Published online by Cambridge University Press: 19 January 2004
The fresh-water crustacean Daphnia magna may acquire an infection with the microsporidium Octosporea bayeri either by ingesting spores from the water (horizontally), or directly from its mother (vertically). Due to differences in the time and mechanisms of transmission, horizontal and vertical infections may lead to differences in the growth of the parasite within the host. This may influence parasite virulence, transmission to new hosts, and, consequently, epidemiology and evolution. Here we describe the within-host dynamics of 3 spore-types of O.bayeri from infections that were acquired either horizontally or vertically. In all treatments the number of spores increased exponentially until spore density reached a plateau, suggesting density-dependent within-host growth. The spore types seen differ in their growth dynamics, suggesting different roles in the parasite life-cycle. Horizontally-infected hosts harboured significantly fewer spores than vertically-infected hosts. Further, host survival was affected by infection route, with mortality being higher in horizontal infections than in vertical infections. Our results suggest that different routes of infection have an immediate effect on within-host parasite growth and thus on parasite fitness and epidemiology.
Parasites that infect their hosts horizontally as well as vertically are model organisms for the study of the evolution of virulence. They allow us to compare, within a single parasite species, the different selection pressures imposed by horizontal and vertical transmission (e.g. Kover & Clay, 1998; Jaenike, 2000). Among microsporidia, parasites using both routes of transmission are common (Becnel & Andreadis, 1999; Dunn & Smith, 2001), and the relationship between transmission route and virulence has been studied in some species (e.g. Sweeney, Doggett & Gullick, 1989; Solter, Maddox & Onstad, 1991; Kurtti et al. 1994; Agnew & Koella, 1997).
Horizontal and vertical routes of infection may differ in several mechanistic aspects which can potentially influence their evolution. In microsporidia, for example, differences can be found in the particular environment where the infection occurs (e.g. the gut of the host versus the ovaries of the mother in the case of transovarial infections, Agnew & Koella, 1997), the cellular damage produced by the different routes (e.g. cell piercing in horizontal infections, Frixione et al. 1997, versus transport with the yolk in transovarial infection, Becnel & Andreadis, 1999), the time of infection (usually at a later stage of host life in horizontal infections) and the amount and genetic diversity of the parasites (which we expect to be higher in horizontal infections, all else being equal, as horizontally infecting parasites may come from more than one host; see Frank, 1996). All of these may have direct effects on the survival and reproduction of both host and parasite.
Within-host dynamics have been incorporated into models of host–parasite interactions (e.g. Antia, Levin & May, 1994; Anderson, 1998; Frank, 2000; Hoshen et al. 2000), and can be an important factor in the epidemiology and evolution of pathogens (Ganusov, Bergstrom & Antia, 2002; Galvani, 2003). Experimental data are scarce (Anderson, 1998), and come mainly from pathogens of vertebrates (e.g. malaria, Hetzel & Anderson, 1996). The route of infection may have an effect on the within-host dynamics of a parasite, thus influencing its epidemiology and evolution. To determine whether the route of infection has any effect on the within-host dynamics of a parasite, we experimentally manipulated infection route and followed the growth of the parasite within its host.
Daphnia magna Straus 1820, is a fresh-water crustacean usually found in eutrophic shallow ponds. It reproduces by cyclic parthenogenesis, and thus can be maintained clonally in laboratory conditions. In nature it is frequently infected with a large number of ecto- and endoparasites (Green, 1974; Stirnadel & Ebert, 1997; Ebert, Hottinger & Pajunen, 2001), and has been used as a model host for the study of several aspects of host–parasite interactions (Ebert, 1994; Ebert, Payne & Weisser, 1997; Ebert, Zschokke-Rohringer & Carius, 1998; Ebert, Lipsitch & Mangin, 2000; Little & Ebert, 2000; Carius, Little & Ebert, 2001). The microsporidium Octosporea bayeri Jírovec 1936, is an obligate intracellular parasite of D. magna with high prevalences in rock-pool Daphnia populations of the Tvärminne Archipelago in Southern Finland (Ebert et al. 2001). It can infect its host vertically (from mother to parthenogenetic or sexual offspring) and horizontally (through water-borne