Introduction
The Taita Hills are part of the Eastern Arc Mountains that range from south-eastern Kenya to eastern Tanzania. The montane cloud forests of this area are known for their high degree of biodiversity and endemism, and they are recognized as one of the biodiversity hotspots of the world (Rogo & Oguge Reference Rogo and Oguge2000; Burgess et al. Reference Burgess, Butynski, Cordeiro, Doggart, Fjeldså, Howell, Kilahama, Loader, Lovett and Mbilinyi2006; Lange Reference Lange2006; Malonza et al. Reference Malonza, Lötters and Measey2010). This rich and unique ecosystem is an outcome of long isolation as well as favourable climatic conditions. The mountains rise abruptly from the surrounding plain and native vegetation effectively captures precipitation from clouds and mist developed by the relatively cool air rising from the Indian Ocean.
The forests in the Taita Hills are influenced considerably by human action and have become highly fragmented. The remaining indigenous forests are mainly found on the hilltops and continue to shrink year by year. According to Pellikka et al. (Reference Pellikka, Lotjonen, Siljander and Lens2009), the total area of indigenous forest diminished by 50% between 1955 and 2004. Today, the largest remaining indigenous forests are on the mountains of Mbololo (220 ha), Ngangao (124 ha) and Chawia (50 ha) (Burgess et al. Reference Burgess, Butynski, Cordeiro, Doggart, Fjeldså, Howell, Kilahama, Loader, Lovett and Mbilinyi2006; Rogers et al. Reference Rogers, O'Connell, Mwang'ombe, Madoffe and Hertel2008; Pellikka et al. Reference Pellikka, Lotjonen, Siljander and Lens2009). The total forest area of the Taita Hills has, however, only reduced by 2%. This is due to exotic forest plantations that have replaced large areas of the indigenous forest comprising Acacia mearnsii, Cupressus lusitanica, Eucalyptus saligna and Pinus patula stands growing side by side, or even intermixed with natural forests. Planted forests are usually less efficient in capturing moisture and more susceptible to forest fires and, therefore, may permanently change the whole ecosystem towards a drier one (Pellikka et al. Reference Pellikka, Lotjonen, Siljander and Lens2009).
Following on from several historical works (e.g. Zahlbruckner Reference Zahlbruckner1926; Cengia Sambo Reference Cengia1938; Santesson Reference Santesson1952; Maas Geesteranus Reference Maas Geesteranus1955; Klement Reference Klement1962), a critical and comprehensive study of lichens in East Africa including Kenya was conducted by Swinscow & Krog (Reference Swinscow and Krog1988), and their work has since been continued by several authors (e.g. Farkas Reference Farkas1987; Farkas & Vězda Reference Farkas and Vězda1993; Jørgensen Reference Jørgensen1994; Kalb & Vězda Reference Kalb and Vězda1994; Frisch & Hertel Reference Frisch and Hertel1998; Frisch Reference Frisch1999; Marbach Reference Marbach2000; Lücking & Kalb Reference Lücking and Kalb2002; Alstrup & Aptroot Reference Alstrup and Aptroot2005; Alstrup & Christensen Reference Alstrup and Christensen2006; Yeshitela Reference Yeshitela2008; Yeshitela et al. Reference Yeshitela, Fischer, Killmann and Sérusiaux2009; Rikkinen Reference Rikkinen, Johansson, Pellikka and Sorvali2010; Kirika et al. Reference Kirika, Mugambi, Lücking and Lumbsch2012; Farkas & Flakus Reference Farkas and Flakus2015; Bjelland et al. Reference Bjelland, Bendiksby and Frisch2017; Suija et al. Reference Suija, Kaasalainen, Kirika and Rikkinen2018). However, the genus Micarea Fr., with over 100 species known worldwide (International Mycological Association 2019), is largely overlooked in Africa (but see Coppins Reference Coppins1999; Brand et al. Reference Brand, van den Boom and Sérusiaux2014) and its species have not been collected in the Taita Hills until now. In Australasia, Europe and the Russian Far East, the taxonomy and systematics of the genus has recently received much scientific interest (e.g. Czarnota Reference Czarnota2007; van den Boom et al. Reference van den Boom, Brand, Coppins and Sérusiaux2017; Guzow-Krzemińska et al. Reference Guzow-Krzemińska, Sérusiaux, van den Boom, Brand, Launis, Łubek and Kukwa2019; Kantvilas & Coppins Reference Kantvilas and Coppins2019; Konoreva et al. Reference Konoreva, Chesnokov, Kuznetsova and Stepanchikova2019; Launis et al. Reference Launis, Pykälä, van den Boom, Sérusiaux and Myllys2019a, Reference Launis, Malicek, Svensson, Tsurykau, Sérusiaux and Myllysb).
Recent molecular phylogenies show that Micarea is paraphyletic (Andersen & Ekman Reference Andersen and Ekman2005; Sérusiaux et al. Reference Sérusiaux, Brand, Motiejūnaitè, Orange and Coppins2010), even after the introduction of the new genera Brianaria S. Ekman & M. Svensson for the M. sylvicola group (Ekman & Svensson Reference Ekman and Svensson2014) and Leimonis Harris & Lendemer for the M. erratica group (Harris Reference Harris2009). The M. prasina group, which includes the type species M. prasina Fr., forms a monophyletic core group in the genus. The group is characterized by a ‘micareoid’ photobiont (a coccoid green alga with cells of 4–7.5 μm diam.), immarginate small apothecia, a hyaline hypothecium, branched paraphyses, and an ascus of the Micarea type, with a K/I+ blue amyloid tholus and a more lightly staining axial body often with a darkly stained lining (Hafellner Reference Hafellner1984; Czarnota Reference Czarnota2007; Ekman et al. Reference Ekman, Andersen and Wedin2008). Many species develop effuse thalli composed of goniocysts and produce Sedifolia-grey pigment (K+ violet, C+ violet), which is typically present in the epihymenium of the apothecia as well as the pycnidia (Coppins Reference Coppins1983; Czarnota & Guzow-Krzemińska Reference Czarnota and Guzow-Krzemińska2010).
