striegel-Moore, Fairburn, Wilfley, Pike, Dohm and Kraemer2005; Degortes et al. Reference Degortes, Santonastaso, Zanetti, Tenconi, Veronese and Favaro2014; Hilbert et al. Reference Hilbert, Pike, Goldschmidt, Wilfley, Fairburn, Dohm, Walsh and Striegel Weissman2014). Parental over-focus on eating, weight, and shape, and weight-related teasing by family members and peers represent specific risk factors for LOC eating in childhood and binge-type EDs in adolescence and young adulthood (Haines & Neumark-Sztainer, Reference Haines and Neumark-Sztainer2006; Keel & Forney, Reference Keel and Forney2013). Furthermore, perceived social pressures to be thin or lose weight and internalization of the sociocultural ‘thin ideal’ body size, which likely lead to ego-threatening social comparisons with others’ bodies, predict the emergence of LOC eating and binge-type EDs in adolescents (Stice et al. Reference Stice, Presnell and Spangler2002; Field et al. Reference Field, Sonneville, Micali, Crosby, Swanson, Laird, Treasure, Solmi and Horton2012; Sonneville et al. Reference Sonneville, Calzo, Horton, Haines, Austin and Field2012). Childhood exposure to sociocultural stressors, especially those involving acute threat and body-related social-evaluative threats, may give rise to altered gene expression and NVS neurophysiological disturbances that contribute to LOC eating in vulnerable individuals.
The age of LOC eating onset influences development. Individuals reporting LOC eating onset during childhood, relative to adulthood, are more likely to endorse severe binge eating, use of multiple purging behaviors, and a BN diagnosis (Brewerton et al. Reference Brewerton, Rance, Dansky, O'Neil and Kilpatrick2014). Similarly, early relative to later-onset binge-type EDs manifest greater binge eating and purging frequencies, weight/shape concerns, and mood disturbances (Marcus et al. Reference Marcus, Moulton and Greeno1995; Sullivan et al. Reference Sullivan, Bulik, Carter and Joyce1996). Taken together, this suggests that early onset increases risk for more severe and persistent pathology. Other data link childhood onset of LOC eating to familial emotional problems and trauma (Marcus et al. Reference Marcus, Moulton and Greeno1995; Brewerton et al. Reference Brewerton, Rance, Dansky, O'Neil and Kilpatrick2014), further demonstrating the ways in which NVS construct disturbances relate to binge-type EDs.
Towards a NVS model of binge-type eating disorders
NVS disturbances appear to increase risk for LOC eating and the development of binge-type EDs; Fig. 1 illustrates a framework considered preliminary due to the early stage of developmental research in this area.

Fig. 1. Working developmental negative valence systems (NVS) model of binge-type eating disorders. LOC, Loss of control; BED, binge-eating disorder; BN, bulimia nervosa. a NVS stressors encompass all Research Domain Criteria NVS constructs, including situations involving acute threat, potential harm, frustrative non-reward, and loss. See Table 1 for description and examples of these stressors. b Negative affect and dissociation represent two of the most common psychopathological responses to NVS stressors linked to LOC eating. See Table 1 for additional examples of clinical manifestations of NVS responses.
Genetic factors are hypothesized to influence expression of negative emotionality traits and emotional-eating tendencies that emerge early in life, are stable throughout development, and predispose youth to LOC eating. Indeed, genetic effects account for approximately 38–77% of the associations between LOC eating and temperaments characterized by persistent negative mood and negative urgency, which refers to the tendency to act impulsively in response to negative affective states (Racine et al. Reference Racine, Keel, Burt, Sisk, Neale, Boker and Klump2013; Koren et al. Reference Koren, Munn-Chernoff, Duncan, Bucholz, Madden, Heath and Agrawal2014). Additionally, emotional eating is considered as a heritable appetitive trait (Carnell et al. Reference Carnell, Benson, Pryor and Driggin2013) that contributes to LOC eating onset in children (Allen et al. Reference Allen, Byrne, La Puma, McLean and Davis2008) and adolescents (Stice et al. Reference Stice, Presnell and Spangler2002). Evidence suggests that genetic predispositions toward altered signaling of serotonin, catecholamines, glucocorticoids, and/or BDNF, which impact corticolimbic circuitry and neuroendocrine systems mediating NVS responses, may play an important role in the causal relationship between NVS trait disturbances and the early development of LOC eating. These genetic vulnerabilities may also predispose youth to experience stressors linked to LOC eating more frequently, either through selecting environments or eliciting reactions from others that are consistent with NVS traits (Trace et al. Reference Trace, Baker, Peñas-Lledó and Bulik2013). Since not all children with LOC eating develop binge-type EDs (Tanofsky-Kraff et al. Reference Tanofsky-Kraff, Shomaker, Olsen, Roza, Wolkoff, Columbo, Raciti, Zocca, Wilfley, Yanovski and Yanovski2011a , Reference Tanofsky-Kraff, Yanovski, Yanovski, Striegel-Moore, Wonderlich, Walsh and Mitchell b ; Hilbert et al. Reference Hilbert, Hartmann, Czaja and Schoebi2013), youth with genetic vulnerability to aberrant NVS responses and environmental stressors present early in development are likely at highest risk for the emergence of such EDs.