spores). O. bayeri is heterosporous, with 3 spore-types distinguishable using a phase-contrast microscope (300×). Two of these spore-types are present in most infections (Fig. 1), and correspond morphologically with the common spore-types described for different microsporidia: ‘few-coils’ (FC) and ‘many-coils’ (MC) spores (Iwano & Kurtti, 1995). The denomination refers to the differences in length of the polar tube, which is coiled inside the spore and everts during germination. FC spores, with a relatively thin spore wall and a pear-like shape, are commonly associated with within-host propagation, while MC spores, with a thick spore wall and an oval shape, are associated with horizontal transmission (Iwano & Kurtti, 1995; Dunn & Smith, 2001). The third spore-type is of intermediate refringency, elongated, and very variable in size. Its frequency is more variable than the other two spore-types, and its possible role in the life-cycle is unclear.
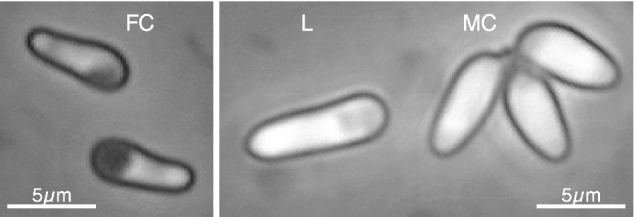
Fig. 1. Types of spores found in Octosporea bayeri infections. The pictures were taken at 400× magnification using a phase-contrast microscope. MC, many-coils spore; FC, few-coils spore (see text for details); L, long spore.
In this study we describe the infection dynamics of O. bayeri in a sympatric clone of D. magna experimentally infected either horizontally or vertically with 3 different parasite isolates. We determine whether the route of infection has an effect on the production of spores over time, and if the dynamics of the 2 spore types studied vary according to their predicted roles in the life-cycle of the parasite.
As host we chose a single clone of Daphnia magna from the Tvärminne Archipelago in Southern Finland. This clone was the product of out-crossing 2 clones collected from 2 small rock-pool populations. The clone was kept for several generations under standard laboratory conditions: in artificial medium (Klüttgen et al. 1994; modified after Ebert et al. 1998), fed regularly with the unicellular green alga Scenedesmus sp., in a light[ratio ]dark cycle of 8[ratio ]16 h, and at 20 °C temperature.
The parasites used in this experiment were obtained from field samples by allowing single infected females to reproduce parthenogenetically in the laboratory. The parasites coming from one such female clone are referred to as an isolate, and considered as a population of unknown genetic diversity. Three isolates were used, herein referred to as Ob1, Ob2, and Ob3, which were collected from 3 different rock-pools in the Tvärminne Archipelago. Pilot experiments showed differences in spore production and virulence among these 3 isolates, suggesting that they are genetically distinct.
In order to keep the genetic environment of the parasite isolates equal, the 3 isolates were transferred from their original hosts to the experimental host clone. To do so, spore suspensions of each isolate were prepared by homogenizing dying or freshly dead infected Daphnia from the original cultures and exposing uninfected Daphnia to these spore suspensions. Four replicate lines were produced for each parasite isolate, and were kept under standardized laboratory conditions (with a predominance of vertical transmission, but allowing horizontal infections to occur) for more than 3 host generations. From these lines, females of similar age (2 to 3 days old) were isolated to constitute the mothers of the vertically infected hosts. Simultaneously, and under the same conditions, uninfected females were raised as the mothers of horizontally infected hosts. We thus had 6 treatments, with all the combinations between parasite isolate (Ob1, Ob2, and Ob3) and route of infection (horizontal and vertical). Three days before the inoculation, newly born female Daphnia from infected and uninfected mothers were isolated and randomized to balance for line and jar effects, 120 individuals per treatment, a total of 720 Daphnia. Finally, dying or freshly dead individuals from the infected cultures were homogenized with a plastic pestle in vials containing 1·5 ml of medium to produce the spore suspensions.
The inoculation was performed in 2·5 ml of medium by adding 50000 spores from one of the spore suspensions. The vertically infected Daphnia were given a placebo made of homogenized uninfected Daphnia to compensate for potential nutrient and/or chemical effects. All animals were placed randomly in 24-well cell-culture plates. The Daphnia were fed on the day of infection and after 2 days with 1·5 million algae cells. On day 6 post-infection the Daphnia were transferred individually to 100 ml of fresh medium and changed to new medium every 3 days, or whenever reproduction occurred. The individuals were fed with 2 million algae cells and checked for mortality every day.