In this study, we explored the diversity and systematics of Micarea species in the Taita Hills of Kenya. We used phenotypic characters and molecular DNA sequence data from three loci (nuclear rDNA internal transcribed spacer region (ITS1-5.8S-ITS2 = ITS), mitochondrial rDNA small subunit (mtSSU) and replication licensing factor Mcm7). We also continued to investigate the use of crystalline granules as a character for species delimitation (Guzow-Krzemińska et al. Reference Guzow-Krzemińska, Sérusiaux, van den Boom, Brand, Launis, Łubek and Kukwa2019; Launis et al. Reference Launis, Pykälä, van den Boom, Sérusiaux and Myllys2019a, Reference Launis, Malicek, Svensson, Tsurykau, Sérusiaux and Myllysb). The focus of this study was the epiphytic and epixylic Micarea species in indigenous and planted forests of the two mountains, Ngangao and Vuria. This study increases our knowledge of the diversity of lichens in the Taita Hills, and also reveals the suitability of plantation forest habitats for Micarea species.
Material and Methods
Taxon sampling
Fresh specimens were collected on the mountains of Ngangao (c. 1952 m) and Vuria (2228 m) in Kenya, during an expedition in 2017. According to Pellikka et al. (Reference Pellikka, Lotjonen, Siljander and Lens2009), the intensity of human disturbance in the Ngangao forests is moderate, whereas it is relatively higher in Vuria, where only 1 ha of indigenous forest remains (Wilder et al. Reference Wilder, Brooks and Lens1998). Type material of related Micarea species from the herbaria H-NYL and UPS were studied for comparison. The samples used in phylogenetic analyses are listed in Table 1 and include a total of 52 specimens of 42 taxa.
Table 1. List of Micarea specimens used in the phylogenetic analyses with locality, voucher information and GenBank Accession numbers. New species and new sequences generated for the current study are marked in bold.

DNA extraction, polymerase chain reaction and DNA sequencing
Genomic DNA was extracted from 1–3 apothecia of specimens stored for a maximum of 1 year, using the DNeasy Blood & Tissue Kit (Qiagen, Maryland, USA) following the protocol described by Myllys et al. (Reference Myllys, Velmala, Holien, Halonen, Wang and Goward2011). Polymerase chain reactions (PCRs) were prepared using PuReTaq Ready-To-Go PCR Beads (GE Healthcare, Chicago, Illinois, USA). Each 25 μl reaction volume contained 19 μl distilled water (dH2O), 1 μl of each primer (10 μM), and 4 μl extracted DNA. The primers listed below were used for PCR amplification and sequencing.
For the ITS region, PCR was run under the following conditions: initial denaturation for 5 min at 95 °C followed by five cycles of 30 s at 95 °C (denaturation), 30 s at 58 °C (annealing), and 1 min at 72 °C (extension); for the remaining 40 cycles, the annealing temperature was decreased to 56 °C; the PCR program ended with a final extension for 7 min at 72 °C. The primers used were ITS1-LM (Myllys et al. Reference Myllys, Lohtander, Källersjö and Tehler1999) and ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990).
For the mtSSU gene, PCR was run under the following conditions: initial denaturation for 10 min at 95 °C followed by six cycles of 1 min at 95 °C (denaturation), 1 min at 62 °C (annealing), and 1 min 45 s at 72 °C (extension); for the remaining 35 cycles, the annealing temperature was decreased to 56 °C; the PCR program ended with a final extension of 10 min at 72 °C. The primers used were mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999).
For the Mcm7 gene, PCR was run under two different conditions depending on the primers selected. For the first protocol, an initial denaturation for 10 min at 94 °C was followed by 38 cycles of 45 s at 94 °C (denaturation), 50 s at 55 °C (annealing), and 1 min at 72 °C (extension), with the PCR program ending with a final extension for 5 min at 72 °C. The primers used were MCM7_AL1r and MCM7_AL2f (Launis et al. Reference Launis, Pykälä, van den Boom, Sérusiaux and Myllys2019a). The second protocol used an initial denaturation for 10 min at 94 °C, followed by 38 cycles of 45 s at 94 °C (denaturation), 50 s at 56 °C (annealing), and 1 min at 72 °C (extension); the PCR program ended with a final extension for 5 min at 72 °C. The primers used were x.Mcm7.f (Leavitt et al. Reference Leavitt, Johnson, Goward and St. Clair2011) and Mcm7.1348R (Schmitt et al. Reference Schmitt, Crespo, Divakar, Fankhauser, Herman-Sackett, Kalb, Nelsen, Nelson, Rivas-Plata and Shimp2009).
PCR products were cleaned and sequenced by Macrogen Inc. (Amsterdam, The Netherlands; www.macrogen.com).
Phylogenetic analyses
In order to examine the phylogenetic position of our study species within Micarea s. lat., we made a preliminary analysis of a combined mtSSU + Mcm7 data set using Psora decipiens (Hedw.) Hoffm. from the family Psoracaeae as an outgroup, based on a study by Andersen & Ekman (Reference Andersen and Ekman2005). ITS regions were too variable and could not be included in the analysis. In the phylogeny (tree not shown) our new samples fall within the Micarea prasina group as delimited by van den Boom et al. (Reference van den Boom, Brand, Coppins and Sérusiaux2017), Launis & Myllys (Reference Launis and Myllys2019), Launis et al. (Reference Launis, Pykälä, van den Boom, Sérusiaux and Myllys2019a, Reference Launis, Malicek, Svensson, Tsurykau, Sérusiaux and Myllysb) and Guzow-Krzemińska et al. (Reference Guzow-Krzemińska, Sérusiaux, van den Boom, Brand, Launis, Łubek and Kukwa2019), except for one specimen, M. taitensis sp. nov., which appears as basal to the M. prasina group.