Childhood stress may shape development of corticolimbic circuitry and neuroendocrine stress response systems that influence LOC eating behaviors. Findings from animal and human studies reveal that sexual and physical abuse, maternal separation, and social isolation from peers predict significant structural and functional remodeling of corticolimbic neurons (Lupien et al. Reference Lupien, McEwen, Gunnar and Heim2009; Eiland & Romeo, Reference Eiland and Romeo2013), altered functional connectivity between corticolimbic regions (Burghy et al. Reference Burghy, Stodola, Ruttle, Molloy, Armstrong, Oler, Fox, Hayes, Kalin and Essex2012), SAM and HPA axis hyperreactivity to stressors, and diminished inhibitory effects of cortisol on the HPA axis (Sinha & Jastreboff, Reference Sinha and Jastreboff2013; Sominsky & Spencer, Reference Sominsky and Spencer2014). These disturbances are linked to abnormal NVS responses often observed among individuals with binge-type EDs and psychological risk factors for LOC eating. Such stressor-induced biological alterations may serve as mechanisms through which sustained childhood stressors increase risk for binge-type EDs (Steiger et al. Reference Steiger, Gauvin, Israël, Koerner, Kin, Paris and Young2001; Díaz-Marsá et al. Reference Díaz-Marsá, Carrasco, Basurte, Pastrana, Sáiz-Ruiz and López-lbor2007). Childhood appears to represent a sensitive period for the impact of chronic stressors, as more persistent and atypical neurodevelopmental alterations in corticolimbic circuitry emerge when sustained threats and losses occur at an earlier age and for a longer duration (Lupien et al. Reference Lupien, McEwen, Gunnar and Heim2009).
The effects of early sustained threats and losses on corticolimbic and neuroendocrine dysregulation often are not apparent until adolescence and are more robust in females (Shin & Liberzon, Reference Shin and Liberzon2010; Burghy et al. Reference Burghy, Stodola, Ruttle, Molloy, Armstrong, Oler, Fox, Hayes, Kalin and Essex2012), which may partly account for the increased risk for binge-type EDs in adolescent females. Puberty may be a necessary driving force for these relationships. Indeed, genetic effects on disordered eating symptoms, including LOC eating, do not emerge until post-puberty in females (Klump et al. Reference Klump, Culbert, Slane, Burt, Sisk and Nigg2012) and with higher levels of estradiol (Klump et al. Reference Klump, Keel, Sisk and Burt2010). Estradiol is proposed to mediate the relationship between genetic risk and binge-type EDs since it is involved in gene expression and provides signals for the profound neural development and reorganization that occurs during adolescence (Klump, Reference Klump2013). Estradiol promotes the expression of genes linked to NVS functioning (Klump, Reference Klump2013). Therefore, it is possible that epigenetic effects stemming from childhood stressors do not influence NVS perturbations linked to LOC eating until adolescence, particularly in girls. Similar to children at high risk for anxiety and depression (Casey et al. Reference Casey, Jones and Hare2008), children with LOC eating may have a pre-existing imbalance between subcortical limbic and prefrontal cortical structures or dysfunctional neuroendocrine stress systems that is amplified during adolescence. Vulnerable youth, such as those entering puberty with LOC eating, a history of sustained childhood stressors, and/or NVS gene variants, may be at particularly high risk for disproportionate increases in negative mood intensity, affect lability, emotion dysregulation, exacerbated LOC eating patterns, and the early emergence and maintenance of binge-type EDs.
Future directions
Numerous areas of exploration are needed to further elucidate our proposed developmental NVS model of binge-type EDs. The field would benefit from an increased focus on individual differences and traits that are known to promote risk for binge-type EDs. Since LOC eating appears to be an early behavioral marker of worsening eating disturbance, NVS construct dysfunctions that predict LOC eating also require examination. Longitudinal studies of brain development and interactions with genetic, physiological, and behavioral levels of analysis will be crucial to distinguish cause from consequence. Since not all youth with LOC eating develop binge-type EDs, prospective studies will also identify trajectories associated with LOC eating cessation and may inform novel prevention targets. Additionally, developmental trajectories that differentiate individuals who develop BN v. BED would also be beneficial for improved outcome prediction. RDoC may provide an exciting opportunity to conduct such systematic studies by examining constructs within the NVS-domain.
Consistent with the RDoC approach, we believe that binge-type EDs represent a behavioral outcome of NVS dysfunction as well as disturbed functioning in other RDoC domains. RDoC stresses the importance of heterogeneous contributions to similar phenotypes. Binge-type EDs, which are diagnoses derived from symptom-based categories, are likely comprised of individuals with diverse neurophysiological dysfunction. However, dysregulation in the NVS-domain clearly plays an important role in many binge-type ED vulnerabilities and should be a focus of future work. Incorporating interactions with other RDoC domains into developmental NVS models for binge-type EDs will also be important. RDoC's ambitious approach provides a framework that may prove crucial for identifying, and ultimately intervening with, those at greatest risk for developing binge-type EDs.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S003329171500104X.
Acknowledgements
Intramural Research Program, NIH, grant 1ZIAHD000641 from the NICHD with supplemental funding from NIMHD; Intramural Research Program, NIMH.
The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of USUHS, the U.S. Public Health Service, or the U.S. Department of Defense.