From day zero post-infection, 5 Daphnia from each treatment were killed at determined intervals to assess the amount of spores harboured by a host. The sampling frequency was each second day between days 0 and 20 post-infection (including day 0), each third day between days 20 and 29 post-infection, each fourth day between days 29 and 37 post-infection, and each ninth day between days 37 and 55 post-infection, for a total of 18 sampling dates. All individuals from each sampling date were chosen at random before the start of the experiment. Due to differential mortality, not all treatments had 5 individuals per sampling date, and 1 sampling date was lost for 1 treatment (horizontally infected Ob3 on day 46 post-infection) and thus removed from the analyses. Each individual was homogenized in a standard volume of medium, and the concentration of spores determined by counting a subsample of 2·2 μl on a counting chamber (Neubauer Improved) under phase-contrast at 600×. The last sampling took place 55 days post-infection.
The amount of spores per sampling date for each spore-type represents the spore-load, i.e. the spores carried by the host at that particular time. All spore-load data were ln-transformed. The means and standard errors of the ln-transformed spore-loads per sampling date were calculated for each treatment.
To determine whether the route of transmission had an effect on the start of the spore production, we compared the proportion of hosts with spores on the first 3 sampling dates after the first spores were detected, using a Logistic Regression Analysis with parasite isolate and infection route as effect variables (Kleinbaum et al. 1998, pp. 656–686). The significance of each effect variable was determined using Likelihood Ratio tests (Kleinbaum et al. 1998, pp. 649–652).
One treatment group on day 45 post-infection was lost due to mortality. To maintain the orthogonality of the design, that date was removed from the results. In order to determine whether the spore production had stabilized at the end of the experiment, we compared the amount of spores in the last 2 sampling dates (days 37 and 55 post-infection, 18 days apart) using a three-way ANOVA with parasite isolate, infection route, and sampling date (time) as factors. We pooled the interaction sum of squares with the error sum of squares before testing the mean squares of the main effects if the interaction was not significant (Sokal & Rohlf, 1998, p. 337). To detect differences between treatments in the spore-load during the whole period, we used a two-way ANCOVA with parasite isolate and infection route as factors, and time as a covariate. As time was non-linearly correlated with spore-load, its square was incorporated in the model (Kleinbaum et al. 1998, p. 282). Further, to determine the influence of time on the proportion of FC spores relative to the total, an ANCOVA with parasite isolate and infection route as factors, and time as a covariate was used. The dependent variable (proportion of FC spores) was normalized with the arcsine transformation (Zar, 1999, p. 282). All assumptions of the ANOVAs and ANCOVAs were tested, and fulfilled with the transformed data. Data points with zero values were excluded in both tests.
A survival curve was calculated for each treatment using the proportion of individuals alive at each sampling date. We determined if Daphnia from the different treatments differed in their survival using Cox's proportional hazards method, with infection route, parasite isolate, and their interaction as factors. The Daphnia that did not die naturally (i.e. those individuals sampled or surviving at the end of the experiment) were censored. The analysis was performed using the proportional hazards survival platform of JMP 4.0 (SAS Institute Inc., 2000).
Figure 2 shows the ln-transformed spore-load over time for FC, MC, and long (L) spores. Spores were first detected when hosts were 11 days old (8 days after horizontal infection) in all vertically infected treatments, but only in 1 horizontally infected treatment (Table 1). The difference in the proportion of individuals of each infection route found infected in that date is thus highly significant (Likelihood-Ratio Chi-square=11·2, P=0·0008). This difference, however, disappeared from day 12 onwards after horizontal infection (Likelihood-Ratio Chi-square=1·3, P=0·25). No significant effect of the isolate on the proportion of infected individuals was found for any of these dates. As the threshold of detection is rather high (1000 spores/host), the appearance of the first spores might have occurred earlier than observed.