The final phylogenies comprising 33 ITS, 52 mtSSU and 40 Mcm7 sequences were first aligned separately with MUSCLE v.3.8.31 (Edgar Reference Edgar2004) using the European Molecular Biology Laboratory, European Bioinformatics Institute's (EMBL-EBI) freely available web server (http://www.ebi.ac.uk/Tools/msa/muscle/). Phylogenetic analyses for each gene region were performed as below for the concatenated data set. The single gene trees did not show any strongly supported conflicts according to the approach of Kauff & Lutzoni (Reference Kauff and Lutzoni2002) (with threshold bootstrap values ≥ 75%) and the three data sets were combined into a concatenated matrix in PhyDE® (Phylogenetic Data Editor, http://www.phyde.de/index.html). Based on our previous studies (Launis et al. Reference Launis, Pykälä, van den Boom, Sérusiaux and Myllys2019a, Reference Launis, Malicek, Svensson, Tsurykau, Sérusiaux and Myllysb) and our preliminary phylogenetic reconstruction, Micarea peliocarpa (Anzi) Coppins & R. Sant. was used as an outgroup. The hypervariable region at the end of the mtSSU and the ambiguously aligned region at the end of the ITS2 were removed from the analyses. The concatenated data set, including 52 terminals, was subjected to maximum parsimony (MP) analysis as implemented in TNT v.1.1 (Goloboff et al. Reference Goloboff, Farris and Nixon2008) and to maximum likelihood (ML) analysis using RAxML 8.1.15 (Stamatakis Reference Stamatakis2014) on the CSC-IT Center for Science server (http://www.csc.fi/home). The MP analysis was performed using ‘traditional search’ with random addition of sequences with 100 replicates and the tree bisection reconnection (TBR) branch swapping algorithm. Ten trees were saved for each replicate and gaps were treated as missing data. Node support was estimated by bootstrapping (Felsenstein Reference Felsenstein1985) with 1000 replicates. Bootstrap values > 75% were considered significant. For the ML analysis, the combined data set was assigned to seven partitions: ITS1, 5.8S, ITS2, mtSSU, and each of three codon positions of Mcm7. An independent GTR + G model was used for each subset, and branch lengths were assumed to be proportional across subsets. Node support was estimated with 1000 bootstrap replicates using the rapid bootstrap algorithm. The alignments are available from the Dryad Digital Repository (https://doi.org/10.5061/dryad.vmcvdncqv).
Morphology and chemistry
Hand-cut apothecial sections and squashed thallus preparations were examined with a dissecting and compound microscope. Ascospores and other anatomical details were studied, and measurements made on material mounted in water or in 10% potassium hydroxide (K) to relax features. Measurements are given in the format of minimum and maximum values. Rare minimum or maximum measurements of ascospores are given in parentheses. Chemical spot tests were performed under a compound microscope using sodium hypochlorite (C) and K (Orange et al. Reference Orange, James and White2010). Pigments were defined following Coppins (Reference Coppins1983), Meyer & Printzen (Reference Meyer and Printzen2000) and Czarnota (Reference Czarnota2007). The chemistry of the samples was further studied using thin-layer chromatography (TLC) in solvent system ‘C’, following Culberson & Kristinsson (Reference Culberson and Kristinsson1970) and Orange et al. (Reference Orange, James and White2010). The crystalline granules were investigated using a compound microscope with polarization filters.
Results
The multilocus data matrix from sequences of 52 specimens included 1793 aligned nucleotide characters, with 776 positions in the mtSSU, 592 positions in the Mcm7 gene and 425 positions in the ITS regions. Since the topologies of the ML and MP analyses did not have any strongly supported conflicts, only the tree obtained from the ML analysis is shown (Fig. 1).

Fig. 1. Phylogenetic positions of Micarea pumila sp. nov., M. stellaris sp. nov., M. taitensis sp. nov. and M. versicolor sp. nov. (shown in bold) in a maximum likelihood phylogram obtained from the RAxML analysis (Stamatakis Reference Stamatakis2014) based on the combined ITS, mtSSU and Mcm7 data set. Branches supported with bootstrap values ≥ 70% in both analyses (RAxML and TNT (Goloboff et al. Reference Goloboff, Farris and Nixon2008)) are indicated in bold. Bootstrap values ≥ 70% supported only in the maximum likelihood analysis are shown above nodes.
The highly resolved phylogeny agrees with that already presented in earlier studies (Guzow-Krzemińska et al. Reference Guzow-Krzemińska, Czarnota, Łubek and Kukwa2016; van den Boom et al. Reference van den Boom, Brand, Coppins and Sérusiaux2017; Launis & Myllys Reference Launis and Myllys2019; Launis et al. Reference Launis, Pykälä, van den Boom, Sérusiaux and Myllys2019a, Reference Launis, Malicek, Svensson, Tsurykau, Sérusiaux and Myllysb). However, it should be noted that our new accessions of Micarea eximia Hedl. form a basal clade in the phylogeny after M. incrassata Hedl., and the M. eximia sequence obtained from GenBank groups instead with M. misella (Nyl.) Hedl. Our sequences of M. eximia are extracted from reliably identified specimens collected in 2015 from central Finland, and the species has been collected several times since. Micarea eximia is a rarely collected species and most of the specimens are from Fennoscandia and northern Scotland. The GenBank accession is most probably obtained from an undescribed species or is a sequence of M. misella. A North American species, M. endocyanea (Tuck. ex Willey) R. C. Harris, is analyzed here for the first time and is closely related to M. elachista (Körb.) Coppins & R. Sant. The species has a darkly pigmented hypothecium, which is a rare exception amongst its relatives.
The Micarea prasina group is strongly supported (97%) and a clade including M. tomentosa Czarnota & Coppins and M. pusilla Launis et al. appears as basal. The remaining taxa of the M. prasina group are divided into two clades: the first, strongly supported clade (99%) includes M. hedlundii Coppins, M. xanthonica Coppins & Tønsberg and species referred to the M. byssacea and M. micrococca complexes (see Czarnota & Guzow-Krzemińska Reference Czarnota and Guzow-Krzemińska2010; Launis et al. Reference Launis, Pykälä, van den Boom, Sérusiaux and Myllys2019a); the second clade remains unsupported and consists of species from the M. prasina complex (see Launis et al. Reference Launis, Malicek, Svensson, Tsurykau, Sérusiaux and Myllys2019b).
Our new material was found in four separate lineages and is supported by unique molecular and phenotypic characters. The main distinguishing morphological characters are presented in a species synopsis (Table 2). Micarea stellaris sp. nov., represented by one specimen in our phylogeny, is nested in the M. micrococca complex and forms a strongly supported clade with M. levicula (Nyl.) Coppins (a specimen collected from the island of Réunion by Brand et al. (Reference Brand, van den Boom and Sérusiaux2014)). Micarea versicolor sp. nov., including four specimens, and M. pumila sp. nov. with one specimen are both members of the M. prasina complex. The two species form a strongly supported group but are separated from each other by rather long branches and also from other members of the complex. The fourth new species discovered in our study, M. taitensis sp. nov., appears as a basal taxon to the M. prasina group.
Table 2. A species synopsis representing the main distinguishing morphological characters for the new Micarea species and for their closest relatives or morphologically similar species.

Small crystalline granules, soluble in K, were studied in polarized light and are shown in detail in Fig. 3. The granules were detected in the hymenium of all four new species. In M. stellaris, the granules appear as an intense belt-like continuum across the lower hymenium. In M. pumila, M. taitensis and M. versicolor, the granules are scattered across the hymenium, sometimes clustered (M. taitensis) or occasionally not visible at all (M. pumila).

Fig. 2. Morphological and anatomical features. Micarea pumila (Kantelinen 4630, holotype, H): A, habit; B, apothecial section. Micarea stellaris (Kantelinen 4625, holotype, H): C, habit; D, apothecial section. Micarea taitensis (Kantelinen 4623, holotype, H): E, habit; F, apothecial section. Micarea versicolor (Kantelinen 4626, holotype, H): G, habit; H, apothecial section. Scales: A, C, E & G = 1 mm; B, D, F & H = 100 μm.
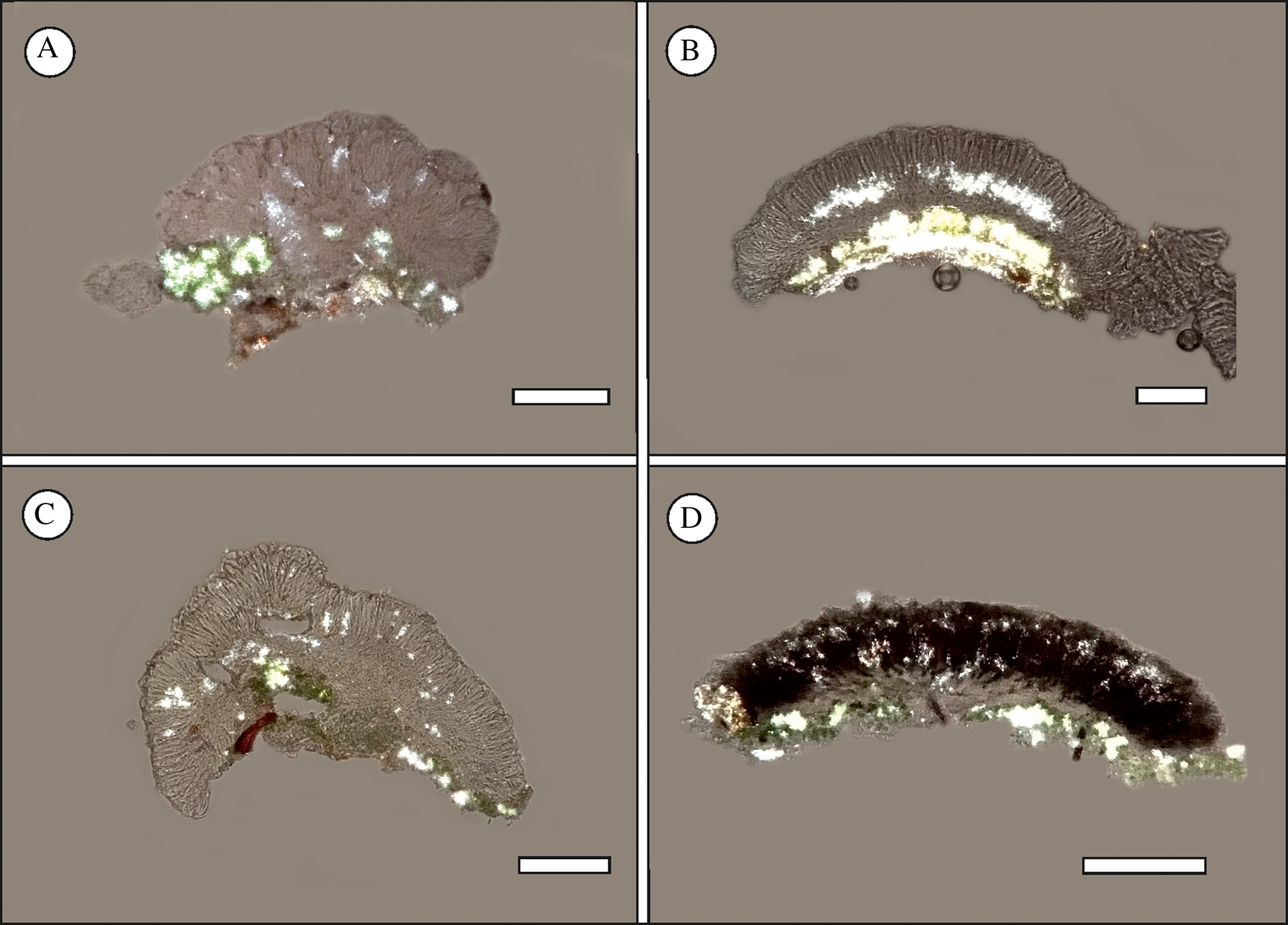
Fig. 3. Crystalline granules detected in apothecial sections in polarized light. A, Micarea pumila (Kantelinen 4630, holotype, H). B, Micarea stellaris (Kantelinen 4625, holotype, H). C, Micarea taitensis (Kantelinen 4623, holotype, H). D, Micarea versicolor (Kantelinen 4626, holotype, H). Scales = 100 μm. In colour online.