Fig. 2. Means of the ln-transformed spore-load per sampling date, for (A), FC spores; (B), MC spores; and (C), L spores. The arrows indicate the day of horizontal infection. Filled and empty symbols represent vertical and horizontal infections respectively. Different symbols represent different parasite isolates (square=Ob1, rhombi=Ob2, and circles=Ob3). Standard errors are indicated by bars.
Table 1. Appearance of spores in hosts (The number of days after infection when spores were first observed, for each isolate and route of infection, are shown. Below the number of days, in parentheses, the proportion of hosts in which spores were found is given.)

The comparison between spore-load on the last 2 sampling dates revealed that the amount of FC spores reaches a plateau (F-ratio for days=1·29, D.F.=1, P=0·26, n=44), as did L spores (F-ratio for days=1·56, D.F.=1, P=0·22, n=39) whereas the MC spore-load increased 10% of the total (F-ratio for days=5·78, D.F.=1, P=0·01, n=42). There was no significant interaction between sampling date and the experimental factors, route of infection and parasite isolate.
During the whole sampling period vertically infected hosts had higher spore-loads than horizontally infected hosts, for FC spores (Fig. 2A; F-ratio for route=30·6, D.F.=1, P=0·03, n=272), MC spores (Fig. 2B; F-ratio for route=263·3, D.F.=1, P=0·004, n=217), and L spores (Fig. 2C; F-ratio for route=57·9, D.F.=1, P=0·017, n=188). The parasite isolate had no significant effect on the amount of any of the spore-types (F-ratio for isolate on FC spores=1·2, D.F.=2, P=0·46; F-ratio for isolate on MC spores=1·5, D.F.=2, P=0·94; F-ratio for isolate on L spores=1·1, D.F.=2, P=0·47). The interaction between parasite isolate and infection route was significant for FC and L spores, due to a decrease in the difference between horizontal and vertical infections in isolate Ob3 (F-ratio for interaction, FC spores=9·5, D.F.=2, P=0·0001; L spores=3·7, D.F.=2, P=0·027). No interaction was found for MC spores (F-ratio for interaction=1·5, D.F.=2, P=0·23, n=217).
At the beginning of the infection most of the observed spores were of the FC type, but their percentage decreased strongly over time (Fig. 3; ANCOVA test on the transformed-transformed proportion of FC spores: F-ratio for time=59·5, D.F.=1, P<0·0001).