Discussion
Based on new collections from the Taita Hills, Kenya, we describe four species: M. pumila sp. nov., M. stellaris sp. nov., M. taitensis sp. nov. and M. versicolor sp. nov. Three of the new species are nested in the M. prasina group, and the fourth (M. taitensis) resolves as a basal taxon to this group. The phylogenetic placement of our new species is also supported by their phenotypic characters.
We continued to investigate the use of crystalline granules as a character for species delimitation (Guzow-Krzemińska et al. Reference Guzow-Krzemińska, Sérusiaux, van den Boom, Brand, Launis, Łubek and Kukwa2019; Launis et al. Reference Launis, Pykälä, van den Boom, Sérusiaux and Myllys2019a, Reference Launis, Malicek, Svensson, Tsurykau, Sérusiaux and Myllysb). The granules were found to be highly informative for the description of M. stellaris, in which they appear as an intense belt-like continuum across the lower hymenium. However, they were not found to be particularly useful for the delimitations of M. pumila, M. taitensis and M. versicolor. In those species, the granules are either occasionally absent (M. pumila) or simply scattered across the hymenium, sometimes clustered (M. taitensis). The chemical composition of the crystalline granules remains unclear.
Half (50%) of the indigenous forests of the Taita Hills were replaced with exotic tree plantations between 1955 and 2004 (Pellikka et al. Reference Pellikka, Lotjonen, Siljander and Lens2009), and it is estimated that the natural forest coverage in the region has diminished by up to 90% within the last 200 years (Rodgers Reference Rodgers, Lovett and Wasser1993). Therefore, it is important to understand how well epiphytic lichen diversity could survive in the exotic forest plantations. Concerning Micarea, examples of such translocations between natural and planted forests have already been observed in the Azores and Réunion (Purvis & James Reference Purvis and James1993; Brand et al. Reference Brand, van den Boom and Sérusiaux2014). In Tasmania, however, Micarea species were found to be sensitive to ecological change, such as logging, silvicultural treatments and fire. Interestingly, the Tasmanian examples vividly demonstrate how species in this genus are adapted to a wide range of ecological niches: almost all the species of Micarea from unlogged forest were replaced by other Micarea species that proliferated in just 3–5 years after the logging (Jarman & Kantvilas Reference Jarman and Kantvilas2001a, Reference Jarman and Kantvilasb; Kantvilas & Jarman Reference Kantvilas and Jarman2006; Kantvilas et al. Reference Kantvilas, Jarman and Minchin2015).
Our results from Ngangao and Vuria show that at least some Micarea species have found favourable habitats in the Pinus patula plantations. In these locations, the species were encountered frequently, especially on dead wood. This result is not particularly surprising, since the Pinus plantations are quite similar to the environments epiphytic Micarea species inhabit elsewhere in the world, that is habitats with medium to high precipitation and substrata of low pH such as bark and wood of coniferous trees (e.g. Coppins Reference Coppins1983; Czarnota Reference Czarnota2007). Micarea is also recognized as one of the major lichen groups on dead wood in North America and Fennoscandia (Spribille et al. Reference Spribille, Thor, Bunnell, Goward and Björk2008), which is in line with our discoveries from the Taita Hills.
Since the majority of our collections are from Pinus plantations, the question arises: are the new species in fact native or unintentionally transported together with the exotic central American tree species? Unfortunately, we cannot answer this question conclusively. However, a native origin is suggested for the following reasons: 1) Micarea stellaris and M. taitensis appear to share close relationships with two species collected from Réunion, an island in the Indian Ocean east of Madagascar; 2) three specimens of M. versicolor were indeed collected from indigenous forests that had low human disturbance; 3) our survey effort was limited by time and resources, which particularly impacted the exploration of the indigenous forests. In these localities, finding the specific tree species with favourable pH and bark structure among the rich tropical biodiversity was challenging. Hence, it is possible that we just did not find the suitable tree species and that they do exist and host the source populations for the Micarea species found in the Pinus plantations.
Based on this study, and that of Brand et al. (Reference Brand, van den Boom and Sérusiaux2014), the diversity of the genus Micarea in eastern Africa and the islands in the Indian Ocean is rich, though understudied. Broader phylogeographical investigations are needed to understand the distribution, endemism and speciation of the group in this unique geographical region.
The Species
Micarea pumila Kantelinen & Myllys sp. nov.
MycoBank No.: MB 836919
Thallus olive green to bright green, minutely granular; apothecia numerous, cream-white or pale brownish, 0.2–0.4 mm diam., plane, convex or hemispherical, simple or tuberculate, K− and C−; ascospores oblong-ellipsoid or obovoid, 0–1-septate, 7.0–10.5 × 2.5–3.2(–3.5) μm; prasinic acid.
Type: Kenya, Taita Taveta, Taita Hills, Ngangao forest, near top of the mountain, Pinus patula plantation, on wood of dead standing Pinus patula (c. 180 cm tall), 3.355015°S, 38.338873°E, 1868 m a.s.l., 23 November 2017, Annina Kantelinen 4630 (H—holotype; NAI—isotype). GenBank Accession number: MT982140 (mtSSU).
Thallus effuse, olive green to bright green, minutely granular, composed of goniocysts, 12–30 μm diam., usually coalescing to form larger granules; photobiont micareoid, algal cells 4.5–7.5 μm diam.
Apothecia numerous, cream-white or pale brownish, 0.2–0.4 mm diam., plane, convex or hemispherical, simple or sometimes tuberculate, K− and C−; hypothecium hyaline; hymenium hyaline, c. 40–52 μm high; epihymenium hyaline; paraphyses numerous, 1.2–2.0 μm wide with apices not wider or increasing up to 2.7 μm, mostly branched, sometimes branched 1–2 times from the apices resulting in a fork-like appearance; asci clavate, Micarea-type, 8-spored, 25–35 × 8–10 μm; ascospores oblong-ellipsoid or obovoid, 0–1-septate, 7.0–10.5 × 2.5–3.2(–3.5) μm.
Pycnidia of one type; mesopycnidia, sessile or immersed within goniocysts, whitish, K− and C−, globose or barrel-shaped, up to 90 μm wide; mesoconidia cylindrical or cylindrical-fusiform, 4.0–5.2 × 1.0–1.5 μm.
Crystals (studied in polarized light) spread across the hymenium, often rather weakly polarizing, or not visible at all. Soluble in K (Fig. 3A).
Chemistry
Prasinic acid.
Etymology
‘Pumila’ (Latin) meaning small/dwarf, referring to the small size and inconspicuous appearance of the species.
Habitat and distribution
Micarea pumila is known from a Pinus patula plantation near the top of Ngangao Mountain. The species was collected from two trunks of fallen decaying Pinus patula (Fig. 4C & D).

Fig. 4. Habitats. A, indigenous forest on Ngangao Mountain. B, indigenous forest on Vuria Mountain. C, Pinus patula plantation near top of Ngangao. D, collection site of M. pumila in the Pinus patula plantation. E, landscape seen from the top of Vuria (2228 m a.s.l.). In colour online.
Notes
Micarea pumila is characterized by a minutely granular thallus, small cream-white or pale brownish apothecia and small ascospores. It resembles species in the M. prasina complex, especially M. fallax Launis & Myllys and M. prasinastra Coppins & Kantvilas. The small whitish apothecia are also similar to M. micrococca and M. pseudomicrococca. The main morphological characters separating M. pumila from the other species are the smaller ascospore size and wider paraphyses, with the exception of M. prasinastra which has a similar ascospore size but the thallus contains gyrophoric acid. In addition, the geographical distribution of these species is not known to overlap.
In the phylogenetic analysis, M. pumila is nested within the M. prasina complex. The species forms a well-supported group with another new species from the Taita Hills, M. versicolor. The close relationship probably reflects a geographical and evolutionary isolation that these two species have encountered in the mountains. However, M. pumila and M. versicolor are morphologically quite distinct. They can be separated by the structure of the thalli (minutely granular vs warted-areolate, respectively), size and pigmentation of the apothecia (bigger and K+ violet when pigmented in M. versicolor), ascospore size (7.0–10.5 × 2.5–3.2(–3.5) μm vs 9.5–13.0 × 3.2–4.0 μm, respectively) and secondary metabolites (prasinic acid vs methoxymicareic acid).
Additional specimen examined
Kenya: Taita Taveta: Taita Hills, Ngangao forest, west side, near top of the mountain, Pinus plantation, by a forest path, on wood of fallen decaying Pinus patula trunk, 3.366105°S, 38.341582°E, 1850 m a.s.l., 2017, Annina Kantelinen 4632 (H, NAI).
Micarea stellaris Kantelinen & Myllys sp. nov.
MycoBank No.: MB 836920
Thallus whitish green to bright green, warted-areolate; apothecia numerous, cream-white, usually darker at the centre, 0.3–0.5 (–0.6) mm diam., adnate, convex, simple; hymenium light grey or brownish, pigment dissolving in K; crystalline granules intense, appearing as a belt-like continuum across lower hymenium; ascospores, oblong-ellipsoid or obovoid, 0–1-septate, 10.0–14.0 × 3.8–5.0 μm; methoxymicareic acid.
Type: Kenya, Taita Taveta, Taita Hills, Ngangao forest, east side, near road, by a path in the indigenous forest, on wood of decaying fallen tree trunk, 3.370467°S, 38.340808°E, 1808 m a.s.l., 24 November 2017, Annina Kantelinen 4625 (H—holotype; NAI—isotype). GenBank Accession numbers: MT981448 (Mcm7), MT982139 (mtSSU).
Thallus effuse, whitish green to bright green, warted-areolate; photobiont micareoid, algal cells 4.5–7.5 μm diam.
Apothecia numerous, cream-white, usually darker at the centre, 0.3–0.5(–0.6)mm diam., adnate, convex, simple; hypothecium hyaline or slightly pigmented near hymenium; hymenium light grey or brownish, pigment K− and dissolving (possibly Elachista-brown pigment), 50–60 μm high; epihymenium hyaline; paraphyses numerous, mostly branched, 0.8–1.2 (–1.5) μm wide, sometimes slightly wider at the apices; asci clavate, Micarea-type, 8-spored, 42–50 × 10–13 μm; ascospores oblong-ellipsoid or obovoid, 0–1-septate, 10.0–14.0 × 3.8–5.0 μm.
Pycnidia of one type; micropycnidia immersed in thallus, small and inconspicuous, whitish, K− and C−, globose, up to 80 μm wide; microconidia filiform to narrowly fusiform, straight or slightly curved, 6.5–8.0 × 0.8–1.0 μm.
Crystals (studied in polarized light) intense, appearing as a belt-like continuum across lower hymenium. Soluble in K (Fig. 3B).
Chemistry
Methoxymicareic acid.
Etymology
‘Stellaris’ (Latin) meaning star, referring to the intensely shining crystalline granules.
Habitat and distribution
Micarea stellaris is known from two localities on Ngangao Mountain: an indigenous forest (Fig. 4A) and a Pinus patula plantation (Fig. 4C). In both localities the new species grew on wood of dead fallen tree trunks (Pinus patula and an unidentified, likely native tree species).
Notes
Micarea stellaris is characterized by a warted-areolate thallus, light grey or brownish (K−) pigment in the hymenium, and intensely polarizing crystals appearing as a belt-like continuum across the lower hymenium. It resembles Micarea taitensis and M. versicolor but differs in the production of hymenial pigmentation and the intense crystals. Based on our phylogenetic analysis, the three species are not particularly closely related (Fig. 1).