Fig. 3. Percentage of FC spores as a function of host age in vertically and horizontally infected hosts. Different symbols represent different parasite isolates (square=Ob1, rhombi=Ob2, and circles=Ob3). Standard errors are indicated by bars. Data points on each date have been spaced over the x-axis for clarity.
Figure 4 shows the survival curves and the mean spore-load over time (FC plus MC spores) for each treatment. Hosts died at a faster rate when infected horizontally (Likelihood-Ratio-Test for infection route, χ2=39·2, D.F.=1, P<0·0001, n=688), regardless of parasite isolate (Likelihood-Ratio-Test for isolate, χ2=2·7, D.F.=2, P=0·25). The interaction between infection route and isolate on the survival of the hosts was significant (Likelihood-Ratio-Test for the interaction, χ2=20·36, D.F.=2, P<0·0001), due to a wider difference in the survival of hosts infected horizontally and vertically with isolate Ob3 in both the initial mortality rates and the survival at the end of the experiment. In fact, when removing Ob3 hosts from the test, the interaction was not significant (Likelihood-Ratio-Test for the interaction, χ2=1·9, D.F.=2, P=0·16, n=462).

Fig. 4. Spore production and survival curves over time, per treatment. The amount of spores per host (all spore-types included, ln-transformed) is shown with continuous lines, while the proportion of hosts alive is shown with stippled lines.
The difference in timing, growth and maximal spore-load between FC and MC spores of O. bayeri is consistent with the hypothesis that FC spores are responsible for within-host parasite proliferation, while MC spores accumulate for horizontal infection. While within-host proliferation, and thus FC spores, will likely reach a limit when most of the host's tissue is infected, MC spores remain being accumulated. MC spores were observed in the hosts as early as FC spores appeared. An earlier start of production of FC spores makes sense under the hypothesis that the parasite would spread through the host before producing MC spores. For O. bayeri, however, producing MC spores from an early age would provide assurance of some horizontal transmission, given the high mortality observed during the first 10 days. L spores present a high variability in their number and presence, and their role in parasite life-cycle remains unclear.
An alternative explanation for our results is that what we define here as FC spores are actually immature stages of MC spores. Spores usually become more refringent as they mature, corresponding to the description of both types and thus making this alternative plausible. The frequent germination observed for both spore types, and their different size and shape (Vizoso & Ebert, manuscript in preparation), however, suggests that they are not different stages of the same spore type. Moreover, non-refringent spores of size and shape similar to MC spores are frequently found at low densities (D. Vizoso & S. Lass, personal observations).
The route of infection had a strong effect on the within-host spore dynamics of O. bayeri. Spore production in horizontal infections started later than in vertical infections. Vertically infected hosts acquire the parasite before they are released into the brood chamber (about 3 days before birth) and thus the within-host proliferation can start earlier than in horizontally infected hosts, as the latter were exposed to the parasite 3 days after birth. Thus, we would expect a difference in the appearance of spores of about 6 days. Our data show a shorter difference (of about 2 days), suggesting that spore production starts relatively faster in horizontal infections (at least 8 days after exposure) than in vertical infections (about 14 days after infection). This could be explained by slower growth in early stages of proliferation in vertical infections, as observed in other microsporidia (Dunn & Smith, 2001). A slow growth of the parasite, especially during the early stages of the host, will reduce the harm to the host. Thus, it has been suggested that the reduced parasite growth in vertical infections is an adaptation of the parasite to increase host survival, and the likelihood of host (and parasite) reproduction (Dunn, Terry & Smith, 2001). Exclusively vertical microsporidia infecting the crustacean Gammarus duebeni, for example, seem to restrict their spore production to the ovarian tissues (Terry, Dunn & Smith, 1997), perhaps through a non-random sorting of parasites in the host cell lineages during the early stages of infection (Dunn, Terry & Taneyhill, 1998). This efficient use of the host is expected to reduce the metabolic cost of infecting other tissues that would not lead to vertical infection, and thus decrease the parasite's virulence. In a parasite like O. bayeri, however, selection for mechanisms to decrease the harm to the host might be weak, as the parasite can also be spread horizontally. Moreover, the infection of tissues other than the ovaries is necessary for the subsequent horizontal infection.
Horizontal infections can be more harmful to the host than vertical infections for diverse reasons, including faster parasite reproduction, concomitant infections, and a stronger immune reaction. A stronger effect of horizontal infection is reflected in the higher proportion of hosts dying earlier when the infection was acquired horizontally, as reflected by the survival analysis. This higher mortality in horizontally infected hosts does not seem to be a direct effect of parasite proliferation, as the initial spore-load of vertically infected hosts is significantly higher. Therefore, our data support the previous suggestion that the increase of virulence in horizontal infections is to a large extent caused by secondary effects of infection and not by parasite growth (Vizoso & Ebert, manuscript submitted). Within treatments, though, parasite growth seems to increase mortality, as depicted by the coupling of changes in the spore production rate and the host survival rate.
Horizontal infections appeared to be more variable in their spore-load. A simple explanation for this is that the amount of spores is lower in horizontal infections, thus increasing both the measurement error and the natural variation. Nevertheless, we would expect that horizontal transmission itself is variable, as the amount, quality, and diversity of spores will be different between hosts. Vertical transmission, on the other hand, should be less variable, as it occurs in the rather protected environment of the mother host.
In conclusion, our data show that in O. bayeri 2 different types of spore follow different growth patterns, suggesting that they correspond to spore-types described in other microsporidia, FC and MC spores. The observed dynamics for each type are consistent with the idea that FC spores are responsible for within-host proliferation and MC spores for horizontal transmission. Ultimate tests of this hypothesis, though, would imply experimental infections with both types of spores (with the difficulty of segregating them) or ultrastructural studies of the life-cycle of this parasite. Horizontal infections seem to inflict a greater harm to the host than vertical infections. This could explain the low spore-load and early host death observed in horizontal infections. Despite its apparent low within-host performance when compared with vertical transmission, the ecological significance of horizontal transmission for parasite fitness in the case of O. bayeri is clear: it allows the parasite to spread.
D.B.V. thanks Hans-Joachim Carius for helpful discussions on the experimental design and methodological enlightenment; Patrick Mucklow and Olga Sakwinska for invaluable help in the lab; Philip Agnew, Jacob Koella and Lukas Schärer for useful suggestions and discussion. D.B.V. was supported by CONICIT (Venezuela) during the experimental phase and the Roche Research Foundation (Switzerland) during writing (Grant no. 2002-111).

Fig. 1. Types of spores found in Octosporea bayeri infections. The pictures were taken at 400× magnification using a phase-contrast microscope. MC, many-coils spore; FC, few-coils spore (see text for details); L, long spore.

Fig. 2. Means of the ln-transformed spore-load per sampling date, for (A), FC spores; (B), MC spores; and (C), L spores. The arrows indicate the day of horizontal infection. Filled and empty symbols represent vertical and horizontal infections respectively. Different symbols represent different parasite isolates (square=Ob1, rhombi=Ob2, and circles=Ob3). Standard errors are indicated by bars.

Table 1. Appearance of spores in hosts

Fig. 3. Percentage of FC spores as a function of host age in vertically and horizontally infected hosts. Different symbols represent different parasite isolates (square=Ob1, rhombi=Ob2, and circles=Ob3). Standard errors are indicated by bars. Data points on each date have been spaced over the x-axis for clarity.

Fig. 4. Spore production and survival curves over time, per treatment. The amount of spores per host (all spore-types included, ln-transformed) is shown with continuous lines, while the proportion of hosts alive is shown with stippled lines.