In the phylogenetic analysis, M. stellaris resolves as a sister to Micarea levicula, and is nested within the M. micrococca complex. The specimen of M. levicula in our analysis was originally collected from a natural stand of Acacia heterophylla on the island of Réunion by Brand et al. (Reference Brand, van den Boom and Sérusiaux2014). However, we also collected a specimen of M. levicula from the Taita Hills, Vuria, and it is new to Kenya (specimen Annina Kantelinen 4648 & Marko Hyvärinen, see below for details). The two species resemble each other in many respects, such as the similar ecological preferences, and the shape and size of the apothecia. The main morphological features separating them are the structure of the thallus, ascospore size, pigmentation in the apothecia and secondary metabolites. Micarea levicula forms a thallus of delicately coralloid goniocysts which is distinctly different to the warted-areolate thallus of M. stellaris. The apothecia of M. levicula are non-pigmented throughout, the ascospores are thinner (3.7–4.1 μm vs 3.8–5.0 μm wide in M. stellaris) and it produces gyrophoric acid instead of methoxymicareic acid.
Selected specimens examined
Kenya: Taita Taveta: Taita Hills, Ngangao forest, west side, near Ngangao Forest Camp and by a forest path, indigenous forest, on wood of decaying fallen tree trunk, 3.370565°S, 38.346693°E, 1834 m a.s.l., 2017, Annina Kantelinen 4633 (H); ibid., near top of the mountain, indigenous forest, by a forest path, on wood of a small fallen decaying tree trunk, 3.368355°S, 38.343012°E, 1844 m a.s.l., 2017, Annina Kantelinen 4634 (H, NAI).
Micarea levicula specimen examined
Kenya: Taita Taveta: Taita Hills, Vuria, NE slope of the mountain, indigenous forest, near road to the hilltop, on wood of a decaying stump c. 1 m tall, in shade, together with M. versicolor, 3.39969444°S, 38.36472222°E, 2040 m a.s.l., 2017, Annina Kantelinen 4648 & Marko Hyvärinen (H).
Micarea taitensis Kantelinen & Myllys sp. nov.
MycoBank No.: MB 836921
Thallus whitish green to bright green, warted-areolate or sometimes membranate; apothecia numerous, cream-white or yellowish, often with a greyish tinge because of the Sedifolia-grey pigment (K± violet and C± violet), 0.4–0.6 mm diam., adnate, convex, simple; ascospores oblong-ellipsoid or obovoid, (0–)1(–2)-septate, when 1-septate often slightly constricted at the septum, 10.0–14.0 × 4.0–4.7(–5.0) μm; methoxymicareic acid.
Type: Kenya, Taita Taveta, Taita Hills, Ngangao forest, Pinus plantation near top of the mountain, on bark of fallen decaying Pinus patula trunk, 3.368355°S, 38.343012°E, 1850 m a.s.l., 24 November 2017, Annina Kantelinen 4623 (H—holotype; NAI—isotype). GenBank Accession numbers: MT981446 (Mcm7), MT982137 (mtSSU).
Thallus effuse, whitish green to bright green, warted-areolate or sometimes membranate, bright green especially in parts distinctly warted; photobiont micareoid, algal cells 4.5–7.5 μm diam.
Apothecia numerous, cream-white or yellowish, often with a greyish tinge because of the Sedifolia-grey pigment (K± violet and C± violet), 0.4–0.6 mm diam., adnate, convex, simple; hypothecium hyaline; hymenium hyaline, c. 45–60 μm high; epihymenium hyaline; paraphyses numerous, branched or straight, 0.8–1.2 μm wide, apices not widening; asci clavate, Micarea-type, 8-spored, 30–48 × 13–17 μm; ascospores oblong-ellipsoid or obovoid, (0–)1(–2)-septate, when 1-septate often slightly constricted at the septum, 10.0–14.0 × 4.0–4.7(–5.0) μm.
Pycnidia of one type; micropycnidia immersed in thallus, small and inconspicuous, whitish, K− and C−, globose, up to 80 μm wide; microconidia filiform to narrowly fusiform, straight or slightly curved, 6.5–8.0 × 0.8–1.0 μm.
Crystals (studied in polarized light) visible in hymenium, sometimes clustered. Soluble in K (Fig. 3C).
Chemistry
Methoxymicareic acid.
Etymology
The name M. taitensis refers to the type locality, the Taita Hills.
Habitat and distribution
Micarea taitensis was found on the bark of Pinus patula from Ngangao Mountain. The type locality is a mature Pinus patula plantation near the top of the mountain, and it is so far known only from that locality (Fig. 4C).
Notes
Micarea taitensis is characterized by a warted-areolate thallus and pale cream or yellowish apothecia that sometimes produce the Sedifolia-grey pigment. Macroscopically it resembles Micarea stellaris and M. versicolor, two other new species from the Taita Hills. However, the species differ in their microscopic features. Micarea stellaris produces a light grey or brownish pigment in the hymenium, and in polarized light it exhibits intense crystalline granules that appear as a belt-like continuum across the lower hymenium. Micarea versicolor, on the other hand, develops apothecia varying in colour from cream-white to blackish, and it has slightly thinner ascospores (3.2–4.0 μm vs 4.0–4.7 μm in M. taitensis). The phylogenetic relationship of these species is not particularly close, but instead M. taitensis resolves as a basal lineage to the M. prasina group (Fig. 1).
Micarea taitensis is possibly closely related to M. sublithinella, a species known from Madagascar and Réunion (Brand et al. Reference Brand, van den Boom and Sérusiaux2014). These two species share morphological and ecological similarities. Both develop a warted thallus and grow in montane forests on acidic bark (Acacia heterophylla and Pinus patula). However, M. taitensis develops paler apothecia and thinner ascospores (4.0–4.7 μm vs 5.0–5.8 μm), and produces methoxymicareic acid, whereas M. sublithinella produces protolichesterinic acid. So far, their distribution is not known to overlap.
Micarea versicolor Kantelinen, Hyvärinen & Myllys sp. nov.
MycoBank No.: MB 836922
Thallus whitish green to bright green, warted-areolate or continuous crust, sometimes partly granular and then composed of goniocysts; apothecia numerous, cream-white to light grey to dark brownish grey or almost black (Sedifolia-grey and a purplish brown pigment), K+ intensifying purple and K+ violet if pigmented, 0.3–0.6 mm diam., adnate, convex to slightly hemispherical, simple; ascospores oblong-ellipsoid or obovoid, 0–1-septate, 9.5–13.0 × 3.2–4.0(–4.5) μm; methoxymicareic acid.
Type: Kenya, Taita Taveta, Taita Hills, Ngangao forest, west side, near Ngangao Forest Camp and by a forest path, indigenous forest, on wood of decaying fallen tree trunk, 3.370565°S, 38.346693°E, 1834 m a.s.l., 24 November 2017, Annina Kantelinen 4626 (H—holotype; NAI—isotype). GenBank Accession number: MT982144 (mtSSU).
Thallus effuse, whitish green to bright green, quite thin, warted-areolate or continuous crust, sometimes partly granular and then composed of goniocysts of 18–40 μm diam; photobiont micareoid, algal cells 4.5–7.5 μm diam.
Apothecia numerous, cream-white or light grey to dark brownish grey to almost black (Sedifolia-grey and a purplish brown pigment), K+ intensifying purple and K+ violet when pigmented, 0.3–0.6 mm diam., adnate, convex to slightly hemispherical, simple; hypothecium hyaline; hymenium hyaline or purplish brown, K+ purple intensifying when pigmented, c. 42–50 μm high. Epihymenium hyaline to purplish brown or greenish grey, K+ violet; paraphyses numerous, branched, 1.2–2.0 μm wide, sometimes slightly wider from the apices; asci clavate, Micarea-type, 8-spored, 32–45 × 10–17 μm; ascospores oblong-ellipsoid or obovoid, 0–1-septate, 9.5–13.0 × 3.2–4.0(–4.5) μm.
Pycnidia of one type; micropycnidia numerous, sessile or immersed in thallus, cream-white, K− and C−, globose or barrel-like, sometimes with gaping ostiole, up to 100 μm wide; microconidia filiform to narrowly fusiform, straight or slightly curved, 7.0–9.0 × 0.8–1.0 μm.
Crystals (studied in polarized light) visible in hymenium and upper part of hypothecium. Soluble in K (Fig. 3D).
Chemistry
Methoxymicareic acid.
Etymology
The epithet versicolor refers to the coloration of the apothecia that vary considerably from cream-white to pale grey and to almost black.
Habitat and distribution
Micarea versicolor is known from four localities: two are in the indigenous forest on Ngangao Mountain, the third in a Pinus patula plantation near the top of Ngangao and the fourth in a small patch of indigenous forest on Vuria Mountain (Fig. 4B). In all localities the species grew on dead wood of fallen or standing tree species (Pinus patula and unidentified native trees).
Notes
Micarea versicolor is characterized by the warted-areolate, sometimes partly granular thallus and apothecia that vary in colour from cream-white to light grey to blackish. This considerable variation in the coloration of the apothecia is probably caused by a mixture of pigments that can occur independently of one another: 1) Sedifolia-grey pigment in the epihymenium (K+ violet, C+ violet), which is a common pigment in the M. prasina group and produced especially in response to sunlight; 2) a purplish brown pigment in the hymenium (K+ intensifying purple) that may either be the same as that known from the hypothecium of M. melaena, or an unknown pigment somewhat similar to that found in the hymenium of Bacidia schweinitzii s. str. (a species also known for having high variation in the coloration of its apothecia; see Ekman (Reference Ekman1996) and Lendemer et al. (Reference Lendemer, Harris and Ladd2016)). This mixture of pigments causes the apothecia of M. versicolor to appear as cream-white (no pigments), greyish (Sedifolia-grey pigment only), or dark brownish grey to blackish (purplish brown pigment + Sedifolia-grey pigment).
Macroscopically M. versicolor resembles two other new species from the Taita Hills, M. stellaris and M. taitensis. These species can be separated by microscopic features that include the pigmentation of the apothecia and hymenium, crystalline granules in polarized light and ascospore width (see more details under M. taitensis).
Based on our phylogenetic analysis, M. versicolor is not closely related to M. stellaris or M. taitensis. Instead it is sister to M. pumila and nested within the M. prasina complex, although with low support in the latter. Morphologically the sister species M. versicolor and M. pumila are quite distinct: M. pumila develops a minutely granular thallus composed of goniocysts, has smaller unpigmented apothecia (K− and C−) and smaller ascospores (7.0–10.5 × 2.5–3.2(–3.5) μm vs 9.5–13.0 × 3.2–4.0(–4.5) μm). They also produce different secondary metabolites: M. versicolor produces methoxymicareic acid, whereas M. pumila produces prasinic acid.
Selected specimens examined
Kenya: Taita Taveta: Taita Hills, Ngangao forest, west side, near top of the mountain, Pinus plantation, by a forest path, on wood of fallen decaying Pinus patula trunk, 3.366105°S, 38.341582°E, 1850 m a.s.l., 2017, Annina Kantelinen 4624 (H, NAI); ibid., indigenous forest, by a forest path, on wood of a small fallen decaying tree truck, 3.368355°S, 38.343012°E, 1844 m a.s.l., 2017, Annina Kantelinen 4627 (H, NAI); ibid., Vuria, NE slope of the mountain, indigenous forest, near road to the hilltop, on wood of a decaying stump c. 1 m tall, in shade, 3.39969444°S, 38.36472222°E, 2040 m a.s.l., 2017, Annina Kantelinen 4647 & Marko Hyvärinen (H, NAI).
Acknowledgements
Financial support for this study was provided by the Societas pro Fauna et Flora Fennica, the University of Helsinki LUOVA travel grants for doctoral candidates (personal grants for AK), and the Academy of Finland (Grant 323711). We thank the two anonymous reviewers for improving the manuscript. We would also like to acknowledge the organizers and participants of the expedition to the Taita Hills in 2017, namely Prof. Leif Schulman, head gardener Pertti Pehkonen and doctoral candidate Anton Savchenko. We also thank the Taita Research Station (University of Helsinki) for providing access to facilities and logistics during the stay. The staff and local people are warmly thanked for their helpfulness and hospitality.
Author ORCIDs
Annina Kantelinen, 0000-0001-8664-7662; Leena Myllys, 0000-0002-9566-9473.









